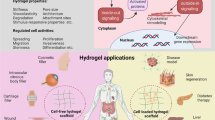
Abstract
Hydrogels — water-insoluble, three-dimensional networks of polymer chains — are used as biomaterials in various biomedical and clinical applications. Their modularity and versatility have led to the development of increasingly complex hydrogels, which can dynamically respond to their environment, release drugs and regenerate cells and tissues. In this Review, we present a model-based modular hydrogel design framework that is application-driven and considers clinical translation early in the design process. In this approach, every component of the hydrogel formulation is optimized towards multifaceted design criteria of the target application, identifying how multiple properties can be integrated into a single formulation. We highlight the fundamental models of polymer physics that provide the basis of modular hydrogel design and examine how synthetic polymer precursors can be integrated to achieve such modularity. Finally, we discuss clinically approved hydrogel formulations, and investigate how challenges in clinical translation may be addressed by a modular design approach.
Key points
-
Hydrogels can be applied as biomaterials for various applications, benefiting from their versatility, their mechanical and structural properties, and their modularity.
-
The clinical translation of hydrogels may be accelerated by a model-driven modular design approach, considering how modular changes affect multiple structure–property interactions.
-
Modular components can be consistently incorporated into hydrogels using fundamental models to optimize their multiple properties.
-
Theoretical models need to be refined to predict relevant properties of hydrogels, and validated with a diverse dataset of well characterized and newly designed hydrogels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wu, D. T., Jeffreys, N., Diba, M. & Mooney, D. J. Viscoelastic biomaterials for tissue regeneration. Tissue Eng. C 28, 289–300 (2022).
Blache, U. et al. Engineered hydrogels for mechanobiology. Nat. Rev. Meth. Prim. 2, 98 (2022).
Yuk, H., Wu, J. & Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 7, 935–952 (2022).
Correa, S. et al. Translational applications of hydrogels. Chem. Rev. 121, 11385–11457 (2021).
Yin, S. & Cao, Y. Hydrogels for large-scale expansion of stem cells. Acta Biomater. 128, 1–20 (2021).
Aguado, B. A., Grim, J. C., Rosales, A. M., Watson-Capps, J. J. & Anseth, K. S. Engineering precision biomaterials for personalized medicine. Sci. Transl. Med. 10, eaam8645 (2018).
Charlet, A., Bono, F. & Amstad, E. Mechanical reinforcement of granular hydrogels. Chem. Sci. 13, 3082–3093 (2022).
Clegg, J. R. et al. Modular fabrication of intelligent material-tissue interfaces for bioinspired and biomimetic devices. Prog. Mater. Sci. 106, 100589 (2019).
Wancura, M. et al. PEG-based hydrogel coatings: design tools for biomedical applications. Ann. Biomed. Eng. https://doi.org/10.1007/s10439-023-03154-9 (2023).
Suthiwanich, K. & Hagiwara, M. Localization of multiple hydrogels with multiCUBE platform spatially guides 3D tissue morphogenesis in vitro. Adv. Mater. Technol. 8, 2201660 (2023).
de Paiva Narciso, N. et al. Design parameters for injectable biopolymeric hydrogels with dynamic covalent chemistry crosslinks. Adv. Healthc. Mater. 12, e2301265 (2023).
Richbourg, N. R. & Peppas, N. A. The swollen polymer network hypothesis: quantitative models of hydrogel swelling, stiffness, and solute transport. Prog. Polym. Sci. 105, 101243 (2020).
Richbourg, N. R., Ravikumar, A. & Peppas, N. A. Solute transport dependence on 3D geometry of hydrogel networks. Macromol. Chem. Phys. 222, 2100138 (2021).
Graham, T. XXXV. — On the properties of silicic acid and other analogous colloidal substances. J. Chem. Soc. 17, 318–327 (1864).
Flory, P. J. Principles of Polymer Chemistry (Cornell Univ. Press, 1953).
Flory, P. J. & Rehner, J. Statistical mechanics of cross‐linked polymer networks. I. Rubberlike elasticity. J. Chem. Phys. 11, 512–520 (1943).
Flory, P. J. & Rehner, J. Statistical mechanics of cross‐linked polymer networks. II. Swelling. J. Chem. Phys. 11, 521–526 (1943).
Wichterle, O. & LÍM, D. Hydrophilic gels for biological use. Nature 185, 117–118 (1960).
Wichterle, O. & Lim, D. Process for producing shaped articles from three-dimensional hydrophilic high polymers. US patent US2976576A (1961).
Treloar, L. R. G. The Physics of Rubber Elasticity (Oxford Univ. Press, 1975).
Peppas, N. A. & Merrill, E. W. Crosslinked poly(vinyl alcohol) hydrogels as swollen elastic networks. J. Appl. Polym. Sci. 21, 1763–1770 (1977).
Bray, J. C. & Merrill, E. W. Poly(vinyl alcohol) hydrogels. Formation by electron beam irradiation of aqueous solutions and subsequent crystallization. J. Appl. Polym. Sci. 17, 3779–3794 (1973).
Erman, B. & Mark, J. E. Structures and Properties of Rubberlike Networks (Oxford Univ. Press, 1997).
Amsden, B. Solute diffusion within hydrogels. mechanisms and models. Macromolecules 31, 8382–8395 (1998).
Flory, P. J. Statistical Mechanics of Chain Molecules (Interscience, 1980).
Canal, T. & Peppas, N. A. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J. Biomed. Mater. Res. 23, 1183–1193 (1989).
De Gennes, P.-G. Scaling Concepts in Polymer Physics (Cornell Univ. Press, 1979).
Lustig, S. R. & Peppas, N. A. Solute diffusion in swollen membranes. IX. Scaling laws for solute diffusion in gels. J. Appl. Polym. Sci. 36, 735–747 (1988).
Ogston Alexander, G., Preston, B. N., Wells, J. D. & Snowden, J. M. On the transport of compact particles through solutions of chain-polymers. Proc. R. Soc. London. A 333, 297–316 (1973).
Axpe, E. et al. A multiscale model for solute diffusion in hydrogels. Macromolecules 52, 6889–6897 (2019).
Richbourg, N. R. et al. Precise control of synthetic hydrogel network structure via linear, independent synthesis-swelling relationships. Sci. Adv. 7, eabe3245 (2021).
Richbourg, N. R. & Peppas, N. A. High-throughput FRAP analysis of solute diffusion in hydrogels. Macromolecules 54, 10477–10486 (2021).
Richbourg, N. R. & Peppas, N. A. Solute diffusion and partitioning in multi-arm poly(ethylene glycol) hydrogels. J. Mater. Chem. B https://doi.org/10.1039/D2TB02004A (2023).
Richbourg, N. R., Rausch, M. K. & Peppas, N. A. Cross-evaluation of stiffness measurement methods for hydrogels. Polymer 258, 125316 (2022).
Richbourg, N. R. & Peppas, N. A. Structurally decoupled stiffness and solute transport in multi-arm poly(ethylene glycol) hydrogels. Biomaterials 301, 122272 (2023).
Bello, A. B., Kim, D., Kim, D., Park, H. & Lee, S.-H. Engineering and functionalization of gelatin biomaterials: from cell culture to medical applications. Tissue Eng. B 26, 164–180 (2020).
Spearman, B. S. et al. Tunable methacrylated hyaluronic acid-based hydrogels as scaffolds for soft tissue engineering applications. J. Biomed. Mater. Res. A 108, 279–291 (2020).
Padmavathi, N. C. & Chatterji, P. R. Structural characteristics and swelling behavior of poly(ethylene glycol) diacrylate hydrogels. Macromolecules 29, 1976–1979 (1996).
Tibbitt, M. W., Kloxin, A. M., Sawicki, L. A. & Anseth, K. S. Mechanical properties and degradation of chain and step-polymerized photodegradable hydrogels. Macromolecules 46, 2785–2792 (2013).
Beamish, J. A., Zhu, J., Kottke-Marchant, K. & Marchant, R. E. The effects of monoacrylated poly(ethylene glycol) on the properties of poly(ethylene glycol) diacrylate hydrogels used for tissue engineering. J. Biomed. Mater. Res. A 92, 441–450 (2010).
Pathak, C. P., Sawhney, A. S. & Hubbell, J. A. Rapid photopolymerization of immunoprotective gels in contact with cells and tissue. J. Am. Chem. Soc. 114, 8311–8312 (1992).
Hossainy, S. F. A. & Hubbell, J. A. Molecular weight dependence of calcification of polyethylene glycol hydrogels. Biomaterials 15, 921–925 (1994).
Cruise, G. M., Scharp, D. S. & Hubbell, J. A. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials 19, 1287–1294 (1998).
Cha, C., Jeong, J. H., Shim, J. & Kong, H. Tuning the dependency between stiffness and permeability of a cell encapsulating hydrogel with hydrophilic pendant chains. Acta Biomater. 7, 3719–3728 (2011).
Browning, M. B., Wilems, T., Hahn, M. S. & Cosgriff-Hernandez, E. Compositional control of poly(ethylene glycol) hydrogel modulus independent of mesh size. J. Biomed. Mater. Res. A 98, 268–273 (2011).
Munoz-Pinto, D. J., Samavedi, S., Grigoryan, B. & Hahn, M. S. Impact of secondary reactive species on the apparent decoupling of poly(ethylene glycol) diacrylate hydrogel average mesh size and modulus. Polymer 77, 227–238 (2015).
Nguyen, K. T. & West, J. L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 23, 4307–4314 (2002).
Choi, J. R., Yong, K. W., Choi, J. Y. & Cowie, A. C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. BioTechniques 66, 40–53 (2019).
Moore, E. M. & West, J. L. Bioactive poly(ethylene glycol) acrylate hydrogels for regenerative engineering. Regen. Eng. Transl. Med. 5, 167–179 (2019).
Hamidi, M., Azadi, A. & Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug. Deliv. Rev. 60, 1638–1649 (2008).
Huang, Y., Szleifer, I. & Peppas, N. A. A molecular theory of polymer gels. Macromolecules 35, 1373–1380 (2002).
Sakai, T. et al. Design and fabrication of a high-strength hydrogel with ideally homogeneous network structure from tetrahedron-like macromonomers. Macromolecules 41, 5379–5384 (2008).
Andreopoulos, F. M. et al. Photoscissable hydrogel synthesis via rapid photopolymerization of novel PEG-based polymers in the absence of photoinitiators. J. Am. Chem. Soc. 118, 6235–6240 (1996).
Zhao, X. & Harris, J. M. Novel degradable poly(ethylene glycol) hydrogels for controlled release of protein. J. Pharm. Sci. 87, 1450–1458 (1998).
Keys, K. B., Andreopoulos, F. M. & Peppas, N. A. Poly(ethylene glycol) star polymer hydrogels. Macromolecules 31, 8149–8156 (1998).
Lutolf, M. P. & Hubbell, J. A. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules 4, 713–722 (2003).
Fairbanks, B. D. et al. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv. Mater. 21, 5005–5010 (2009).
Raza, A. & Lin, C.-C. The influence of matrix degradation and functionality on cell survival and morphogenesis in PEG-based hydrogels. Macromol. Biosci. 13, 1048–1058 (2013).
Phelps, E. A. et al. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv. Mater. 24, 64–70 (2012).
Darling, N. J., Hung, Y.-S., Sharma, S. & Segura, T. Controlling the kinetics of thiol-maleimide Michael-type addition gelation kinetics for the generation of homogenous poly(ethylene glycol) hydrogels. Biomaterials 101, 199–206 (2016).
Jansen, L. E., Negrón-Piñeiro, L. J., Galarza, S. & Peyton, S. R. Control of thiol-maleimide reaction kinetics in PEG hydrogel networks. Acta Biomater. 70, 120–128 (2018).
Paez, J. I., Farrukh, A., Valbuena-Mendoza, R., Włodarczyk-Biegun, M. K. & del Campo, A. Thiol-methylsulfone-based hydrogels for 3D cell encapsulation. ACS Appl. Mater. Interf. 12, 8062–8072 (2020).
Rizzo, R., Petelinšek, N., Bonato, A. & Zenobi-Wong, M. From free-radical to radical-free: a paradigm shift in light-mediated biofabrication. Adv. Sci. 10, 2205302 (2023).
Farahani, P. E., Adelmund, S. M., Shadish, J. A. & DeForest, C. A. Photomediated oxime ligation as a bioorthogonal tool for spatiotemporally-controlled hydrogel formation and modification. J. Mater. Chem. B 5, 4435–4442 (2017).
Lee, S., Tong, X. & Yang, F. Effects of the poly(ethylene glycol) hydrogel crosslinking mechanism on protein release. Biomater. Sci. 4, 405–411 (2016).
Rehmann, M. S. et al. Tuning and predicting mesh size and protein release from step growth hydrogels. Biomacromolecules 18, 3131–3142 (2017).
Yavitt, F. M. et al. The effect of thiol structure on allyl sulfide photodegradable hydrogels and their application as a degradable scaffold for organoid passaging. Adv. Mater. 32, 1905366 (2020).
Jansen, L. E. et al. A poly(ethylene glycol) three-dimensional bone marrow hydrogel. Biomaterials 280, 121270 (2022).
Galarza, S., Crosby, A. J., Pak, C. & Peyton, S. R. Control of astrocyte quiescence and activation in a synthetic brain hydrogel. Adv. Healthc. Mater. 9, 1901419 (2020).
Koetting, M. C., Peters, J. T., Steichen, S. D. & Peppas, N. A. Stimulus-responsive hydrogels: theory, modern advances, and applications. Mater. Sci. Eng. R 93, 1–49 (2015).
Qu, M. et al. Stimuli-responsive delivery of growth factors for tissue engineering. Adv. Healthc. Mater. 9, 1901714 (2020).
Culver, H. R., Clegg, J. R. & Peppas, N. A. Analyte-responsive hydrogels: intelligent materials for biosensing and drug delivery. Acc. Chem. Res. 50, 170–178 (2017).
Sharpe, L. A., Daily, A. M., Horava, S. D. & Peppas, N. A. Therapeutic applications of hydrogels in oral drug delivery. Expert. Opin. Drug. Deliv. 11, 901–915 (2014).
Alvarez-Lorenzo, C., Anguiano-Igea, S., Varela-García, A., Vivero-Lopez, M. & Concheiro, A. Bioinspired hydrogels for drug-eluting contact lenses. Acta Biomater. 84, 49–62 (2018).
Webber, M. J. & Tibbitt, M. W. Dynamic and reconfigurable materials from reversible network interactions. Nat. Rev. Mater. 127, 167–184 (2022).
Dimatteo, R., Darling, N. J. & Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug. Deliv. Rev. 127, 167–184 (2018).
Bovone, G., Dudaryeva, O. Y., Marco-Dufort, B. & Tibbitt, M. W. Engineering hydrogel adhesion for biomedical applications via chemical design of the junction. ACS Biomater. Sci. Eng. 7, 4048–4076 (2021).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020).
Jons, C. K. et al. Yield-stress and creep control depot formation and persistence of injectable hydrogels following subcutaneous administration. Adv. Funct. Mater. 32, 2203402 (2022).
Muir, V. G. & Burdick, J. A. Chemically modified biopolymers for the formation of biomedical hydrogels. Chem. Rev. 121, 10908–10949 (2021).
Li, F., Tang, J., Geng, J., Luo, D. & Yang, D. Polymeric DNA hydrogel: design, synthesis and applications. Prog. Polym. Sci. 98, 101163 (2019).
Gačanin, J., Synatschke, C. V. & Weil, T. Biomedical applications of DNA-based hydrogels. Adv. Funct. Mater. 30, 1906253 (2020).
Lee, K. Y. & Mooney, D. J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 37, 106 (2012).
Bai, R., Yang, J. & Suo, Z. Fatigue of hydrogels. Eur. J. Mech. A 74, 337–370 (2019).
Zhao, X. Designing toughness and strength for soft materials. Proc. Natl Acad. Sci. USA 114, 8138 (2017).
Freedman, B. R. et al. Degradable and removable tough adhesive hydrogels. Adv. Mater. 33, 2008553 (2021).
Lanier, O. L., D’Andrea, A. P., Shodeinde, A. & Peppas, N. A. siRNA delivery from cationic nanocarriers prepared by diffusion-assisted loading in the presence and absence of electrostatic interactions. J. Appl. Polym. Sci. 141, e55029 (2024).
Clegg, J. R., Ludolph, C. M. & Peppas, N. A. QCM-D assay for quantifying the swelling, biodegradation, and protein adsorption of intelligent nanogels. J. Appl. Polym. Sci. 137, 48655 (2019).
Zhong Justin, X., Clegg John, R., Ander Eric, W. & Peppas Nicholas, A. Tunable poly(methacrylic acid‐co‐acrylamide) nanoparticles through inverse emulsion polymerization. J. Biomed. Mater. Res. A 106, 1677–1686 (2018).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug. Discov. 20, 101–124 (2021).
Ramirez-Velez, I. & Belardi, B. Storming the gate: new approaches for targeting the dynamic tight junction for improved drug delivery. Adv. Drug. Deliv. Rev. 199, 114905 (2023).
Cuggino, J. C., Blanco, E. R. O., Gugliotta, L. M., Alvarez Igarzabal, C. I. & Calderón, M. Crossing biological barriers with nanogels to improve drug delivery performance. J. Control. Release 307, 221–246 (2019).
Daly, A. C., Riley, L., Segura, T. & Burdick, J. A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 5, 20–43 (2020).
Wechsler, M. E. et al. Engineered microscale hydrogels for drug delivery, cell therapy, and sequencing. Biomed. Microdevices 21, 31 (2019).
Riley, L. et al. Void volume fraction of granular scaffolds. Small 19, 2303466 (2023).
Muir, V. G., Qazi, T. H., Shan, J., Groll, J. & Burdick, J. A. Influence of microgel fabrication technique on granular hydrogel properties. ACS Biomater. Sci. Eng. 7, 4269–4281 (2021).
Muir, V. G. et al. Influence of microgel and interstitial matrix compositions on granular hydrogel composite properties. Adv. Sci. 10, 2206117 (2023).
Zhao, Z. et al. Composite hydrogels in three-dimensional in vitro models. Front. Bioeng. Biotechnol. 8, 611 (2020).
Widener, A. E., Roberts, A. & Phelps, E. A. Single versus dual microgel species for forming guest–host microporous annealed particle PEG-MAL hydrogel. J. Biomed. Mater. Res. A 111, https://doi.org/10.1002/jbm.a.37540 (2023).
Lavrador, P., Esteves, M. R., Gaspar, V. M. & Mano, J. F. Stimuli-responsive nanocomposite hydrogels for biomedical applications. Adv. Funct. Mater. 31, 2005941 (2021).
Omar, J., Ponsford, D., Dreiss, C. A., Lee, T.-C. & Loh, X. J. Supramolecular hydrogels: design strategies and contemporary biomedical applications. Chem. Asian J. 17, e202200081 (2022).
Bernhard, S. & Tibbitt, M. W. Supramolecular engineering of hydrogels for drug delivery. Adv. Drug. Deliv. Rev. 171, 240–256 (2021).
Li, Y., Zhu, C., Dong, Y. & Liu, D. Supramolecular hydrogels: mechanical strengthening with dynamics. Polymer 210, 122993 (2020).
Bovone, G. et al. Supramolecular reinforcement of polymer–nanoparticle hydrogels for modular materials design. Adv. Mater. 34, 2106941 (2021).
Kirchhof, S., Goepferich, A. M. & Brandl, F. P. Hydrogels in ophthalmic applications. Eur. J. Pharm. Biopharm. 95, 227–238 (2015).
Lee, K. Y. & Mooney, D. J. Hydrogels for tissue engineering. Chem. Rev. 101, 1869–1880 (2001).
Fogg, K. C. et al. Roadmap on biomaterials for women’s health. J. Phys. Mater. 6, 012501 (2022).
Oyen, M. L. Biomaterials science and engineering to address unmet needs in women’s health. MRS Bull. 47, 864–871 (2022).
Cascone, S. & Lamberti, G. Hydrogel-based commercial products for biomedical applications: a review. Int. J. Pharm. 573, 118803 (2020).
Mitura, S., Sionkowska, A. & Jaiswal, A. Biopolymers for hydrogels in cosmetics: review. J. Mater. Sci. Mater. Med. 31, 50 (2020).
Stapleton, F., Stretton, S., Papas, E., Skotnitsky, C. & Sweeney, D. F. Silicone hydrogel contact lenses and the ocular surface. Ocul. Surf. 4, 24–43 (2006).
DiPasquale, S. A. et al. One week sustained in vivo therapeutic release and safety of novel extended-wear silicone hydrogel contact lenses. Adv. Healthc. Mater. 11, 2101263 (2022).
DiPasquale, S. A., Uricoli, B., DiCerbo, M. C., Brown, T. L. & Byrne, M. E. Controlled release of multiple therapeutics from silicone hydrogel contact lenses for post-cataract/post-refractive surgery and uveitis treatment. Transl. Vis. Sci. Technol. 10, 5 (2021).
Pall, B., Gomes, P., Yi, F. & Torkildsen, G. Management of ocular allergy itch with an antihistamine-releasing contact lens. Cornea 38, 713–717 (2019).
Takamatsu, T., Chen, Y., Yoshimasu, T., Nishizawa, M. & Miyake, T. Highly efficient, flexible wireless-powered circuit printed on a moist, soft contact lens. Adv. Mater. Technol. 4, 1800671 (2019).
Mandal, A., Clegg, J. R., Anselmo, A. C. & Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 5, e10158 (2020).
Townsend, J. M., Beck, E. C., Gehrke, S. H., Berkland, C. J. & Detamore, M. S. Flow behavior prior to crosslinking: the need for precursor rheology for placement of hydrogels in medical applications and for 3D bioprinting. Prog. Polym. Sci. 91, 126–140 (2019).
Peppas, N. A. & Hoffman, A. S. In Biomaterials Science 4th edn (eds Wagner, W. R., Sakiyama-Elbert, S. E., Zhang, G. & Yaszemski, M. J.) 153–166 (Academic Press, 2020).
Reisener, M.-J., Pumberger, M., Shue, J., Girardi, F. P. & Hughes, A. P. Trends in lumbar spinal fusion — a literature review. J. Spine Surg. 6, https://doi.org/10.21037/jss-20-492 (2020).
Vernengo, J., Fussell, G. W., Smith, N. G. & Lowman, A. M. Evaluation of novel injectable hydrogels for nucleus pulposus replacement. J. Biomed. Mater. Res. B 84, 64–69 (2008).
Fusco, A. et al. 168. Minimally invasive hydrogel nucleoplasty in a goat model of moderate severity disc degeneration. Spine J. 21, S84 (2021).
Hunter, D. J. Viscosupplementation for osteoarthritis of the knee. N. Engl. J. Med. 372, 1040–1047 (2015).
Finelli, I., Chiessi, E., Galesso, D., Renier, D. & Paradossi, G. Gel-like structure of a hexadecyl derivative of hyaluronic acid for the treatment of osteoarthritis. Macromol. Biosci. 9, 646–653 (2009).
Alonso, J. M., Andrade del Olmo, J., Perez Gonzalez, R. & Saez-Martinez, V. Injectable hydrogels: from laboratory to industrialization. Polymers 13, 650 (2021).
Finn, J. C. & Cox, S. Fillers in the periorbital complex. Facial Plastic Surg. Clin. North. Am. 15, 123–132 (2007).
Sokol Eric, R., Karram Mickey, M. & Dmochowski, R. Efficacy and safety of polyacrylamide hydrogel for the treatment of female stress incontinence: a randomized, prospective, multicenter North American Study. J. Urol. 192, 843–849 (2014).
Lose, G., Mouritsen, L. & Nielsen, J. B. A new bulking agent (polyacrylamide hydrogel) for treating stress urinary incontinence in women. BJU Int. 98, 100–104 (2006).
Freedman, B. R. & Mooney, D. J. Biomaterials to mimic and heal connective tissues. Adv. Mater. 31, 1806695 (2019).
Kehoe, S., Zhang, X. F. & Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury 43, 553–572 (2012).
Serban, M. A. Translational biomaterials — the journey from the bench to the market — think ‘product’. Curr. Opin. Biotechnol. 40, 31–34 (2016).
Peppas, N. A. & Khademhosseini, A. Make better, safer biomaterials. Nature 540, 335 (2016).
Madl, C. M., Katz, L. M. & Heilshorn, S. C. Tuning bulk hydrogel degradation by simultaneous control of proteolytic cleavage kinetics and hydrogel network architecture. ACS Macro Lett. 7, 1302–1307 (2018).
Benoit, D. S. W., Durney, A. R. & Anseth, K. S. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 12, 1663–1673 (2006).
Li, X. et al. Precise control and prediction of hydrogel degradation behavior. Macromolecules 44, 3567–3571 (2011).
Reid, B. et al. PEG hydrogel degradation and the role of the surrounding tissue environment. J. Tissue Eng. Regen. Med. 9, 315–318 (2015).
Pearce, H. A. et al. Evaluating the physicochemical effects of conjugating peptides into thermogelling hydrogels for regenerative biomaterials applications. Regen. Biomater. 8, rbab073 (2021).
Shen, J. et al. Hydrolytically degradable POSS-PEG hybrid hydrogels prepared in aqueous phase with tunable mechanical properties, swelling ratio and degradation rate. Reactive Funct. Polym. 123, 91–96 (2018).
Huang, W. et al. Maleimide–thiol adducts stabilized through stretching. Nat. Chem. 11, 310–319 (2019).
Bretherton, R. C. et al. User-controlled 4D biomaterial degradation with substrate-selective sortase transpeptidases for single-cell biology. Adv. Mater. 35, e2209904 (2023).
Gilchrist, A. E. et al. Encapsulation of murine hematopoietic stem and progenitor cells in a thiol-crosslinked maleimide-functionalized gelatin hydrogel. Acta Biomater. 131, 138–148 (2021).
Wiley, K. L., Sutherland, B. P., Ogunnaike, B. A. & Kloxin, A. M. Rational design of hydrogel networks with dynamic mechanical properties to mimic matrix remodeling. Adv. Healthc. Mater. 11, 2101947 (2022).
Zhang, W. et al. Fracture toughness and fatigue threshold of tough hydrogels. ACS Macro Lett. 8, 17–23 (2019).
Lin, S. et al. Anti-fatigue-fracture hydrogels. Sci. Adv. 5, eaau8528 (2019).
Rodriguez-Rivera, G. J., Green, M., Shah, V., Leyendecker, K. & Cosgriff-Hernandez, E. A user’s guide to degradation testing of polyethylene glycol-based hydrogels: from in vitro to in vivo studies. J. Biomed. Mater. Res. A https://doi.org/10.1002/jbm.a.37609 (2023).
Swartzlander, M. D. et al. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials 41, 26–36 (2015).
Vegas, A. J. et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol. 34, 345–352 (2016).
Nian, G., Kim, J., Bao, X. & Suo, Z. Making highly elastic and tough hydrogels from doughs. Adv. Mater. 34, e2206577 (2022).
GhavamiNejad, A., Ashammakhi, N., Wu, X. Y. & Khademhosseini, A. Crosslinking strategies for 3D bioprinting of polymeric hydrogels. Small 16, e2002931 (2020).
Riley, L., Schirmer, L. & Segura, T. Granular hydrogels: emergent properties of jammed hydrogel microparticles and their applications in tissue repair and regeneration. Curr. Opin. Biotechnol. 60, 1–8 (2019).
Kharaziha, M., Baidya, A. & Annabi, N. Rational design of immunomodulatory hydrogels for chronic wound healing. Adv. Mater. 33, 2100176 (2021).
Lee, B. M., Park, S. J., Noh, I. & Kim, C.-H. The effects of the molecular weights of hyaluronic acid on the immune responses. Biomater. Res. 25, 27 (2021).
Browne, S., Hossainy, S. & Healy, K. Hyaluronic acid macromer molecular weight dictates the biophysical properties and in vitro cellular response to semisynthetic hydrogels. ACS Biomater. Sci. Eng. 6, 1135–1143 (2020).
Snetkov, P., Zakharova, K., Morozkina, S., Olekhnovich, R. & Uspenskaya, M. Hyaluronic acid: the influence of molecular weight on structural, physical, physico-chemical, and degradable properties of biopolymer. Polymers 12, 1800 (2020).
Qazi, T. H. et al. Programming hydrogels to probe spatiotemporal cell biology. Cell Stem Cell https://doi.org/10.1016/j.stem.2022.03.013 (2022).
Zhang, K. et al. Evidence-based biomaterials research. Bioact. Mater. 15, 495–503 (2022).
Axpe, E., Orive, G., Franze, K. & Appel, E. A. Towards brain-tissue-like biomaterials. Nat. Commun. 11, 3423 (2020).
Bej, R. & Haag, R. Mucus-inspired dynamic hydrogels: synthesis and future perspectives. J. Am. Chem. Soc. 144, 20137–20152 (2022).
Bierman-Duquette, R. D. et al. Engineering tissues of the central nervous system: interfacing conductive biomaterials with neural stem/progenitor cells. Adv. Healthc. Mater. 11, 2101577 (2022).
Gilchrist, A. E. & Harley, B. A. C. Engineered tissue models to replicate dynamic interactions within the hematopoietic stem cell niche. Adv. Healthc. Mater. 11, e2102130 (2021).
Caliari, S. R. & Burdick, J. A. A practical guide to hydrogels for cell culture. Nat. Methods 13, 405–414 (2016).
Li, J., Xing, R., Bai, S. & Yan, X. Recent advances of self-assembling peptide-based hydrogels for biomedical applications. Soft Matter 15, 1704–1715 (2019).
Yang, C. et al. An injectable antibiotic hydrogel that scavenges proinflammatory factors for the treatment of severe abdominal trauma. Adv. Funct. Mater. 32, 2111698 (2022).
Tu, Z. et al. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 7, 557–574 (2022).
Francis, R. M. & DeForest, C. A. 4D biochemical photocustomization of hydrogel scaffolds for biomimetic tissue engineering. Acc. Mater. Res. 8, 704–715 (2023).
Dhand, A. P., Galarraga, J. H. & Burdick, J. A. Enhancing biopolymer hydrogel functionality through interpenetrating networks. Trends Biotechnol. 39, 519–538 (2021).
Wang, H. & Heilshorn, S. C. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv. Mater. 27, 3717–3736 (2015).
Anthis, A. H. C. et al. Modular stimuli-responsive hydrogel sealants for early gastrointestinal leak detection and containment. Nat. Commun. 13, 7311 (2022).
Wang, Y., Yan, J. & Goult, B. T. Force-dependent binding constants. Biochemistry 58, 4696–4709 (2019).
Lele, T. P., Brock, A. & Peyton, S. R. Emerging concepts and tools in cell mechanomemory. Ann. Biomed. Eng. 48, 2103–2112 (2020).
Mussel, M., Basser, P. J. & Horkay, F. Ion-induced volume transition in gels and its role in biology. Gels 7, 20 (2021).
Acknowledgements
This work was supported by the Office of the Dean of the Cockrell School of Engineering at The University of Texas at Austin for the Institute for Biomaterials, Drug Delivery and Regenerative Medicine.
Author information
Authors and Affiliations
Contributions
All authors contributed to drafting and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Matthew Webber and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Swollen polymer network model: www.hydrogeldesign.org
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Richbourg, N., Wechsler, M.E., Rodriguez-Cruz, J.J. et al. Model-based modular hydrogel design. Nat Rev Bioeng (2024). https://doi.org/10.1038/s44222-024-00167-4
Accepted:
Published:
DOI: https://doi.org/10.1038/s44222-024-00167-4