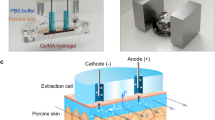
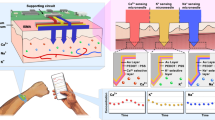
Abstract
Microneedles (MNs) are microscopic needles that are applied to the skin in a minimally invasive way to facilitate transdermal drug delivery and/or uptake of interstitial fluid from the skin, which contains a variety of metabolites that can serve as biomarkers. The collection of interstitial fluid can be followed by post-sampling analysis or in situ real-time biosensing for disease diagnosis and drug monitoring. The painless and easy administration of MNs is appealing to patients, especially for long-term monitoring. In this Review, we discuss the use of MNs for biosensing purposes. We highlight the different types of MNs and sensing technologies used to develop MN-based biosensors. In addition, we discuss the potential to integrate MNs with wearable devices for real-time monitoring to improve point-of-care testing. Finally, we review the translational hurdles to be considered in bringing this technology from benchtop to bedside.
Key points
-
Skin, as the largest body organ, houses a wide range of metabolites that may be identified as biomarkers for disease prognosis and monitoring.
-
Microneedle (MN) technology is primarily used as a drug-delivery tool; however, there is a paradigm shift toward utilizing MNs for biosensing purposes.
-
MN-based biosensors can provide a powerful platform for high-throughput and rapid disease state diagnosis and monitoring.
-
Developing new sensing modalities can improve the accuracy, precision and sensitivity of MN-based biosensors.
-
MN-based biosensors offer a wide range of benefits for patients who require continuous and convenient health surveillance; however, multiple translational hurdles must be overcome before MN-based biosensors reach the market.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Houten, S. M. Metabolomics: unraveling the chemical individuality of common human diseases. Ann. Med. 41, 402–407 (2009).
Wishart, D. S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 15, 473–484 (2016).
Lu, H., Zada, S., Yang, L. & Dong, H. Microneedle-based device for biological analysis. Front. Bioeng. Biotechnol. 10, 851134 (2022).
Xue, P. et al. Blood sampling using microneedles as a minimally invasive platform for biomedical diagnostics. Appl. Mater. Today 13, 144–157 (2018).
Corrie, S. R., Coffey, J. W., Islam, J., Markey, K. A. & Kendall, M. A. F. Blood, sweat, and tears: developing clinically relevant protein biosensors for integrated body fluid analysis. Analyst 140, 4350–4364 (2015).
Luszczynska, A., Benight, C. C. & Cieslak, R. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: a systematic review. Eur. Psychol. 14, 51–62 (2009).
Slupsky, C. M. et al. Investigations of the effects of gender, diurnal variation, and age in human urinary metabolomic profiles. Anal. Chem. 79, 6995–7004 (2007).
Liu, Y., Yu, Q., Luo, X., Yang, L. & Cui, Y. Continuous monitoring of diabetes with an integrated microneedle biosensing device through 3D printing. Microsyst. Nanoeng. 7, 75 (2021).
Chung, M., Fortunato, G. & Radacsi, N. Wearable flexible sweat sensors for healthcare monitoring: a review. J. R. Soc. Interface 16, 20190217 (2019).
Bandodkar, A. J. & Wang, J. Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol. 32, 363–371 (2014).
Paliwal, S., Hwang, B. H., Tsai, K. Y. & Mitragotri, S. Diagnostic opportunities based on skin biomarkers. Eur. J. Pharm. Sci. 50, 546–556 (2013).
Himawan, A. et al. Where microneedle meets biomarkers: futuristic application for diagnosing and monitoring localized external organ diseases. Adv. Healthc. Mater. 12, 2202066 (2023).
Jarmusch, A. K. et al. Initial development toward non-invasive drug monitoring via untargeted mass spectrometric analysis of human skin. Anal. Chem. 91, 8062–8069 (2019).
Bodenlenz, M. et al. Open flow microperfusion as a dermal pharmacokinetic approach to evaluate topical bioequivalence. Clin. Pharmacokinet. 56, 91–98 (2017).
Miller, P. R. et al. Extraction and biomolecular analysis of dermal interstitial fluid collected with hollow microneedles. Commun. Biol. 1, 173 (2018).
Samant, P. P. et al. Sampling interstitial fluid from human skin using a microneedle patch. Sci. Transl. Med. 12, eaaw0285 (2020).
McKenna, P. E. et al. Polymeric microarray patches for enhanced transdermal delivery of the poorly soluble drug olanzapine. ACS Appl. Mater. Interfaces 15, 31300–31319 (2023).
Paris, J. L., Vora, L. K., Torres, M. J., Mayorga, C. & Donnelly, R. F. Microneedle array patches for allergen-specific immunotherapy. Drug Discov. Today 28, 103556 (2023).
Tekko, I. A. Novel bilayer microarray patch‐assisted long‐acting micro‐depot cabotegravir intradermal delivery for HIV pre‐exposure prophylaxis. Adv. Funct. Mater. 32, 2106999 (2022).
Vora, L. K., Courtenay, A. J., Tekko, I. A., Larrañeta, E. & Donnelly, R. F. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int. J. Biol. Macromol. 146, 290–298 (2020).
Chambers, R. Microdissection studies, III. Some problems in the maturation and fertilization of the echinoderm egg. Biol. Bull. 41, 318–350 (1921).
Rawson, T. M. et al. Microneedle biosensors for real-time, minimally invasive drug monitoring of phenoxymethylpenicillin: a first-in-human evaluation in healthy volunteers. Lancet Digit. Health 1, e335–e343 (2019).
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022).
MarketsandMarkets. Biosensors Market Size, Share, Industry Growth Analysis Report by Type, Product (Wearable, Non-wearable), Technology, Application (POC, Home Diagnostics, Research Lab, Environmental Monitoring, Food & Beverages, Biodefense), Global Growth Driver and Industry Forecast to 2026 https://www.marketsandmarkets.com/Market-Reports/biosensors-market-798.html (2023).
Larrañeta, E., Lutton, R. E. M., Woolfson, A. D. & Donnelly, R. F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R. Rep. 104, 1–32 (2016).
Rodgers, A. M., Cordeiro, A. S., Kissenpfennig, A. & Donnelly, R. F. Microneedle arrays for vaccine delivery: the possibilities, challenges and use of nanoparticles as a combinatorial approach for enhanced vaccine immunogenicity. Expert Opin. Drug Deliv. 15, 851–867 (2018).
Vora, L. K. et al. Microneedle array systems for long-acting drug delivery. Eur. J. Pharm. Biopharm. 159, 44–76 (2021).
Cárcamo-Martínez, Á. et al. Hollow microneedles: a perspective in biomedical applications. Int. J. Pharm. 599, 120455 (2021).
Larrañeta, E. & Vora, L. In Microneedles for Drug and Vaccine Delivery and Patient Monitoring (ed. Donnelly, R. F.) 177–205 (Wiley, 2018).
Gowers, S. A. N. et al. Development of a minimally invasive microneedle-based sensor for continuous monitoring of β-lactam antibiotic concentrations in vivo. ACS Sens. 4, 1072–1080 (2019).
Huang, X. et al. 3D-assembled microneedle ion sensor-based wearable system for the transdermal monitoring of physiological ion fluctuations. Microsyst. Nanoeng. 9, 25 (2023).
Henry, S., McAllister, D. V., Allen, M. G. & Prausnitz, M. R. Microfabricated microneedles: a novel approach to transdermal drug delivery. J. Pharm. Sci. 87, 922–925 (1998).
Kim, S., Lee, M. S., Yang, H. S. & Jung, J. H. Enhanced extraction of skin interstitial fluid using a 3D printed device enabling tilted microneedle penetration. Sci. Rep. 11, 14018 (2021).
Chen, L. et al. Local extraction and detection of early stage breast cancers through a microneedle and nano-Ag/MBL film based painless and blood-free strategy. Mater. Sci. Eng. C. 109, 110402 (2020).
Corrie, S. R. et al. Surface-modified microprojection arrays for intradermal biomarker capture, with low non-specific protein binding. Lab Chip 10, 2655–2658 (2010).
Zhang, B. L., Yang, Y., Zhao, Z. Q. & Guo, X. D. A gold nanoparticles deposited polymer microneedle enzymatic biosensor for glucose sensing. Electrochim. Acta 358, 136917 (2020).
Tortolini, C., Cass, A. E. G., Pofi, R., Lenzi, A. & Antiochia, R. Microneedle-based nanoporous gold electrochemical sensor for real-time catecholamine detection. Microchim. Acta 189, 180 (2022).
Ming, D. K. et al. Real-time continuous measurement of lactate through a minimally invasive microneedle patch: a phase I clinical study. BMJ Innov. 8, 87–94 (2022).
Chinnadayyala, S. R. & Cho, S. Porous platinum black-coated minimally invasive microneedles for non-enzymatic continuous glucose monitoring in interstitial fluid. Nanomaterials 11, 37 (2020).
Wang, Q. et al. Intradermal glycine detection with a wearable microneedle biosensor: the first in vivo assay. Anal. Chem. 94, 11856–11864 (2022).
Dervisevic, M., Alba, M., Adams, T. E., Prieto-Simon, B. & Voelcker, N. H. Electrochemical immunosensor for breast cancer biomarker detection using high-density silicon microneedle array. Biosens. Bioelectron. 192, 113496 (2021).
Senel, M., Dervisevic, M. & Voelcker, N. H. Gold microneedles fabricated by casting of gold ink used for urea sensing. Mater. Lett. 243, 50–53 (2019).
Lee, W. et al. Conformable microneedle pH sensors via the integration of two different siloxane polymers for mapping peripheral artery disease. Sci. Adv. 7, eabi6290 (2021).
Downs, A. M. et al. Microneedle electrochemical aptamer-based sensing: real-time small molecule measurements using sensor-embedded, commercially-available stainless steel microneedles. Biosens. Bioelectron. 236, 115408 (2023).
Lin, S. et al. Wearable microneedle-based electrochemical aptamer biosensing for precision dosing of drugs with narrow therapeutic windows. Sci. Adv. 8, eabq4539 (2023).
Zou, Y. et al. Minimally invasive electrochemical continuous glucose monitoring sensors: recent progress and perspective. Biosens. Bioelectron. 225, 115103 (2023).
Parrilla, M., Detamornrat, U., Domínguez-Robles, J., Donnelly, R. F. & De Wael, K. Wearable hollow microneedle sensing patches for the transdermal electrochemical monitoring of glucose. Talanta 249, 123695 (2022).
Dervisevic, M. et al. Transdermal electrochemical monitoring of glucose via high-density silicon microneedle array patch. Adv. Funct. Mater. 32, 2009850 (2022).
Seaton, B. T. & Heien, M. L. Biocompatible reference electrodes to enhance chronic electrochemical signal fidelity in vivo. Anal. Bioanal. Chem. 413, 6689–6701 (2021).
Bartsch, H. et al. Surface properties and biocompatibility of thick film materials used in ceramic bioreactors. Materialia 5, 100213 (2019).
Ju, J. et al. Surface enhanced raman spectroscopy based biosensor with a microneedle array for minimally invasive in vivo glucose measurements. ACS Sens. 5, 1777–1785 (2020).
Wang, Z. et al. Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat. Biomed. Eng. 5, 64–76 (2021).
Zeng, Y. et al. Colloidal crystal microneedle patch for glucose monitoring. Nano Today 35, 100984 (2020).
Al Sulaiman, D. et al. Hydrogel-coated microneedle arrays for minimally-invasive sampling and sensing of specific circulating nucleic acids from skin interstitial fluid. ACS Nano 13, 9620 (2019).
Gao, J., Huang, W., Chen, Z., Yi, C. & Jiang, L. Simultaneous detection of glucose, uric acid and cholesterol using flexible microneedle electrode array-based biosensor and multi-channel portable electrochemical analyzer. Sens. Actuators B Chem. 287, 102–110 (2019).
Nagamine, K., Kubota, J., Kai, H., Ono, Y. & Nishizawa, M. An array of porous microneedles for transdermal monitoring of intercellular swelling. Biomed. Microdevices 19, 68 (2017).
Abe, Y. et al. Porous microneedle-based wearable device for monitoring of transepidermal potential. Biomed. Eng. Adv. 1, 100004 (2021).
Lee, H. et al. Porous microneedles on a paper for screening test of prediabetes. Med. Devices Sens. 3, e10109 (2020).
Liu, P. et al. Polymer microneedles with interconnected porous structures via a phase inversion route for transdermal medical applications. J. Mater. Chem. B 8, 2032–2039 (2020).
Detamornrat, U., McAlister, E., Hutton, A. R. J., Larrañeta, E. & Donnelly, R. F. The role of 3D printing technology in microengineering of microneedles. Small 18, 2106392 (2022).
Bollella, P., Sharma, S., Cass, A. E. G., Tasca, F. & Antiochia, R. Minimally invasive glucose monitoring using a highly porous gold microneedles-based biosensor: characterization and application in artificial interstitial fluid. Catalysts 9, 580 (2019).
Li, C. G. et al. One-touch-activated blood multidiagnostic system using a minimally invasive hollow microneedle integrated with a paper-based sensor. Lab Chip 15, 3286–3292 (2015).
Ranamukhaarachchi, S. A., Padeste, C., Häfeli, U. O., Stoeber, B. & Cadarso, V. J. Design considerations of a hollow microneedle-optofluidic biosensing platform incorporating enzyme-linked assays. J. Micromech. Microeng. 28, 024002 (2018).
Goud, K. Y. et al. Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: toward parkinson management. ACS Sens. 4, 2196–2204 (2019).
Joshi, P., Riley, P. R., Mishra, R., Azizi Machekposhti, S. & Narayan, R. Transdermal polymeric microneedle sensing platform for fentanyl detection in biofluid. Biosensors 12, 198 (2022).
Miller, P. R. et al. Multiplexed microneedle-based biosensor array for characterization of metabolic acidosis. Talanta 88, 739–742 (2012).
Miller, P. R. et al. Microneedle-based transdermal sensor for on-chip potentiometric determination of K+. Adv. Healthc. Mater. 3, 876–881 (2014).
Jiang, X. & Lillehoj, P. B. Microneedle-based skin patch for blood-free rapid diagnostic testing. Microsyst. Nanoeng. 6, 96 (2020).
Windmiller, J. R. et al. Microneedle array-based carbon paste amperometric sensors and biosensors. Analyst 136, 1846–1851 (2011).
Donnelly, R. F. et al. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv. Funct. Mater. 22, 4879–4890 (2012).
Tekko, I. A. et al. Development and characterisation of novel poly (vinyl alcohol)/poly (vinyl pyrrolidone)-based hydrogel-forming microneedle arrays for enhanced and sustained transdermal delivery of methotrexate. Int. J. Pharm. 586, 119580 (2020).
Al-Kasasbeh, R. et al. Evaluation of the clinical impact of repeat application of hydrogel-forming microneedle array patches. Drug Deliv. Transl. Res. 10, 690–705 (2020).
Caffarel-Salvador, E. et al. Hydrogel-forming microneedle arrays allow detection of drugs and glucose in vivo: potential for use in diagnosis and therapeutic drug monitoring. PLoS One 10, e0145644 (2016).
Chang, H. et al. A swellable microneedle patch to rapidly extract skin interstitial fluid for timely metabolic analysis. Adv. Mater. 29, 1702243 (2017).
Zhu, J. et al. Gelatin methacryloyl microneedle patches for minimally invasive extraction of skin interstitial fluid. Small 16, 1905910 (2020).
Romanyuk, A. V. et al. Collection of analytes from microneedle patches. Anal. Chem. 86, 10520–10523 (2014).
He, R. et al. A hydrogel microneedle patch for point‐of‐care testing based on skin interstitial fluid. Adv. Healthc. Mater. 9, 1901201 (2020).
Xu, N. et al. Swellable PVA/PVP hydrogel microneedle patches for the extraction of interstitial skin fluid toward minimally invasive monitoring of blood glucose level. Analyst 147, 1478–1491 (2022).
Vicente-Perez, E. M. et al. Repeat application of microneedles does not alter skin appearance or barrier function and causes no measurable disturbance of serum biomarkers of infection, inflammation or immunity in mice in vivo. Eur. J. Pharm. Biopharm. 117, 400–407 (2017).
Beauchamp, R. O. et al. A critical review of the toxicology of glutaraldehyde. Crit. Rev. Toxicol. 22, 143–174 (1992).
Zheng, M. et al. Osmosis-powered hydrogel microneedles for microliters of skin interstitial fluid extraction within minutes. Adv. Healthc. Mater. 9, 1901683 (2020).
Babity, S. et al. Rational design of a fluorescent microneedle tattoo for minimally invasive monitoring of lymphatic function. J. Control. Rel. 327, 350–359 (2020).
He, R. et al. A colorimetric dermal tattoo biosensor fabricated by microneedle patch for multiplexed detection of health‐related biomarkers. Adv. Sci. 8, 2103030 (2021).
Vora, L. K. Novel bilayer dissolving microneedle arrays with concentrated plga nano-microparticles for targeted intradermal delivery: proof of concept. J. Control. Rel. 265, 93–101 (2017).
Li, M., Vora, L. K., Peng, K. & Donnelly, R. F. Trilayer microneedle array assisted transdermal and intradermal delivery of dexamethasone. Int. J. Pharm. 612, 121295 (2021).
Vora, L. K., Vavia, P. R., Larrañeta, E., Bell, S. E. & Donnelly, R. F. Novel nanosuspension-based dissolving microneedle arrays for transdermal delivery of a hydrophobic drug. J. Interdiscip. Nanomed. 3, 89–101 (2018).
Li, S., Kim, Y., Lee, J. W. & Prausnitz, M. R. Microneedle patch tattoos. iScience 25, 105014 (2022).
McCrudden, M. T. C. et al. Design, formulation, and evaluation of novel dissolving microarray patches containing rilpivirine for intravaginal delivery. Adv. Healthc. Mater. 8, 1801510 (2019).
Donnelly, R. F. et al. Hydrogel-forming microneedle arrays can be effectively inserted in skin by self-application: a pilot study centred on pharmacist intervention and a patient information leaflet. Pharm. Res. 31, 1989–1999 (2014).
Ripolin, A. et al. Successful application of large microneedle patches by human volunteers. Int. J. Pharm. 521, 92–101 (2017).
Vicente-Pérez, E. M. et al. The use of a pressure-indicating sensor film to provide feedback upon hydrogel-forming microneedle array self-application in vivo. Pharm. Res. 33, 3072–3080 (2016).
Li, H. et al. Microneedle-based potentiometric sensing system for continuous monitoring of multiple electrolytes in skin interstitial fluids. ACS Sens. 6, 2181–2190 (2021).
Yang, B., Fang, X. & Kong, J. Engineered microneedles for interstitial fluid cell-free DNA capture and sensing using iontophoretic dual-extraction wearable patch. Adv. Funct. Mater. 30, 2000591 (2020).
Damiati, S. & Schuster, B. Electrochemical biosensors based on S-layer proteins. Sensors 20, 1721 (2020).
Chinnadayyala, S. R., Park, J., Satti, A. T., Kim, D. & Cho, S. Minimally invasive and continuous glucose monitoring sensor based on non-enzymatic porous platinum black-coated gold microneedles. Electrochim. Acta 369, 137691 (2021).
Zheng, Y. et al. A wearable microneedle-based extended gate transistor for real-time detection of sodium in interstitial fluids. Adv. Mater. 34, 2108607 (2022).
Wang, R., Jiang, X., Wang, W. & Li, Z. A microneedle electrode array on flexible substrate for long-term EEG monitoring. Sens. Actuators B Chem. 224, 750–758 (2017).
O’Mahony, C. et al. Microneedle-based electrodes with integrated through-silicon via for biopotential recording. Sens. Actuators Phys. 186, 130–136 (2012).
Lozano, J. & Stoeber, B. Fabrication and characterization of a microneedle array electrode with flexible backing for biosignal monitoring. Biomed. Microdevices 23, 53 (2021).
Takeuchi, K., Takama, N., Kinoshita, R., Okitsu, T. & Kim, B. Flexible and porous microneedles of PDMS for continuous glucose monitoring. Biomed. Microdevices 22, 79 (2020).
Valdés-Ramírez, G. et al. Microneedle-based self-powered glucose sensor. Electrochem. Commun. 47, 58–62 (2014).
Zhao, L., Wen, Z., Jiang, F., Zheng, Z. & Lu, S. Silk/polyols/GOD microneedle based electrochemical biosensor for continuous glucose monitoring. RSC Adv. 10, 6163–6171 (2020).
Bollella, P., Sharma, S., Cass, A. E. G. & Antiochia, R. Microneedle-based biosensor for minimally-invasive lactate detection. Biosens. Bioelectron. 123, 152–159 (2019).
Windmiller, J. R. et al. Bicomponent microneedle array biosensor for minimally-invasive glutamate monitoring. Electroanalysis 23, 2302–2309 (2011).
Mohan, A. M. V., Windmiller, J. R., Mishra, R. K. & Wang, J. Continuous minimally-invasive alcohol monitoring using microneedle sensor arrays. Biosens. Bioelectron. 91, 574–579 (2017).
Ciui, B. et al. Wearable wireless tyrosinase bandage and microneedle sensors: toward melanoma screening. Adv. Healthc. Mater. 7, 1701264 (2018).
Fang, L. et al. Differential amperometric microneedle biosensor for wearable levodopa monitoring of Parkinson’s disease. Biosensors 12, 102 (2022).
Park, S. et al. Highly-sensitive single-step sensing of levodopa by swellable microneedle-mounted nanogap sensors. Biosens. Bioelectron. 220, 114912 (2023).
Mishra, R. K., Vinu Mohan, A. M., Soto, F., Chrostowski, R. & Wang, J. A microneedle biosensor for minimally-invasive transdermal detection of nerve agents. Analyst 142, 918–924 (2017).
Rawson, T. M. et al. Towards a minimally invasive device for beta-lactam monitoring in humans. Electrochem. Commun. 82, 1–5 (2017).
Seo, J. W. et al. Real-time monitoring of drug pharmacokinetics within tumor tissue in live animals. Sci. Adv. 8, eabk2901 (2022).
Jina, A. et al. Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J. Diabetes Sci. Technol. 8, 483–487 (2014).
Miller, P. et al. Towards an integrated microneedle total analysis chip for protein detection. Electroanalysis 28, 1305–1310 (2016).
García-Guzmán, J. J., Pérez-Ràfols, C., Cuartero, M. & Crespo, G. A. Toward in vivo transdermal pH sensing with a validated microneedle membrane electrode. ACS Sens. 6, 1129–1137 (2021).
Ranamukhaarachchi, S. A. et al. Integrated hollow microneedle-optofluidic biosensor for therapeutic drug monitoring in sub-nanoliter volumes. Sci. Rep. 6, 29175 (2016).
Rachim, V. P. & Chung, W. Y. Wearable-band type visible-near infrared optical biosensor for non-invasive blood glucose monitoring. Sens. Actuators B Chem. 286, 173–180 (2019).
Kim, J., Campbell, A. S., de Ávila, B. E. F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Erickson, D., Mandal, S., Yang, A. H. J. & Cordovez, B. Nanobiosensors: optofluidic, electrical and mechanical approaches to biomolecular detection at the nanoscale. Microfluid. Nanofluidics 4, 33–52 (2008).
Fan, X. & White, I. M. Optofluidic microsystems for chemical and biological analysis. Nat. Photonics 5, 591–597 (2011).
Liu, Y. et al. Skin-interfaced deep-tissue sensing patch via microneedle waveguides. Adv. Mater. Technol. 7, 2200468 (2022).
Zhang, P. et al. Wearable transdermal colorimetric microneedle patch for Uric acid monitoring based on peroxidase-like polypyrrole nanoparticles. Anal. Chim. Acta 1212, 339911 (2022).
Zhang, X., Chen, G., Bian, F., Cai, L. & Zhao, Y. Encoded microneedle arrays for detection of skin interstitial fluid biomarkers. Adv. Mater. 31, 1902825 (2019).
Linh, V. T. N. et al. Bioinspired plasmonic nanoflower-decorated microneedle for label-free intradermal sensing. Appl. Surf. Sci. 551, 149411 (2021).
Strambini, L. M. et al. Self-powered microneedle-based biosensors for pain-free high-accuracy measurement of glycaemia in interstitial fluid. Biosens. Bioelectron. 66, 162–168 (2015).
Blicharz, T. M. et al. Microneedle-based device for the one-step painless collection of capillary blood samples. Nat. Biomed. Eng. 2, 151–157 (2018).
Li, C. G., Dangol, M., Lee, C. Y., Jang, M. & Jung, H. A self-powered one-touch blood extraction system: a novel polymer-capped hollow microneedle integrated with a pre-vacuum actuator. Lab Chip 15, 382–390 (2015).
Siemens Healthineers. DCA Vantage® Analyzer https://www.siemens-healthineers.com/en-uk/diabetes/diabetes/dca-vantage-analyzer (2023).
Abbott. Alere Afiniontm AS100 Analyzer Product Demo Library https://www.globalpointofcare.abbott/en/support/product-demos/afinion.html (2023).
Dixon, R. V. et al. Microneedle-based devices for point-of-care infectious disease diagnostics. Acta Pharm. Sin. B 11, 2344–2361 (2021).
Rao, A. R. et al. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga haematococcus pluvialis. J. Agric. Food Chem. 61, 3842–3851 (2013).
Song, S. et al. A CMOS VEGF sensor for cancer diagnosis using a peptide aptamer-based functionalized microneedle. IEEE Trans. Biomed. Circuits Syst. 13, 1288–1299 (2019).
Yang, H. et al. A swellable bilateral microneedle patch with core-shell structure for rapid lactate analysis and early melanoma diagnosis. Chem. Eng. J. 455, 140730 (2023).
Pundir, C. S., Narwal, V. & Batra, B. Determination of lactic acid with special emphasis on biosensing methods: a review. Biosens. Bioelectron. 86, 777–790 (2016).
Bao, L., Park, J., Qin, B. & Kim, B. Anti-SARS-CoV-2 IgM/IgG antibodies detection using a patch sensor containing porous microneedles and a paper-based immunoassay. Sci. Rep. 12, 10693 (2022).
Sharma, S. et al. A pilot study in humans of microneedle sensor arrays for continuous glucose monitoring. Anal. Methods 10, 2088–2095 (2018).
Takeuchi, K. & Kim, B. Functionalized microneedles for continuous glucose monitoring. Nano Converg. 5, 28 (2018).
Keum, D. H. et al. Microneedle biosensor for real-time electrical detection of nitric oxide for in situ cancer diagnosis during endomicroscopy. Adv. Healthc. Mater. 4, 1153–1158 (2015).
Roberts, J. A. et al. Therapeutic drug monitoring of β-lactams in critically ill patients: proof of concept. Int. J. Antimicrob. Agents 36, 332–339 (2010).
Parrilla, M. et al. Wearable all-solid-state potentiometric microneedle patch for intradermal potassium detection. Anal. Chem. 91, 1578–1586 (2019).
Teymourian, H. et al. Wearable electrochemical sensors for the monitoring and screening of drugs. ACS Sens. 5, 2679–2700 (2020).
Tehrani, F. et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng. 6, 1214–1224 (2022).
Tsai, A. C. et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med. 16, e1002969 (2019).
Mishra, R. K. et al. Continuous opioid monitoring along with nerve agents on a wearable microneedle sensor array. J. Am. Chem. Soc. 142, 5991–5995 (2020).
Kiang, T., Ranamukhaarachchi, S. & Ensom, M. Revolutionizing therapeutic drug monitoring with the use of interstitial fluid and microneedles technology. Pharmaceutics 9, 43 (2017).
Liu, G. S. et al. Microneedles for transdermal diagnostics: recent advances and new horizons. Biomaterials 232, 119740 (2020).
Bal, S. M., Caussin, J., Pavel, S. & Bouwstra, J. A. In vivo assessment of safety of microneedle arrays in human skin. Eur. J. Pharm. Sci. 35, 193–202 (2008).
Li, W. et al. Long-acting reversible contraception by effervescent microneedle patch. Sci. Adv. 5, 2–5 (2019).
Brogden, N. K. et al. Diclofenac delays micropore closure following microneedle treatment in human subjects. J. Control. Rel. 163, 220–229 (2012).
Leone, M. et al. Universal applicator for digitally-controlled pressing force and impact velocity insertion of microneedles into skin. Pharmaceutics 10, 211 (2018).
Battisti, M. et al. Non-invasive production of multi-compartmental biodegradable polymer microneedles for controlled intradermal drug release of labile molecules. Front. Bioeng. Biotechnol. 7, 296 (2019).
Ribet, F., Stemme, G. & Roxhed, N. Real-time intradermal continuous glucose monitoring using a minimally invasive microneedle-based system. Biomed. Microdevices 20, 101 (2018).
Donnelly, R. F. et al. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm. Res. 26, 2513–2522 (2009).
Zaric, M. et al. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly- D, l -Lactide- Co -Glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano 7, 2042–2055 (2013).
McCrudden, M. T. C. et al. Considerations in the sterile manufacture of polymeric microneedle arrays. Drug Deliv. Transl. Res. 5, 3–14 (2015).
Lutton, R. E. M. et al. Microneedle characterisation: the need for universal acceptance criteria and GMP specifications when moving towards commercialisation. Drug Deliv. Transl. Res. 5, 313–331 (2015).
FDA. Regulatory Considerations for Microneedling Products https://www.regulations.gov/docket/FDA-2017-D-4792 (2020).
Matriano, J. A. et al. Macroflux® microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm. Res. 19, 63–70 (2002).
Micron Biomedical. Micron Biomedical Announces Positive Measles and Rubella Vaccination Results from First Clinical Trial of Microarray Injection-Free Vaccine Delivery in Children – Micron Biomedical https://micronbiomedical.com/micron-biomedical-announces-positive-measles-and-rubella-vaccination-results-from-first-clinical-trial-of-microarray-injection-free-vaccine-delivery-in-children/ (2023).
Taylor, N. P. Zosano goes Bankrupt after FDA Rejects Migraine Drug Delivery Patch https://www.fiercepharma.com/pharma/zosano-goes-bankrupt-after-fda-rejects-migraine-drug-delivery-patch (2022).
LTS Lohmann. Microarray Patches https://www.ltslohmann.com/en/our-technologies/map/ (2022).
PERSiSTENCE. Wearable Biosensors Market https://www.persistencemarketresearch.com/market-research/wearable-biosensors-market.asp (2022).
Birchall, J. C., Clemo, R., Anstey, A. & John, D. N. Microneedles in clinical practice-an exploratory study into the opinions of healthcare professionals and the public. Pharm. Res. 28, 95–106 (2011).
Puttaswamy, S. V. et al. Nanophotonic-carbohydrate lab-on-a-microneedle for rapid detection of human cystatin C in finger-prick blood. ACS Nano 14, 11939–11949 (2020).
Chen, W. et al. Reducing false negatives in COVID-19 testing by using microneedle-based oropharyngeal swabs. Matter 3, 1589–1600 (2020).
Yang, B., Fang, X. & Kong, J. In situ sampling and monitoring cell-free DNA of the Epstein–Barr Virus from dermal interstitial fluid using wearable microneedle patches. ACS Appl. Mater. Interfaces 11, 38448–38458 (2019).
Sun, X. et al. A theranostic microneedle array patch for integrated glycemia sensing and self-regulated release of insulin. Biomater. Sci. 10, 1209–1216 (2022).
Yi, K. et al. Aptamer-decorated porous microneedles arrays for extraction and detection of skin interstitial fluid biomarkers. Biosens. Bioelectron. 190, 113404 (2021).
Teymourian, H. et al. Microneedle-based detection of ketone bodies along with glucose and lactate: toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Anal. Chem. 92, 2291–2300 (2020).
Gerstel, M. S. & Place, V. A. Drug Delivery Device. US3964482A (1976).
Clark, J. L. C. Electrochemical Device for Chemical Analysis. US2913386A (1959).
Clark, L. C. Jr. & Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 102, 29–45 (1962).
Guilbault, G. G. & M, J. G. Jr. Urea-specific enzyme electrode. J. Am. Chem. Soc. 8, 2164–2165 (1969).
Suzuki, S., Takahashi, F., Satoh, I. & Sonobe, N. Ethanol and lactic acid sensors using electrodes coated with dehydrogenase—Collagen membranes. Bull. Chem. Soc. Jpn 48, 3246–3249 (1975).
Harder, H. Experiences with a miniaturized needle electrode in electrocochleography. Scand. Audiol. 11, 187–189 (1982).
Liedberg, B., Nylander, C. & Lunström, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators 4, 299–304 (1983).
Hahn, K. M., Waggoner, A. S. & Taylor, D. L. A calcium-sensitive fluorescent analog of calmodulin based on a novel calmodulin-binding fluorophore. J. Biol. Chem. 265, 20335–20345 (1990).
Kong, J. et al. Nanotube molecular wires as chemical sensors. Science 287, 622–625 (2000).
Yu, L. M., Tay, F. E. H., Guo, D. G., Xu, L. & Yap, K. L. A microfabricated electrode with hollow microneedles for ECG measurement. Sens. Actuators Phys. 151, 17–22 (2009).
Acknowledgements
The authors acknowledge support from the Engineering and Physical Sciences Research Council (EPSRC) grant numbers EP/H021647/1 and EP/V047221/1.
Author information
Authors and Affiliations
Contributions
All authors researched the data, discussed the content, and contributed to writing and revising of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Ryan Donnelly is an inventor of patents that have been licensed to companies developing microneedle-based products and is a paid advisor to companies developing microneedle-based products. The resulting potential conflict of interest has been disclosed and is managed by Queen’s University Belfast. The companies had no role in the design of the manuscript, in the collection, analysis or interpretation of the various studies reviewed, in the writing of the manuscript, or in the decision to publish.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Can Dincer, Thanh Nguyen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vora, L.K., Sabri, A.H., McKenna, P.E. et al. Microneedle-based biosensing. Nat Rev Bioeng 2, 64–81 (2024). https://doi.org/10.1038/s44222-023-00108-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44222-023-00108-7