Abstract
Wearable biosensing technologies can provide real-time monitoring of health and disease at the point of care. By integrating flexible microfluidics with wearable biosensors, body fluids can be non-invasively sampled and analysed for reliable, clinically informative, cost-effective and continuous biomedical monitoring. In this Review, we discuss flexible wearable microfluidic sensors for health monitoring and disease diagnosis, highlighting materials and engineering considerations with regard to biofluid collection, analyte calibration, signal interferences reduction, target recognition, and sensor reusability. We outline how such flexible microfluidic-based biosensors can be designed for the analysis of sweat, saliva, tears, interstitial fluid and wound exudate, and examine their applications at the point of care. Finally, we highlight the challenges that remain to be addressed for the clinical translation of wearable flexible microfluidic sensors and discuss future possibilities, including the integration of machine learning and the Internet-of-things.
Key points
-
Flexible microfluidics can be integrated into wearable flexible sensors to facilitate biofluid sampling and sensing.
-
Flexible microfluidic sensors for sweat, interstitial fluid, tears, saliva and wound exudate may advance personalized health care, in particular for metabolic, eye, oral cavity, gastrointestinal and infectious disease management.
-
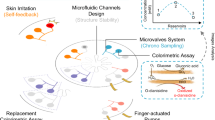
Continuous monitoring by flexible microfluidic sensors can be achieved by exploiting direct or enzyme-based redox reactions or bioaffinity and by applying sensor-regeneration strategies such as programmed electrical stimuli.
-
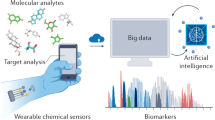
Artificial intelligence can be integrated into flexible microfluidic sensors to design smart point-of-care systems that can aid in medical decision-making by leveraging the Internet-of-things in medicine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sackmann, E. K., Fulton, A. L. & Beebe, D. J. The present and future role of microfluidics in biomedical research. Nature 507, 181–189 (2014).
Whitesides, G. M. The origins and the future of microfluidics. Nature 442, 368–373 (2006).
Yeo, J. C., Kenry & Lim, C. T. Emergence of microfluidic wearable technologies. Lab Chip 16, 4082–4090 (2016).
Li, S., Ma, Z., Cao, Z., Pan, L. & Shi, Y. Advanced wearable microfluidic sensors for healthcare monitoring. Small 16, e1903822 (2020).
Ghaffari, R. et al. Soft wearable systems for colorimetric and electrochemical analysis of biofluids. Adv. Funct. Mater. 30, 1907269 (2019).
Nguyen, N.-T., Wereley, S. T. & Shaegh, S. A. M. Fundamentals and applications of microfluidics (Artech House, 2019).
Gao, W., Ota, H., Kiriya, D., Takei, K. & Javey, A. Flexible electronics toward wearable sensing. Acc. Chem. Res. 52, 523–533 (2019).
Min, J. et al. Skin-interfaced wearable sweat sensors for precision medicine. Chem. Rev. 123, 5049–5138 (2023).
Wu, J., Liu, H., Chen, W., Ma, B. & Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 1, 346–360 (2023).
Sempionatto, J. R., Lasalde-Ramirez, J. A., Mahato, K., Wang, J. & Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 6, 899–915 (2022).
Lin, M., Hu, H., Zhou, S. & Xu, S. Soft wearable devices for deep-tissue sensing. Nat. Rev. Mater. 7, 850–869 (2022).
Ates, H. C. et al. End-to-end design of wearable sensors. Nat. Rev. Mater. 7, 887–907 (2022).
Kim, J., Campbell, A. S., de Ávila, B. E.-F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
He, T. et al. Emerging wearable chemical sensors enabling advanced integrated systems toward personalized and preventive medicine. Anal. Chem. 95, 490–514 (2023).
Luo, Y. et al. Technology roadmap for flexible sensors. ACS Nano 17, 5211–5295 (2023).
Chen, G., Zheng, J., Liu, L. & Xu, L. Application of microfluidics in wearable devices. Small Methods 3, 1900688 (2019).
Jeerapan, I., Moonla, C., Thavarungkul, P. & Kanatharana, P. Lab on a body for biomedical electrochemical sensing applications: the next generation of microfluidic devices. Prog. Mol. Biol. Transl. Sci. 187, 249–279 (2022).
Nyein, H. Y. Y. et al. A wearable patch for continuous analysis of thermoregulatory sweat at rest. Nat. Commun. 12, 1823 (2021). This article reports a wearable patch that contains hydrophilic fillers for rapid sweat uptake, enabling continuous sweat monitoring at rest, including pH, Cl−, levodopa and sweat rate sensing.
Lin, H. et al. A programmable epidermal microfluidic valving system for wearable biofluid management and contextual biomarker analysis. Nat. Commun. 11, 4405–4405 (2020).
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022). This article reports a wearable electrochemical biosensor for continuous metabolite and nutrient analysis, which integrates iontophoresis-based sweat stimulation and potential-initiated in situ electrode regeneration, achieving detection of all essential amino acids and vitamins during physical exercise and at rest.
Kim, S. et al. Soft, skin-interfaced microfluidic systems with integrated immunoassays, fluorometric sensors, and impedance measurement capabilities. Proc. Natl Acad. Sci. USA 117, 27906–27915 (2020).
Fallahi, H., Zhang, J., Phan, H. P. & Nguyen, N. T. Flexible microfluidics: fundamentals, recent developments, and applications. Micromachines https://doi.org/10.3390/mi10120830 (2019).
Coyle, S. et al. Bio-sensing textiles-wearable chemical biosensors for health monitoring. 4th International Workshop on Wearable and Implantable Body Sensor Networks (BSN 2007) March 26–28, 2007 RWTH Aachen University, Germany, 35–39 (Aachen University, 2007).
Coyle, S. et al. BIOTEX-Biosensing textiles for personalised healthcare management. IEEE Trans. Inf. Technol. Biomed. 14, 364–370 (2010).
Curto, V. F. et al. Real-time sweat pH monitoring based on a wearable chemical barcode micro-fluidic platform incorporating ionic liquids. Sens. Actuators B Chem. 171, 1327–1334 (2012).
Rose, D. P. et al. Adhesive RFID sensor patch for monitoring of sweat electrolytes. IEEE Trans. Biomed. Eng. 62, 1457–1465 (2015).
Koh, A. et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl Med. 8, 366ra165 (2016). This article reports the first integrated PDMS-based wearable microfluidic colourimetric sweat monitoring system for analysis of multiple analytes with wireless communication powering modules.
Wang, B. et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 8, eabk0967 (2022). This article reports the accurate detection of cortisol using a label-free field-effect transistor-based sweat sensor.
Gao, Y. et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 7, eabg9614 (2021). This article reports a wireless wound patch with a biomimetic microfluidic exudate collector for multiplexed wound profiling through monitoring of inflammatory mediators, Staphylococcus aureus, temperature and pH.
Klode, J. et al. Investigation of adhesion of modern wound dressings: a comparative analysis of 56 different wound dressings. J. Eur. Acad. Dermatol. Venereol. 25, 933–939 (2011).
Reeder, J. T. et al. Waterproof, electronics-enabled, epidermal microfluidic devices for sweat collection, biomarker analysis, and thermography in aquatic settings. Sci. Adv. 5, eaau6356 (2019).
Choi, J.-Y., Park, D.-W. & Oh, T. S. Variation of elastic stiffness of polydimethylsiloxane (PDMS) stretchable substrates for wearable packaging applications. J. Microelectron. Electron. Packag. 21, 125–131 (2014).
Olanrewaju, A., Beaugrand, M., Yafia, M. & Juncker, D. Capillary microfluidics in microchannels: from microfluidic networks to capillaric circuits. Lab Chip 18, 2323–2347 (2018).
Zhang, Y. et al. Skin-interfaced microfluidic devices with one-opening chambers and hydrophobic valves for sweat collection and analysis. Lab Chip 20, 2635–2645 (2020).
Lim, H., Jafry, A. T. & Lee, J. Fabrication, flow control, and applications of microfluidic paper-based analytical devices. Molecules 24, 2869 (2019).
Shen, L. L., Zhang, G. R. & Etzold, B. J. M. Paper-based microfluidics for electrochemical applications. ChemElectroChem 7, 10–30 (2020).
Zhao, Z. et al. A thread/fabric-based band as a flexible and wearable microfluidic device for sweat sensing and monitoring. Lab Chip 21, 916–932 (2021).
Agustini, D. et al. Microfluidic devices based on textile threads for analytical applications: state of the art and prospects. Anal. Methods 13, 4830–4857 (2021).
Gong, M. M. & Sinton, D. Turning the page: advancing paper-based microfluidics for broad diagnostic application. Chem. Rev. 117, 8447–8480 (2017).
Deroco, P. B., Wachholz Junior, D. & Kubota, L. T. Paper‐based wearable electrochemical sensors: a new generation of analytical devices. Electroanalysis 35, e202200177 (2022).
Abbasiasl, T., Mirlou, F., Istif, E., Ceylan Koydemir, H. & Beker, L. A wearable paper-integrated microfluidic device for sequential analysis of sweat based on capillary action. Sens. Diagn. 1, 775–786 (2022).
Tang, R. H. et al. A review on advances in methods for modification of paper supports for use in point-of-care testing. Mikrochim. Acta 186, 521 (2019).
Li, M. et al. A highly integrated sensing paper for wearable electrochemical sweat analysis. Biosens. Bioelectron. 174, 112828 (2021).
Cao, Q. et al. Three-dimensional paper-based microfluidic electrochemical integrated devices (3D-PMED) for wearable electrochemical glucose detection. RSC Adv. 9, 5674–5681 (2019).
Choi, J., Kang, D., Han, S., Kim, S. B. & Rogers, J. A. Thin, soft, skin-mounted microfluidic networks with capillary bursting valves for chrono-sampling of sweat. Adv. Healthc. Mater. 6, 1601355 (2017).
Kim, S. B. et al. Super‐absorbent polymer valves and colorimetric chemistries for time‐sequenced discrete sampling and chloride analysis of sweat via skin‐mounted soft microfluidics. Small 14, 1703334 (2018).
Choi, J. et al. Soft, skin-integrated multifunctional microfluidic systems for accurate colorimetric analysis of sweat biomarkers and temperature. ACS Sens. 4, 379–388 (2019).
Bandodkar, A. J. et al. Soft, skin‐interfaced microfluidic systems with passive galvanic stopwatches for precise chronometric sampling of sweat. Adv. Mater. 31, 1902109 (2019).
Xiao, J. et al. Microfluidic chip-based wearable colorimetric sensor for simple and facile detection of sweat glucose. Anal. Chem. 91, 14803–14807 (2019).
Mishra, N. et al. A soft wearable microfluidic patch with finger-actuated pumps and valves for on-demand, longitudinal, and multianalyte sweat sensing. ACS Sens. 7, 3169–3180 (2022).
Reeder, J. T. et al. Resettable skin interfaced microfluidic sweat collection devices with chemesthetic hydration feedback. Nat. Commun. 10, 5513 (2019). This article reports a reusable skin-mounted microfluidic sensor, which includes a pinch valve and a suction pump to reset the microfluidics, and an effervescent pump and a chemaesthetic agent for automated warnings of excessive sweat loss.
Zhong, B., Jiang, K., Wang, L. & Shen, G. Wearable sweat loss measuring devices: from the role of sweat loss to advanced mechanisms and designs. Adv. Sci. 9, e2103257 (2022).
Baker, L. B. & Wolfe, A. S. Physiological mechanisms determining eccrine sweat composition. Eur. J. Appl. Physiol. 120, 719–752 (2020).
Sonner, Z. et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 9, 031301 (2015).
Baker, L. B. et al. Skin-interfaced microfluidic system with personalized sweating rate and sweat chloride analytics for sports science applications. Sci. Adv. 6, eabe3929 (2020). This article presents the systematic validation of a wearable microfluidic sweat sensor by testing the device on 312 athletes in various conditions.
Kim, S. B. et al. Soft, skin-interfaced microfluidic systems with wireless, battery-free electronics for digital, real-time tracking of sweat loss and electrolyte composition. Small 14, 1802876 (2018).
Kwon, K. et al. An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time. Nat. Electron. 4, 302–312 (2021).
Pu, Z. et al. A thermal activated and differential self-calibrated flexible epidermal biomicrofluidic device for wearable accurate blood glucose monitoring. Sci. Adv. https://doi.org/10.1126/sciadv.abd0199 (2021).
Bariya, M., Nyein, H. Y. Y. & Javey, A. Wearable sweat sensors. Nat. Electron. 1, 160–171 (2018).
Naik, A. R. et al. Printed microfluidic sweat sensing platform for cortisol and glucose detection. Lab Chip 22, 156–169 (2021).
Kim, J. et al. A skin-interfaced, miniaturized microfluidic analysis and delivery system for colorimetric measurements of nutrients in sweat and supply of vitamins through the skin. Adv. Sci. 9, e2103331 (2022).
Choi, J. et al. Skin-interfaced microfluidic systems that combine hard and soft materials for demanding applications in sweat capture and analysis. Adv. Healthc. Mater. 10, e2000722 (2021).
Zhao, Y. et al. A wearable freestanding electrochemical sensing system. Sci. Adv. 6, eaaz0007 (2020). This article introduces an out-of-plain strain-isolated pathway in microfluidic sensor design, achieving high-fidelity data acquisition.
Dourado, A. H. B. Electric double layer: the good, the bad, and the beauty. Electrochem 3, 789–808 (2022).
Schmickler, W. Electronic effects in the electric double layer. Chem. Rev. 96, 3177–3200 (1996).
Xu, G. et al. Battery-free and wireless epidermal electrochemical system with all-printed stretchable electrode array for multiplexed in situ sweat analysis. Adv. Mater. Technol. 4, 1800658 (2019).
Zhao, Y. et al. Soft strain-insensitive bioelectronics featuring brittle materials. Science 378, 1222–1227 (2022).
Bae, C. W. et al. Fully stretchable capillary microfluidics-integrated nanoporous gold electrochemical sensor for wearable continuous glucose monitoring. ACS Appl. Mater. Interfaces 11, 14567–14575 (2019).
Ji, S. et al. Water-resistant conformal hybrid electrodes for aquatic endurable electrocardiographic monitoring. Adv. Mater. 32, e2001496 (2020).
Xu, S. et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 344, 70–74 (2014).
Lee, H. et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 3, e1601314 (2017).
Hong, Y. J. et al. Multifunctional wearable system that integrates sweat-based sensing and vital-sign monitoring to estimate pre-/post-exercise glucose levels. Adv. Funct. Mater. 28, 1805754 (2018).
Xie, W. et al. Ultra-stretchable, bio-inspired ionic skins that work stably in various harsh environments. J. Mater. Chem. A 6, 24114–24119 (2018).
Sabate Del Rio, J., Henry, O. Y. F., Jolly, P. & Ingber, D. E. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol. 14, 1143–1149 (2019).
Wisniewski, N. & Reichert, M. Methods for reducing biosensor membrane biofouling. Colloids Surf. B 18, 197–219 (2000).
Xu, J. & Lee, H. Anti-biofouling strategies for long-term continuous use of implantable biosensors. Chemosensors https://doi.org/10.3390/chemosensors8030066 (2020).
Lee, H.-B., Meeseepong, M., Trung, T. Q., Kim, B.-Y. & Lee, N.-E. A wearable lab-on-a-patch platform with stretchable nanostructured biosensor for non-invasive immunodetection of biomarker in sweat. Biosens. Bioelectron. 156, 112133 (2020).
Huynh, V. L. et al. Hollow microfibers of elastomeric nanocomposites for fully stretchable and highly sensitive microfluidic immunobiosensor patch. Adv. Funct. Mater. 30, 2004684 (2020).
Xi, W. et al. Soft tubular microfluidics for 2D and 3D applications. Proc. Natl Acad. Sci. USA 114, 10590–10595 (2017).
Xi, W., Yeo, J. C., Yu, L., Zhang, S. & Lim, C. T. Ultrathin and wearable microtubular epidermal sensor for real-time physiological pulse monitoring. Adv. Mater. Technol. 2, 1700016 (2017).
Choi, D. Y. et al. Highly stretchable, hysteresis-free ionic liquid -based strain sensor for precise human motion monitoring. ACS Appl. Mater. Interfaces 9, 1770–1780 (2017).
Bariya, M. et al. Resettable microfluidics for broad-range and prolonged sweat rate sensing. ACS Sens. 7, 1156–1164 (2022).
Li, J. et al. Designing biomimetic liquid diodes. Soft Matter 15, 1902–1915 (2019).
Wang, S. et al. A review of capillary pressure control valves in microfluidics. Biosensors 11, 10 (2021).
Naeimirad, M., Abuzade, R., Babaahmadi, V. & Dabirian, F. Microfluidic through fibrous structures: recent developments and future trends. Mater. Des. Process. Commun. https://doi.org/10.1002/mdp2.78 (2019).
Hu, L., Zhang, Q., Li, X. & Serpe, M. J. Stimuli-responsive polymers for sensing and actuation. Mater. Horiz. 6, 1774–1793 (2019).
Takashima, A., Kojima, K. & Suzuki, H. Autonomous microfluidic control by chemically actuated micropumps and its application to chemical analyses. Anal. Chem. 82, 6870–6876 (2010).
Park, J. & Park, J. K. Integrated microfluidic pumps and valves operated by finger actuation. Lab Chip 19, 2973–2977 (2019).
Bacchin, P., Leng, J. & Salmon, J. B. Microfluidic evaporation, pervaporation, and osmosis: from passive pumping to solute concentration. Chem. Rev. 122, 6938–6985 (2022).
Saha, T., Fang, J., Mukherjee, S., Dickey, M. D. & Velev, O. D. Wearable osmotic-capillary patch for prolonged sweat harvesting and sensing. ACS Appl. Mater. Interfaces 13, 8071–8081 (2021).
Jeon, N. L. et al. Microfluidics section: design and fabrication of integrated passive valves and pumps for flexible polymer 3-dimensional microfluidic systems. Biomed. Microdevices 4, 117–121 (2002).
Gomez, M., Moulton, D. E. & Vella, D. Passive control of viscous flow via elastic snap-through. Phys. Rev. Lett. 119, 144502 (2017).
Cho, H., Kim, H. Y., Kang, J. Y. & Kim, T. S. How the capillary burst microvalve works. J. Colloid Interface Sci. 306, 379–385 (2007).
Jiang, Y. et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01528-3 (2022).
Moreddu, R., Wolffsohn, J. S., Vigolo, D. & Yetisen, A. K. Laser-inscribed contact lens sensors for the detection of analytes in the tear fluid. Sens. Actuators B Chem. 317, 128183 (2020).
Sempionatto, J. R. et al. Eyeglasses-based tear biosensing system: non-invasive detection of alcohol, vitamins and glucose. Biosens. Bioelectron. 137, 161–170 (2019).
García-Carmona, L. et al. Pacifier biosensor: toward noninvasive saliva biomarker monitoring. Anal. Chem. 91, 13883–13891 (2019).
Hou, B. et al. An interactive mouthguard based on mechanoluminescence-powered optical fibre sensors for bite-controlled device operation. Nat. Electron. 5, 682–693 (2022).
Teymourian, H., Tehrani, F., Mahato, K. & Wang, J. Lab under the skin: microneedle based wearable devices. Adv. Healthc. Mater. 10, e2002255 (2021).
Yang, Y. et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 38, 217–224 (2020).
Tehrani, F. et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng. 6, 1214–1224 (2022).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Tai, L.-C. et al. Methylxanthine drug monitoring with wearable sweat sensors. Adv. Mater. 30, e1707442 (2018).
Simmers, P., Li, S. K., Kasting, G. & Heikenfeld, J. Prolonged and localized sweat stimulation by iontophoretic delivery of the slowly-metabolized cholinergic agent carbachol. J. Dermatol. Sci. 89, 40–51 (2018).
Hauke, A. et al. Complete validation of a continuous and blood-correlated sweat biosensing device with integrated sweat stimulation. Lab Chip 18, 3750–3759 (2018).
Martin, A. et al. Epidermal microfluidic electrochemical detection system: enhanced sweat sampling and metabolite detection. ACS Sens. 2, 1860–1868 (2017).
Shirzaei Sani, E. et al. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 9, eadf7388 (2023).
Pal, A. et al. Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages. Biosens. Bioelectron. 117, 696–705 (2018).
Mostafalu, P. et al. A toolkit of thread-based microfluidics, sensors, and electronics for 3D tissue embedding for medical diagnostics. Microsyst. Nanoeng. 2, 16039 (2016).
Liu, W.-T., Cao, Y.-P., Zhou, X.-H. & Han, D. Interstitial fluid behavior and diseases. Adv. Sci. 9, 2100617 (2022).
Friedel, M. et al. Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat. Biomed. Eng. 7, https://doi.org/10.1038/s41551-022-00998-9 (2023).
Saifullah, K. M. & Faraji Rad, Z. Sampling dermal interstitial fluid using microneedles: a review of recent developments in sampling methods and microneedle‐based biosensors. Adv. Mater. Interfaces 10, 2201763 (2023).
Samant, P. P. et al. Sampling interstitial fluid from human skin using a microneedle patch. Sci. Transl Med. 12, eaaw0285 (2020).
Li, X. et al. A fully integrated closed‐loop system based on mesoporous microneedles‐iontophoresis for diabetes treatment. Adv. Sci. 8, 2100827 (2021).
Yang, B., Kong, J. & Fang, X. Programmable CRISPR-Cas9 microneedle patch for long-term capture and real-time monitoring of universal cell-free DNA. Nat. Commun. 13, 3999 (2022).
Wang, Z. et al. Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat. Biomed. Eng. 5, 64–76 (2021).
Ribet, F., Stemme, G. & Roxhed, N. Real-time intradermal continuous glucose monitoring using a minimally invasive microneedle-based system. Biomed. Microdevices 20, 101 (2018).
Jiang, X. & Lillehoj, P. B. Microneedle-based skin patch for blood-free rapid diagnostic testing. Microsyst. Nanoeng. 6, 96 (2020).
Parrilla, M., Detamornrat, U., Dominguez-Robles, J., Donnelly, R. F. & De Wael, K. Wearable hollow microneedle sensing patches for the transdermal electrochemical monitoring of glucose. Talanta 249, 123695 (2022).
Lee, H. et al. Porous microneedles on a paper for screening test of prediabetes. Med. Devices Sens. 3, e10109 (2020).
Kusama, S. et al. Transdermal electroosmotic flow generated by a porous microneedle array patch. Nat. Commun. 12, 658 (2021).
Lee, Y. et al. Wireless, intraoral hybrid electronics for real-time quantification of sodium intake toward hypertension management. Proc. Natl Acad. Sci. USA 115, 5377–5382 (2018).
Mishra, R. K. et al. Simultaneous detection of salivary Δ9-tetrahydrocannabinol and alcohol using a wearable electrochemical ring sensor. Talanta 211, 120757 (2020).
de Castro, L. F. et al. Salivary diagnostics on paper microfluidic devices and their use as wearable sensors for glucose monitoring. Anal. Bioanal. Chem. 411, 4919–4928 (2019).
Lim, H. R. et al. Smart bioelectronic pacifier for real-time continuous monitoring of salivary electrolytes. Biosens. Bioelectron. 210, 114329 (2022). This article reports the first integrated smart pacifier using a hydrophilic microfluidics channel for neonatal intensive care unit monitoring.
Yetisen, A. K. et al. Scleral lens sensor for ocular electrolyte analysis. Adv. Mater. 32, e1906762 (2020).
Yang, X. et al. Flexible, wearable microfluidic contact lens with capillary networks for tear diagnostics. J. Mater. Sci. 55, 9551–9561 (2020).
An, H. et al. Microfluidic contact lenses for unpowered, continuous and non-invasive intraocular pressure monitoring. Sens. Actuators A Phys. 295, 177–187 (2019).
Araci, I. E., Su, B., Quake, S. R. & Mandel, Y. An implantable microfluidic device for self-monitoring of intraocular pressure. Nat. Med. 20, 1074–1078 (2014).
Agaoglu, S. et al. Ultra-sensitive microfluidic wearable strain sensor for intraocular pressure monitoring. Lab Chip 18, 3471–3483 (2018).
Naresh, V. & Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 21, 1906713 (2021).
Campuzano, S., Pedrero, M., Torrente‐Rodríguez, R. M. & Pingarrón, J. M. Affinity‐based wearable electrochemical biosensors: natural versus biomimetic receptors. Anal. Sens. https://doi.org/10.1002/anse.202200087 (2022).
Tu, J. B., Torrente-Rodriguez, R. M., Wang, M. Q. & Gao, W. The era of digital health: a review of portable and wearable affinity biosensors. Adv. Funct. Mater. https://doi.org/10.1002/adfm.201906713 (2020).
Monošík, R., Streďanský, M. & Šturdík, E. Biosensors - classification, characterization and new trends. Acta Chim. Slov. 5, 109–120 (2012).
Ronkainen, N. J., Halsall, H. B. & Heineman, W. R. Electrochemical biosensors. Chem. Soc. Rev. 39, 1747–1763 (2010).
Fiore, L. et al. Microfluidic paper-based wearable electrochemical biosensor for reliable cortisol detection in sweat. Sens. Actuators B Chem. 379, 133258 (2023).
Mazur, F., Tjandra, A. D., Zhou, Y., Gao, Y. & Chandrawati, R. Paper-based sensors for bacteria detection. Nat. Rev. Bioeng. https://doi.org/10.1038/s44222-023-00024-w (2023).
Sadana, A. A kinetic study of analyte-receptor binding and dissociation, and dissociation alone, for biosensor applications: a fractal analysis. Anal. Biochem. 291, 34–47 (2001).
Wu, Y. et al. Microneedle aptamer-based sensors for continuous, real-time therapeutic drug monitoring. Anal. Chem. 94, 8335–8345 (2022).
Singh, N. K., Chung, S., Chang, A. Y., Wang, J. & Hall, D. A. A non-invasive wearable stress patch for real-time cortisol monitoring using a pseudoknot-assisted aptamer. Biosens. Bioelectron. 227, 115097 (2023).
Fercher, C., Jones, M. L., Mahler, S. M. & Corrie, S. R. Recombinant antibody engineering enables reversible binding for continuous protein biosensing. ACS Sens. 6, 764–776 (2021).
Lin, S. et al. Wearable microneedle-based electrochemical aptamer biosensing for precision dosing of drugs with narrow therapeutic windows. Sci. Adv. 8, eabq4539 (2022).
Wang, Z. R. et al. A flexible and regenerative aptameric graphene-nafion biosensor for cytokine storm biomarker monitoring in undiluted biofluids toward wearable applications. Adv. Funct. Mater. 31, 2005958 (2020).
Goode, J. A., Rushworth, J. V. & Millner, P. A. Biosensor regeneration: a review of common techniques and outcomes. Langmuir 31, 6267–6276 (2015).
Flynn, C. D. et al. Biomolecular sensors for advanced physiological monitoring. Nat. Rev. Bioeng. https://doi.org/10.1038/s44222-023-00067-z (2023).
Gosai, A., Ma, X., Balasubramanian, G. & Shrotriya, P. Electrical stimulus controlled binding/unbinding of human thrombin-aptamer complex. Sci. Rep. 6, 37449 (2016).
Aleman, J., Kilic, T., Mille, L. S., Shin, S. R. & Zhang, Y. S. Microfluidic integration of regeneratable electrochemical affinity-based biosensors for continual monitoring of organ-on-a-chip devices. Nat. Protoc. 16, 2564–2593 (2021).
Shin, S. R. et al. Label-free and regenerative electrochemical microfluidic biosensors for continual monitoring of cell secretomes. Adv. Sci. 4, 1600522 (2017).
Ota, H. et al. Highly deformable liquid-state heterojunction sensors. Nat. Commun. 5, 5032 (2014).
Yeo, J. C., Yu, J., Koh, Z. M., Wang, Z. & Lim, C. T. Wearable tactile sensor based on flexible microfluidics. Lab Chip 16, 3244–3250 (2016).
Bandodkar, A. J. et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 5, eaav3294 (2019).
Sekine, Y. et al. A fluorometric skin-interfaced microfluidic device and smartphone imaging module for in situ quantitative analysis of sweat chemistry. Lab Chip 18, 2178–2186 (2018).
He, X., Fan, C., Luo, Y., Xu, T. & Zhang, X. Flexible microfluidic nanoplasmonic sensors for refreshable and portable recognition of sweat biochemical fingerprint. NPJ Flex. Electron. 6, 1 (2022).
Mogera, U. et al. Wearable plasmonic paper-based microfluidics for continuous sweat analysis. Sci. Adv. 8, eabn1736 (2022).
Mei, X., Yang, J., Liu, J. & Li, Y. Wearable, nanofiber-based microfluidic systems with integrated electrochemical and colorimetric sensing arrays for multiplex sweat analysis. Chem. Eng. J. https://doi.org/10.1016/j.cej.2022.140248 (2023).
Schäferling, M. The art of fluorescence imaging with chemical sensors. Angew. Chem. Int. Ed. 51, 3532–3554 (2012).
Stiles, P. L., Dieringer, J. A., Shah, N. C. & Van Duyne, R. P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 1, 601–626 (2008).
Vinoth, R., Nakagawa, T., Mathiyarasu, J. & Mohan, A. M. V. Fully printed wearable microfluidic devices for high-throughput sweat sampling and multiplexed electrochemical analysis. ACS Sens. 6, 1174–1186 (2021).
Nyein, H. Y. Y. et al. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sens. 3, 944–952 (2018).
Elgrishi, N. et al. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 95, 197–206 (2018).
Chen, A. & Shah, B. Electrochemical sensing and biosensing based on square wave voltammetry. Anal. Methods 5, 2158–2173 (2013).
Singh, A., Chowdhury, D. R. & Paul, A. A kinetic study of ferrocenium cation decomposition utilizing an integrated electrochemical methodology composed of cyclic voltammetry and amperometry. Analyst 139, 5747–5754 (2014).
Shaver, A. & Arroyo-Curras, N. The challenge of long-term stability for nucleic acid-based electrochemical sensors. Curr. Opin. Electrochem. https://doi.org/10.1016/j.coelec.2021.100902 (2022).
Daniels, J. S. & Pourmand, N. Label-free impedance biosensors: opportunities and challenges. Electroanalysis 19, 1239–1257 (2007).
Araci, I. E. et al. Flow stabilization in wearable microfluidic sensors enables noise suppression. Lab Chip 19, 3899–3908 (2019).
Bae, C. W., Chinnamani, M. V., Lee, E. H. & Lee, N. E. Stretchable non‐enzymatic fuel cell‐based sensor patch integrated with thread‐embedded microfluidics for self‐powered wearable glucose monitoring. Adv. Mater. Interfaces 9, 2200492 (2022).
Bandodkar, A. J. et al. Sweat-activated biocompatible batteries for epidermal electronic and microfluidic systems. Nat. Electron. 3, 554–562 (2020).
Huang, X. et al. Garment embedded sweat-activated batteries in wearable electronics for continuous sweat monitoring. NPJ Flex. Electron. 6, 1 (2022).
Liu, Y. et al. Stretchable sweat-activated battery in skin-integrated electronics for continuous wireless sweat monitoring. Adv. Sci. 9, e2104635 (2022).
Xiao, G. et al. A weavable and scalable cotton-yarn-based battery activated by human sweat for textile electronics. Adv. Sci. 9, e2103822 (2022).
Baker, L. B. et al. Skin‐interfaced microfluidic system with machine learning‐enabled image processing of sweat biomarkers in remote settings. Adv. Mater. Technol. 7, 2200249 (2022).
Mohr, D. C., Zhang, M. & Schueller, S. M. Personal sensing: understanding mental health using ubiquitous sensors and machine learning. Annu. Rev. Clin. Psychol. 13, 23–47 (2017).
Bohr, A. & Memarzadeh, K. in Artificial Intelligence in Healthcare 25–60 (Elsevier, 2020).
Yang, D. S., Ghaffari, R. & Rogers, J. A. Sweat as a diagnostic biofluid. Science 379, 760–761 (2023).
Oh, S. Y. et al. Skin-attachable, stretchable electrochemical sweat sensor for glucose and pH detection. ACS Appl. Mater. Interfaces 10, 13729–13740 (2018).
Lee, H. et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 11, 566–572 (2016).
Brothers, M. C. et al. Achievements and challenges for real-time sensing of analytes in sweat within wearable platforms. Acc. Chem. Res. 52, 297–306 (2019).
Jagannath, B. et al. A sweat-based wearable enabling technology for real-time monitoring of IL-1β and CRP as potential markers for inflammatory bowel disease. Inflamm. Bowel Dis. 26, 1533–1542 (2020).
Tai, L. C. et al. Wearable sweat band for noninvasive levodopa monitoring. Nano Lett. 19, 6346–6351 (2019).
Raymundo-Pereira, P. A., Gomes, N. O., Machado, S. A. S. & Oliveira, O. N. Wearable glove-embedded sensors for therapeutic drug monitoring in sweat for personalized medicine. Chem. Eng. J. 435, 135047 (2022).
Kim, Y. & Prausnitz, M. R. Sensitive sensing of biomarkers in interstitial fluid. Nat. Biomed. Eng. 5, 3–5 (2021).
Heikenfeld, J. et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37, 407–419 (2019).
Goud, K. Y. et al. Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: toward Parkinson management. ACS Sens. 4, 2196–2204 (2019).
Liu, G. S. et al. Microneedles for transdermal diagnostics: recent advances and new horizons. Biomaterials 232, 119740 (2020).
Himawan, A. et al. Where microneedle meets biomarkers: futuristic application for diagnosing and monitoring localized external organ diseases. Adv. Healthc. Mater. 12, e2202066 (2023).
Loffler, M. W., Schuster, H., Buhler, S. & Beckert, S. Wound fluid in diabetic foot ulceration: more than just an undefined soup? Int. J. Low. Extrem. Wounds 12, 113–129 (2013).
White, R. & Cutting, K. F. Modern exudate management: a review of wound treatments. World Wide Wounds http://www.worldwidewounds.com/2006/september/White/Modern-Exudate-Mgt.html (2006).
Cutting, K. F. Wound exudate: composition and functions. Br. J. Community Nurs. 8, 4–9 (2003).
Fernandez, M. L., Upton, Z., Edwards, H., Finlayson, K. & Shooter, G. K. Elevated uric acid correlates with wound severity. Int. Wound J. 9, 139–149 (2012).
Wang, C., Shirzaei Sani, E. & Gao, W. Wearable bioelectronics for chronic wound management. Adv. Funct. Mater. 32, 2111022 (2021).
Xiong, Z. et al. A wireless and battery-free wound infection sensor based on DNA hydrogel. Sci. Adv. 7, eabj1617 (2021).
Kang, S. M. et al. A matrix metalloproteinase sensing biosensor for the evaluation of chronic wounds. BioChip J. 13, 323–332 (2019).
Xue, M., Le, N. T. & Jackson, C. J. Targeting matrix metalloproteases to improve cutaneous wound healing. Expert Opin. Ther. Targets 10, 143–155 (2006).
Xu, G. et al. Battery‐free and wireless smart wound dressing for wound infection monitoring and electrically controlled on‐demand drug delivery. Adv. Funct. Mater. 31, 2100852 (2021).
Pedersen, A. M. L., Sorensen, C. E., Proctor, G. B., Carpenter, G. H. & Ekstrom, J. Salivary secretion in health and disease. J. Oral. Rehabil. 45, 730–746 (2018).
Li, Y. et al. Oral wearable sensors: health management based on the oral cavity. Biosens. Bioelectron. X 10, 100135 (2022).
Dave, P. K., Rojas-Cessa, R., Dong, Z. & Umpaichitra, V. Survey of saliva components and virus sensors for prevention of COVID-19 and infectious diseases. Biosensors https://doi.org/10.3390/bios11010014 (2020).
Iorgulescu, G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J. Med. Life 2, 303 (2009).
Fan, Y. et al. Dynamic changes in salivary cortisol and secretory immunoglobulin A response to acute stress. Stress Health 25, 189–194 (2009).
Dong, T., Matos Pires, N. M., Yang, Z. & Jiang, Z. Advances in electrochemical biosensors based on nanomaterials for protein biomarker detection in saliva. Adv. Sci. 10, e2205429 (2023).
Moonla, C. et al. Review—Lab-in-a-Mouth and advanced point-of-care sensing systems: detecting bioinformation from the oral cavity and saliva. ECS Sens. Plus 1, 021603 (2022).
Hong, W. & Lee, W. G. Wearable sensors for continuous oral cavity and dietary monitoring toward personalized healthcare and digital medicine. Analyst 145, 7796–7808 (2021).
Esmaeelpour, M., Watts, P. O., Boulton, M. E., Cai, J. & Murphy, P. J. Tear film volume and protein analysis in full-term newborn infants. Cornea 30, 400–404 (2011).
Van Haeringen, N. J. Clinical biochemistry of tears. Surv. Ophthalmol. 26, 84–96 (1981).
Pankratov, D., Gonzalez-Arribas, E., Blum, Z. & Shleev, S. Tear based bioelectronics. Electroanalysis 28, 1250–1266 (2016).
Farandos, N. M., Yetisen, A. K., Monteiro, M. J., Lowe, C. R. & Yun, S. H. Contact lens sensors in ocular diagnostics. Adv. Healthc. Mater. 4, 792–810 (2015).
Occhiutto, M. L., Freitas, F. R., Maranhao, R. C. & Costa, V. P. Breakdown of the blood-ocular barrier as a strategy for the systemic use of nanosystems. Pharmaceutics 4, 252–275 (2012).
Hagan, S., Martin, E. & Enríquez-de-Salamanca, A. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J. 7, 15 (2016).
Çomoğlu, S. S., Güven, H., Acar, M., Öztürk, G. & Koçer, B. Tear levels of tumor necrosis factor-alpha in patients with Parkinson’s disease. Neurosci. Lett. 553, 63–67 (2013).
Li, M. S. et al. Current and future perspectives on microfluidic tear analytic devices. ACS Sens. 7, 1300–1314 (2022).
Keum, D. et al. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 6, eaba3252 (2020).
Kim, J. et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 8, 14997 (2017).
Ku, M. et al. Smart, soft contact lens for wireless immunosensing of cortisol. Sci. Adv. 6, eabb2891 (2020).
Ma, X. et al. Smart contact lenses for biosensing applications. Adv. Intell. Syst. 3, 2000263 (2021).
Khoshmanesh, K. et al. Liquid metal enabled microfluidics. Lab Chip 17, 974–993 (2017).
Wu, C.-Y., Liao, W.-H. & Tung, Y.-C. Integrated ionic liquid-based electrofluidic circuits for pressure sensing within polydimethylsiloxane microfluidic systems. Lab Chip 11, 1740–1746 (2011).
Gao, Y. et al. Wearable microfluidic diaphragm pressure sensor for health and tactile touch monitoring. Adv. Mater. 29, 1701985 (2017).
Jiang, H. et al. A wireless implantable passive intra-abdominal pressure sensing scheme via ultrasonic imaging of a microfluidic device. IEEE Trans. Biomed. Eng. 68, 747–758 (2021).
Zhang, S. et al. A wearable battery-free wireless and skin-interfaced microfluidics integrated electrochemical sensing patch for on-site biomarkers monitoring in human perspiration. Biosens. Bioelectron. 175, 112844 (2021).
Bolat, G. et al. Wearable soft electrochemical microfluidic device integrated with iontophoresis for sweat biosensing. Anal. Bioanal. Chem. 414, 5411–5421 (2022).
Paul Kunnel, B. & Demuru, S. An epidermal wearable microfluidic patch for simultaneous sampling, storage, and analysis of biofluids with counterion monitoring. Lab Chip 22, 1793–1804 (2022).
Kulkarni, M. B., Ayachit, N. H. & Aminabhavi, T. M. Biosensors and microfluidic biosensors: from fabrication to application. Biosensors 12, 543 (2022).
Whitesides, G. M., Ostuni, E., Takayama, S., Jiang, X. & Ingber, D. E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 3, 335–373 (2001).
Beebe, D. J. et al. Microfluidic tectonics: a comprehensive construction platform for microfluidic systems. Proc. Natl Acad. Sci. USA 97, 13488–13493 (2000).
Borok, A., Laboda, K. & Bonyar, A. PDMS bonding technologies for microfluidic applications: a review. Biosensors 11, 8 (2021).
Poulsen, C. E. et al. Laser ablated micropillar energy directors for ultrasonic welding of microfluidic systems. J. Micromech. Microeng. 26, 067001 (2016).
Faghih, M. M. & Sharp, M. K. Solvent-based bonding of PMMA–PMMA for microfluidic applications. Microsyst. Technol. 25, 3547–3558 (2019).
Nyein, H. Y. Y. et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 5, eaaw9906 (2019).
Bariya, M. et al. Roll-to-roll gravure printed electrochemical sensors for wearable and medical devices. ACS Nano 12, 6978–6987 (2018).
Epicore. Gx Sweat Patch Provides Hydration Biomarker Analytics and Recovery Insights. Epicore Biosystems https://www.epicorebiosystems.com/gx-sweat-patch/ (2022).
Nix. Nix Hydration Biosensor. Nix Biosensors https://nixbiosensors.com/pages/product (2022).
L’Oreal Groupe. My Skin Track Ph by La Roche-Posay Won the CES 2019 Innovation Award. Loreal https://www.loreal.com/en/news/research-innovation/my-skin-track-ph-by-la-roche-posay-won-the-ces-2019-innovation-award/ (2019).
Epicore. Continuous real-time hydration monitoring. Epicore Biosystems https://www.epicorebiosystems.com/connected-hydration/ (2023).
FlexoSense. 3D-printed Insole (Diabetes). FlexoSense flexosense.com/product-page/3d-printed-insole-for-diabetes (2023).
Acknowledgements
This work was supported by the Institute for Health Innovation and Technology (iHealthtech), MechanoBioEngineering Laboratory at the Department of Biomedical Engineering and the Institute for Functional Intelligent Materials (I-FIM) at the National University of Singapore (NUS). We also acknowledge support from the National Research Foundation and Singapore A*STAR under its RIE2020 Industry Alignment Fund – Industry Collaboration Projects (IAF-ICP) Grant (Grant No. I2001E0059) – SIA-NUS Digital Aviation Corp Lab. We also thank Henryk Chan for help in preparing some figure items.
Author information
Authors and Affiliations
Contributions
C.T.L., J.Y.L. and S.W.C. discussed and conceived the idea for the synopsis. C.T.L. and S.W.C. guided all aspects of the work. C.T.L., J.C.Y. and J.Y.L. refined the manuscript. S.W.C., Z.Q. and Y.N. drew most figures and wrote and finalized the content. J.C.Y., J.Y.L., Y.C.L., X.Y.L., S.C.F. and J.M.Q. contributed to writing the first draft. All authors contributed to discussing, editing and finalizing the article.
Corresponding author
Ethics declarations
Competing interests
C.T.L. is an inventor on patents related to a smart insole from FlexoSense and an ARIS muscle analyser from Microtube Technologies. These patents have been licensed to FlexoSense and Microtube Technologies, respectively. C.T.L. is a scientific founder of the above two companies and holds equity in them. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Wei Gao, Roozbeh Ghaffari, Nae-Eung Lee and Tran Quang Trung for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Microtube Technologies: https://microtube.tech/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Qiao, Z., Niu, Y. et al. Wearable flexible microfluidic sensing technologies. Nat Rev Bioeng 1, 950–971 (2023). https://doi.org/10.1038/s44222-023-00094-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44222-023-00094-w
This article is cited by
-
Motion artefact management for soft bioelectronics
Nature Reviews Bioengineering (2024)