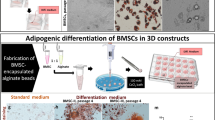
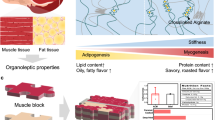
Abstract
The need and demand for sustainable, nutritious and animal-welfare-conscious meat substitutes has spurred research into cultivated meat production. Meat mainly contains muscle and fat tissue, which can be fabricated using various tissue engineering strategies, including monoculture and co-culture approaches in different scaffolds. In this Review, we outline how co-culture approaches commonly used in biomedical tissue engineering can be applied to produce cultured meat. We discuss the relevant cell types and cell sources and examine different co-culture approaches for skeletal muscle and adipose tissue engineering. Finally, we discuss the application of such approaches for animal-free meat production, highlighting their potential to reduce cultured meat production costs, improve the organoleptic properties of cultured meat and increase tissue thickness.
Key points
-
Multiple challenges still stand as barriers to the mass and low-cost production of in vitro meat with desirable nutritional and organoleptic properties.
-
Insights from tissue engineering may advance cultured meat fabrication: in particular, co-culture approaches can promote tissue growth, differentiation and maturation.
-
Co-culture approaches for cultivated meat production may reduce the need for growth factor supplementation, accelerate the fabrication and improve the properties of the final product, making such cultured meat more similar to animal-derived meat.
-
Co-culture may also lead to undesired effects, and scaling up an efficient co-culture system remains technologically challenging.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Goodwin, J. N. & Shoulders, C. W. The future of meat: a qualitative analysis of cultured meat media coverage. Meat Sci. 95, 445–450 (2013).
Mattick, C. S. Cellular agriculture: the coming revolution in food production. Bull. At. Sci. 74, 32–35 (2018).
Ben-Arye, T. & Levenberg, S. Tissue engineering for clean meat production. Front. Sustain. Food Syst. 3, 46 (2019). This review discusses the adjustment needed to create skeletal muscle for cultured meat development using tissue engineering.
World Livestock 2011 — Livestock in Food Security (FAO, 2011).
Hubalek, S., Post, M. J. & Moutsatsou, P. Towards resource-efficient and cost-efficient cultured meat. Curr. Opin. Food Sci. 47, 100885 (2022). This review discusses the hurdles that need to be overcome for the cost-efficient production of cultured meat, describing cost-effective nutrient replacements for cell growth and differentiation, as well as medium recyclability options.
Reddi, A. H. Symbiosis of biotechnology and biomaterials: applications in tissue engineering of bone and cartilage. J. Cell. Biochem. 56, 192–195 (1994).
Mol, A. et al. Tissue engineering of human heart valve leaflets: a novel bioreactor for a strain-based conditioning approach. Ann. Biomed. Eng. 33, 1778–1788 (2005).
Edelman, P. D., McFarland, D. C., Mironov, V. A. & Matheny, J. G. In vitro-cultured meat production. Tissue Eng. 11, 659–662 (2005).
Post, M. J. Cultured beef: medical technology to produce food. J. Sci. Food Agric. 94, 1039–1041 (2014).
Boldrin, L. et al. Efficient delivery of human single fiber-derived muscle precursor cells via biocompatible scaffold. Cell Transpl. 17, 576–584 (2008).
Listrat, A. et al. How muscle structure and composition determine meat quality. Prod. Anim. 28, 125–136 (2015).
Listrat, A. et al. How muscle structure and composition influence meat and flesh quality. Sci. World J. https://doi.org/10.1155/2016/3182746 (2016).
Gomillion, C. T. & Burg, K. J. L. Stem cells and adipose tissue engineering. Biomaterials 27, 6052–6063 (2006).
Yao, R., Zhang, R., Lin, F. & Luan, J. Biomimetic injectable HUVEC-adipocytes/collagen/alginate microsphere co-cultures for adipose tissue engineering. Biotechnol. Bioeng. 110, 1430–1443 (2013).
Vandenburgh, H. et al. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve 37, 438–447 (2008).
Nam, K. H., Smith, A. S. T., Lone, S., Kwon, S. & Kim, D. H. Biomimetic 3D tissue models for advanced high-throughput drug screening. J. Lab. Autom. 20, 201–215 (2015).
Lesman, A., Rosenfeld, D., Landau, S. & Levenberg, S. Mechanical regulation of vascular network formation in engineered matrices. Adv. Drug Deliv. Rev. 96, 176–182 (2016).
Guo, S. et al. Stimulating extracellular vesicles production from engineered tissues by mechanical forces. Nano Lett. 21, 2497–2504 (2021).
Rubio, N. R., Xiang, N. & Kaplan, D. L. Plant-based and cell-based approaches to meat production. Nat. Commun. 11, 6276 (2020).
Stout, A. J. et al. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun. Biol. 5, 466 (2022).
Humbird, D. Scale‐up economics for cultured meat. Biotechnol. Bioeng. 118, 3239–3250 (2021).
Messmer, T. et al. A serum-free media formulation for cultured meat production supports bovine satellite cell differentiation in the absence of serum starvation. Nat. Food 3, 74–85 (2022).
Post, M. J. et al. Scientific, sustainability and regulatory challenges of cultured meat. Nat. Food 1, 403–415 (2020). This review discusses the scientific and social challenges in transforming cultured meat into a viable commercial option.
Fraeye, I., Kratka, M., Vandenburgh, H. & Thorrez, L. Sensorial and nutritional aspects of cultured meat in comparison to traditional meat: much to be inferred. Front. Nutr. 7, 35 (2020).
Bomkamp, C. et al. Scaffolding biomaterials for 3D cultivated meat: prospects and challenges. Adv. Sci. 9, 2102908 (2022).
Haraguchi, Y. & Shimizu, T. Three-dimensional tissue fabrication system by co-culture of microalgae and animal cells for production of thicker and healthy cultured food. Biotechnol. Lett. 43, 1117–1129 (2021).
Kim, H. et al. A novel 3D indirect co-culture system based on a collagen hydrogel scaffold for enhancing the osteogenesis of stem cells. J. Mater. Chem. B 8, 9481–9491 (2020).
Paschos, N. K., Brown, W. E., Eswaramoorthy, R., Hu, J. C. & Athanasiou, K. A. Advances in tissue engineering through stem cell-based co-culture. J. Tissue Eng. Regen. Med. 9, 488–503 (2015). This review discusses the use of stem cells in co-culture systems, describing different methods and emphasizing the advantages of stem cell co-culture strategies and their applications in tissue engineering.
Khademhosseini, A. et al. Layer-by-layer deposition of hyaluronic acid and poly-L-lysine for patterned cell co-cultures. Biomaterials 25, 3583–3592 (2004).
Weizman, A., Michael, I., Wiesel-Motiuk, N., Rezania, A. & Levenberg, S. The effect of endothelial cells on hESC-derived pancreatic progenitors in a 3D environment. Biomater. Sci. 2, 1706–1714 (2014).
Freiman, A. et al. Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Res. Ther. 7, 5 (2016).
Goers, L., Freemont, P. & Polizzi, K. M. Co-culture systems and technologies: taking synthetic biology to the next level. J. R. Soc. Interface 11, 20140065 (2014).
Ben-Arye, T. et al. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 1, 210–220 (2020). This article reports the use of textured soy protein as a scaffold for monoculture and co-culture to create 3D engineered bovine muscle tissue.
Goulet, F., Normand, C. & Morin, O. Cellular interactions promote tissue‐specific function, biomatrix deposition and junctional communication of primary cultured hepatocytes. Hepatology 8, 1010–1018 (1988).
Bian, L., Zhai, D. Y., Mauck, R. L. & Burdick, J. A. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng. Part A 17, 1137–1145 (2011).
Bogliotti, Y. S. et al. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc. Natl Acad. Sci. USA. 115, 2090–2095 (2018).
Lu, Y. et al. Avian-induced pluripotent stem cells derived using human reprogramming factors. Stem Cell Dev. 21, 394–403 (2012).
Ding, S. et al. Maintaining bovine satellite cells stemness through p38 pathway. Sci. Rep. 8, 10808 (2018).
Li, B. J. et al. Isolation, culture and identification of porcine skeletal muscle satellite cells. Asian-Australas. J. Anim. Sci 28, 1171–1177 (2015).
Musina, R. A., Bekchanova, E. S., Belyavskii, A. V. & Sukhikh, G. T. Differentiation potential of mesenchymal stem cells of different origin. Bull. Exp. Biol. Med. 141, 147–151 (2006).
Uezumi, A., Fukada, S. I., Yamamoto, N., Takeda, S. & Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 12, 143–152 (2010).
Biferali, B., Proietti, D., Mozzetta, C. & Madaro, L. Fibro-adipogenic progenitors cross-talk in skeletal muscle: the social network. Front. Physiol. 10, 1074 (2019).
Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 (1965).
Lipsitz, Y. Y., Woodford, C., Yin, T., Hanna, J. H. & Zandstra, P. W. Modulating cell state to enhance suspension expansion of human pluripotent stem cells. Proc. Natl Acad. Sci. USA. 115, 6369–6374 (2018).
Singh, H., Mok, P., Balakrishnan, T., Rahmat, S. N. B. & Zweigerdt, R. Up-scaling single cell-inoculated suspension culture of human embryonic stem cells. Stem Cell Res 4, 165–179 (2010).
Bodiou, V., Moutsatsou, P. & Post, M. J. Microcarriers for upscaling cultured meat production. Front. Nutr. 7, 10 (2020).
Kwok, C. K. et al. Scalable stirred suspension culture for the generation of billions of human induced pluripotent stem cells using single-use bioreactors. J. Tissue Eng. Regen. Med. 12, e1076–e1087 (2018).
Manstein, F., Halloin, C. & Zweigerdt, R. Human pluripotent stem cell expansion in stirred tank bioreactors. Methods Mol. Biol. 1994, 79–91 (2019).
Assou, S. et al. Recurrent genetic abnormalities in human pluripotent stem cells: definition and routine detection in culture supernatant by targeted droplet digital PCR. Stem Cell Rep. 14, 1–8 (2020).
Bar, S. & Benvenisty, N. Epigenetic aberrations in human pluripotent stem cells. EMBO J 38, e101033 (2019).
Evans, M. J. & Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 (1981).
Martin, G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA. 78, 7634–7638 (1981).
Chan, Y. S. et al. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell 13, 663–675 (2013).
Gafni, O. et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 504, 282–286 (2013).
Valamehr, B. et al. Platform for induction and maintenance of transgene-free hiPSCs resembling ground state pluripotent stem cells. Stem Cell Reports 2, 366–382 (2014).
Ware, C. B. et al. Derivation of naïve human embryonic stem cells. Proc. Natl Acad. Sci. USA. 111, 4484–4489 (2014).
Pain, B. et al. Long-term in vitro culture and characterisation of avian embryonic stem cells with multiple morphogenetic potentialities. Development 122, 2339–2348 (1996).
Collodi, P. et al. Culture of cells from zebrafish (Brachydanio rerio) embryo and adult tissues. Cell Biol. Toxicol. 8, 43–61 (1992).
Wakamatsu, Y., Ozato, K. & Sasado, T. Establishment of a pluripotent cell line derived from a medaka (Oryzias latipes) blastula embryo. Mol. Mar. Biol. Biotechnol. 3, 185–191 (1994).
Lavon, N. New technologies for cultivated meat production. Trends Biotechnol. 40, 632–633 (2022).
Karagiannis, P. et al. Induced pluripotent stem cells and their use in human models of disease and development. Physiol. Rev. 99, 79–114 (2019).
Seki, T. Methods of induced pluripotent stem cells for clinical application. World J. Stem Cells 7, 116–125 (2015).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Ezashi, T. et al. Derivation of induced pluripotent stem cells from pig somatic cells. Proc. Natl. Acad. Sci. USA 106, 10993–10998 (2009).
Wu, Z. et al. Generation of pig induced pluripotent stem cells with a drug-inducible system. J. Mol. Cell Biol. 1, 46–54 (2009).
Fuet, A. & Pain, B. Chicken induced pluripotent stem cells: establishment and characterization. Methods Mol. Biol. 1650, 211–228 (2017).
Peng, L. et al. Generation of stable induced pluripotent stem-like cells from adult zebra fish fibroblasts. Int. J. Biol. Sci. 15, 2340–2349 (2019).
Su, Y. et al. Establishment of bovine-induced pluripotent stem cells. Int. J. Mol. Sci. 22, 10489 (2021).
Pawlowski, M. et al. Inducible and deterministic forward programming of human pluripotent stem cells into neurons, skeletal myocytes, and oligodendrocytes. Stem Cell Rep. 8, 803–812 (2017).
Bar-Nur, O., Russ, H. A., Efrat, S. & Benvenisty, N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 9, 17–23 (2011).
Tobin, S. C., Kim, K., De La Rosa, M., Wieland, F. & Just, W. Generating pluripotent stem cells: differential epigenetic changes during cellular reprogramming. FEBS Lett. https://doi.org/10.1016/j.febslet.2012.07.024 (2012).
Pillai, V. V. et al. Induced pluripotent stem cell generation from bovine somatic cells indicates unmet needs for pluripotency sustenance. Anim. Sci. J. 90, 1149–1160 (2019).
Rosselló, R. A. et al. Mammalian genes induce partially reprogrammed pluripotent stem cells in non-mammalian vertebrate and invertebrate species. Elife 2, e00036 (2013).
Roobrouck, V. D., Ulloa-Montoya, F. & Verfaillie, C. M. Self-renewal and differentiation capacity of young and aged stem cells. Exp. Cell Res. 314, 1937–1944 (2008).
Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493–495 (1961).
Verbruggen, S., Luining, D., van Essen, A. & Post, M. J. Bovine myoblast cell production in a microcarriers-based system. Cytotechnology 70, 503–512 (2018).
Sacco, A., Doyonnas, R., Kraft, P., Vitorovic, S. & Blau, H. M. Self-renewal and expansion of single transplanted muscle stem cells. Nature 456, 502–506 (2008).
Sui, M. H. et al. Isolation, culture and myogenic differentiation of muscle stem cells in goat fetal. Sci. Agric. Sin. 51, 1590–1597 (2018).
Dodson, M. V., Martin, E. L., Brannon, M. A., Mathison, B. A. & McFarland, D. C. Optimization of bovine satellite cell-derived myotube formation in vitro. Tissue Cell 19, 159–166 (1987).
Matsuda, R., Spector, D. H. & Strohman, R. C. Regenerating adult chicken skeletal muscle and satellite cell cultures express embryonic patterns of myosin and tropomyosin isoforms. Dev. Biol. 100, 478–488 (1983).
Yablonka-Reuveni, Z., Quinn, L. B. S. & Nameroff, M. Isolation and clonal analysis of satellite cells from chicken pectoralis muscle. Dev. Biol. 119, 252–259 (1987).
Kong, X. et al. Establishment of myoblast cell line and identification of key genes regulating myoblast differentiation in a marine teleost, Sebastes schlegelii. Gene 802, 145869 (2021).
Powell, R. L., Dodson, M. V. & Cloud, J. G. Cultivation and differentiation of satellite cells from skeletal muscle of the rainbow trout Salmo gairdneri. J. Exp. Zool. 250, 333–338 (1989).
Dodson, M. V., McFarland, D. C., Martin, E. L. & Brannon, M. A. Isolation of satellite cells from ovine skeletal muscles. J. Tissue Cult. Methods 10, 233–237 (1986).
Blanton, J. R., Grant, A. L., Mcfarland, D. C., Robinson, J. P. & Bidwell, C. A. Isolation of two populations of myoblasts from porcine skeletal muscle. Muscle Nerve 22, 43–50 (1999).
McFarland, D. C., Doumit, M. E. & Minshall, R. D. The turkey myogenic satellite cell: optimization of in vitro proliferation and differentiation. Tissue Cell 20, 899–908 (1988).
Mouly, V. et al. The mitotic clock in skeletal muscle regeneration, disease and cell mediated gene therapy. Acta Physiol. Scand. 184, 3–15 (2005).
Yin, H., Price, F. & Rudnicki, M. A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 93, 23–67 (2013).
Kolkmann, A. M., Van Essen, A., Post, M. J. & Moutsatsou, P. Development of a chemically defined medium for in vitro expansion of primary bovine satellite cells. Front. Bioeng. Biotechnol. https://doi.org/10.3389/fbioe.2022.895289 (2022).
Haynesworth, S. E., Goshima, J., Goldberg, V. M. & Caplan, A. I. Characterization of cells with osteogenic potential from human marrow. Bone 13, 81–88 (1992).
Pittenger, M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 (1999).
Testa, S. et al. Skeletal muscle-derived human mesenchymal stem cells: influence of different culture conditions on proliferative and myogenic capabilities. Front. Physiol. 11, 553198 (2020).
Bosnakovski, D. et al. Isolation and multilineage differentiation of bovine bone marrow mesenchymal stem cells. Cell Tissue Res 319, 243–253 (2005).
Raoufi, M. F., Tajik, P., Dehghan, M. M., Eini, F. & Barin, A. Isolation and differentiation of mesenchymal stem cells from bovine umbilical cord blood. Reprod. Domest. Anim. 46, 95–99 (2011).
Khatri, M., O’Brien, T. D. & Sharma, J. M. Isolation and differentiation of chicken mesenchymal stem cells from bone marrow. Stem Cells Dev 18, 1495–1492 (2009).
Feyen, D. A. M. et al. Isolation of pig bone marrow-derived mesenchymal stem cells. Methods Mol. Biol. 1416, 225–232 (2016).
Lund, T. C. et al. Sdf1 expression reveals a source of perivascular-derived mesenchymal stem cells in zebrafish. Stem Cells 32, 2767–2779 (2014).
Prockop, D. J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276, 71–74 (1997).
Okamura, L. H. et al. Myogenic differentiation potential of mesenchymal stem cells derived from fetal bovine bone marrow. Anim. Biotechnol. 29, 1–11 (2018).
Meatafora company website. https://meatafora.com/.
Low, M., Eisner, C. & Rossi, F. Fibro/adipogenic progenitors (FAPs): isolation by FACS and culture. Methods Mol. Biol. 1556, 179–189 (2017).
Dohmen, R. G. J. et al. Muscle-derived fibro-adipogenic progenitor cells for production of cultured bovine adipose tissue. NPJ Sci. Food 6, 6 (2022).
Uezumi, A. et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 124, 3654–3664 (2011).
Maqsood, M. I., Matin, M. M., Bahrami, A. R. & Ghasroldasht, M. M. Immortality of cell lines: challenges and advantages of establishment. Cell Biol. Int. 37, 1038–1045 (2013).
Soice, E. & Johnston, J. Immortalizing cells for human consumption. Int. J. Mol. Sci. 22, 11660 (2021).
Kazama, T., Fujie, M., Endo, T. & Kano, K. Mature adipocyte-derived dedifferentiated fat cells can transdifferentiate into skeletal myocytes in vitro. Biochem. Biophys. Res. Commun. 377, 780–785 (2008).
Pasitka, L. et al. Spontaneous immortalization of chicken fibroblasts generates stable, high-yield cell lines for serum-free production of cultured meat. Nat. Food https://doi.org/10.1038/s43016-022-00658-w (2022).
Kuo, H. H. et al. Negligible-cost and weekend-free chemically defined human iPSC Culture. Stem Cell Rep. 14, 256–270 (2020).
Dakhore, S., Nayer, B. & Hasegawa, K. Human pluripotent stem cell culture: Current status, challenges, and advancement. Stem Cells Int. 2018, 7396905 (2018).
Kolkmann, A. M., Post, M. J., Rutjens, M. A. M., van Essen, A. L. M. & Moutsatsou, P. Serum-free media for the growth of primary bovine myoblasts. Cytotechnology 72, 111–120 (2020).
Dai, X. et al. Comparison of the differentiation abilities of bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells toward nucleus pulposus-like cells in three-dimensional culture. Exp. Ther. Med. 22, 1018 (2021).
Rosso, F., Giordano, A., Barbarisi, M. & Barbarisi, A. From cell-ECM interactions to tissue engineering. J. Cell. Physiol. 199, 174–180 (2004).
Scheper, T. Tissue Engineering II: Basics of Tissue Engineering and Tissue Applications (eds Lee, K. & Kaplan, D.) (Springer, 2006).
Bhatia, S. N., Balis, U. J., Yarmush, M. L. & Toner, M. Effect of cell–cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 13, 1883–1900 (1999).
Lu, H. H. & Wang I, E. in Biomedical Nanostructures (ed. Gonsalves, K. E. et al.) Ch. 14 (Wiley, 2007).
Abatangelo, G., Brun, P., Radice, M., Cortiro, R. & Auth, M. K. H. in Integrated Biomaterials Science (ed. Barbucci, R.) 885–945 (Kluwer, 2001).
Li, Y. et al. Myokine IL-15 regulates the crosstalk of co-cultured porcine skeletal muscle satellite cells and preadipocytes. Mol. Biol. Rep. 41, 7543–7553 (2014).
Siddiqui, S. H. et al. Modulatory effects of cell–cell interactions between porcine skeletal muscle satellite cells and fibroblasts on the expression of myogenesis-related genes. J. Appl. Anim. Res. 50, 259–268 (2022).
Krieger, J., Park, B. W., Lambert, C. R. & Malcuit, C. 3D skeletal muscle fascicle engineering is improved with TGF-β1 treatment of myogenic cells and their co-culture with myofibroblasts. PeerJ 2018, e4939 (2018).
Acharya, C. et al. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J. Cell. Physiol. 227, 88–97 (2012).
Guo, S. et al. Prevascularized scaffolds bearing human dental pulp stem cells for treating complete spinal cord injury. Adv. Healthc. Mater. 9, e2000974 (2020).
Luo, Y. et al. Co-culture with TM4 cells enhances the proliferation and migration of rat adipose-derived mesenchymal stem cells with high stemness. Cytotechnology 70, 1409–1422 (2018).
Han, H. W. & Hsu, S. H. Chitosan-hyaluronan based 3D co-culture platform for studying the crosstalk of lung cancer cells and mesenchymal stem cells. Acta Biomater. 42, 157–167 (2016).
Orsi, N. M. & Reischl, J. B. Mammalian embryo co-culture: trials and tribulations of a misunderstood method. Theriogenology 67, 441–458 (2007).
Nishiofuku, M. et al. Modulated differentiation of embryonic stem cells into hepatocyte-like cells by coculture with hepatic stellate cells. J. Biosci. Bioeng. 111, 71–77 (2011).
Campbell, J. J., Davidenko, N., Caffarel, M. M., Cameron, R. E. & Watson, C. J. A multifunctional 3D co-culture system for studies of mammary tissue morphogenesis and stem cell biology. PLoS ONE 6, e25661 (2011).
Wang, I.-N. E. et al. Role of osteoblast–fibroblast interactions in the formation of the ligament-to-bone interface. J. Orthop. Res. 25, 1609–1620 (2007).
Bogdanowicz, D. R. & Lu, H. H. Studying cell–cell communication in co-culture. Biotechnol. J. 8, 395–396 (2013).
Kowalczyk, A. P. & Green, K. J. Structure, function, and regulation of desmosomes. Prog. Mol. Biol. Transl. Sci. 116, 95–118 (2013).
Ou, D. B. et al. Three-dimensional co-culture facilitates the differentiation of embryonic stem cells into mature cardiomyocytes. J. Cell. Biochem. 112, 3555–3562 (2011).
Liu, Y. & Chan-Park, M. B. A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials 31, 1158–1170 (2010).
Arrigoni, C., Bersini, S., Gilardi, M. & Moretti, M. In vitro co-culture models of breast cancer metastatic progression towards bone. Int. J. Mol. Sci. 17, 1405 (2016).
Yuan, Z. et al. Impact of human adipose tissue-derived stem cells on dermatofibrosarcoma protuberans cells in an indirect co-culture: an in vitro study. Stem Cell Res. Ther. 12, 440 (2021).
Zhou, D. et al. A 3D engineered scaffold for hematopoietic progenitor/stem cell co-culture in vitro. Sci. Rep. 10, 11485 (2020).
Osugi, M. et al. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. A 18, 1479–1489 (2012).
Jung, T. H. et al. Application of co-culture technology of epithelial type cells and mesenchymal type cells using nanopatterned structures. PLoS ONE https://doi.org/10.1371/journal.pone.0232899 (2020).
Murphy, S. V. & Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. https://doi.org/10.1038/nbt.2958 (2014).
Kim, J. H. et al. Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function. Nat. Commun. 11, 1025 (2020).
De Giglio, E. et al. Multi-compartment scaffold fabricated via 3D-printing as in vitro co-culture osteogenic model. Sci. Rep. 8, 15130 (2018).
Cho, W. W. et al. Flexible adipose-vascular tissue assembly using combinational 3D printing for volume-stable soft tissue reconstruction. Adv. Healthc. Mater. 10, 1–12 (2021).
Kaji, H., Camci-Unal, G., Langer, R. & Khademhosseini, A. Engineering systems for the generation of patterned co-cultures for controlling cell–cell interactions. Biochim. Biophys. Acta Gen. Subj. 1810, 239–250 (2011).
Bhatia, S. N., Yarmush, M. L. & Toner, M. Controlling cell interactions by micropatterning in co-cultures: hepatocytes and 3T3 fibroblasts. J. Biomed. Mater. Res. 34, 189–199 (1997).
Co, C. C., Wang, Y. C. & Ho, C. C. Biocompatible micropatterning of two different cell types. J. Am. Chem. Soc. 127, 1598–1599 (2005).
Yousaf, M. N., Houseman, B. T. & Mrksich, M. Using electroactive substrates to pattern the attachment of two different cell populations. Proc. Natl Acad. Sci. USA 98, 5992–5996 (2001).
Li, Y. et al. Hierarchical patterning of cells with a microeraser and electrospun nanofibers. Small 12, 1230–1239 (2016).
Zhong, H. et al. Generation of a co-culture cell micropattern model to simulate lung cancer bone metastasis for anti-cancer drug evaluation. RSC Adv. 7, 21837–21847 (2017).
Zagury, Y., Ianovici, I., Landau, S., Lavon, N. & Levenberg, S. Engineered marble-like bovine fat tissue for cultured meat. Commun. Biol. 5, 927 (2022). This article reports the formation of a marble-like construct, composed of engineered bovine adipose and muscle tissues, mimicking inter- and intramuscular fat structures.
Xie, X. et al. A co-culture system of rat synovial stem cells and meniscus cells promotes cell proliferation and differentiation as compared to mono-culture. Sci. Rep. 8, 7693 (2018).
Kovina, M. V., Dyuzheva, T. G., Krasheninnikov, M. E., Yakovenko, S. A. & Khodarovich, Y. M. Co-growth of stem cells with target tissue culture as an easy and effective method of directed differentiation. Front. Bioeng. Biotechnol. 9, 1–11 (2021).
Kuppusamy, P., Kim, D., Soundharrajan, I., Hwang, I. & Choi, K. C. Adipose and muscle cell co-culture system: a novel in vitro tool to mimic the in vivo cellular environment. Biology https://doi.org/10.3390/biology10010006 (2021).
Walenda, T. et al. Co-culture with mesenchymal stromal cells increases proliferation and maintenance of haematopoietic progenitor cells. J. Cell. Mol. Med. 14, 337–350 (2010).
Venter, C. & Niesler, C. A triple co-culture method to investigate the effect of macrophages and fibroblasts on myoblast proliferation and migration. Biotechniques 64, 52–58 (2018).
Shahin-Shamsabadi, A. & Selvaganapathy, P. R. A 3D self-assembled in vitro model to simulate direct and indirect interactions between adipocytes and skeletal muscle cells. Adv. Biosyst. 4, 1–11 (2020).
Orlidge, A. & D’Amore, P. A. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J. Cell Biol. 105, 1455–1462 (1987).
Wang, Z., Wang, Y., Farhangfar, F., Zimmer, M. & Zhang, Y. Enhanced keratinocyte proliferation and migration in co-culture with fibroblasts. PLoS ONE 7, 1–12 (2012).
Ostrovidov, S. et al. Three-dimensional co-culture of C2C12/PC12 cells improves skeletal muscle tissue formation and function. J. Tissue Eng. Regen. Med. 11, 582–595 (2017).
Liu, Y. et al. The effects of different phenotype astrocytes on neural stem cells differentiation in co-culture. Neurosci. Lett. 508, 61–66 (2012).
Frontera, W. R. & Ochala, J. Skeletal muscle: a brief review of structure and function. Behav. Genet. 45, 183–195 (2015).
Levy-Mishali, M., Zoldan, J. & Levenberg, S. Effect of scaffold stiffness on myoblast differentiation. Tissue Eng. A 15, 935–944 (2009).
Kaully, T., Kaufman-Francis, K., Lesman, A. & Levenberg, S. Vascularization — the conduit to viable engineered tissues. Tissue Eng. B 15, 159–169 (2009). This article reviews the progress and recent achievements toward vascularization of engineered tissues, to allow long-term viability of thick 3D-engineered tissue constructs.
Debbi, L. et al. Integrating engineered macro vessels with self-assembled capillaries in 3D implantable tissue for promoting vascular integration in-vivo. Biomaterials 280, 121286 (2022).
Merfeld-Clauss, S., Gollahalli, N., March, K. L. & Traktuev, D. O. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng. A 16, 2953–2966 (2010).
Haug, V., Torio-Padron, N., Stark, G. B., Finkenzeller, G. & Strassburg, S. Comparison between endothelial progenitor cells and human umbilical vein endothelial cells on neovascularization in an adipogenesis mouse model. Microvasc. Res. 97, 159–166 (2015).
Asahara, T. & Isner, J. M. State-of-the-art reviews on vascular stem cells and angiogenesis endothelial progenitor cells for vascular regeneration. J. Hematother. Stem Cell Res. 11, 171–178 (2002).
Perry, L., Ben-Shaul, S., Landau, S. & Levenberg, S. in Vascularization for Tissue Engineering and Regenerative Medicine (eds Holnthoner, W. et al.) 385–413 (Springer, 2021).This article reviews studies integrating co-cultures of endothelial with various types of supporting cells, for the generation of vascularized and functional tissue.
Landau, S., Guo, S. & Levenberg, S. Localization of engineered vasculature within 3D tissue constructs. Front. Bioeng. Biotechnol. 6, 2 (2018).
Von Tell, D., Armulik, A. & Betsholtz, C. Pericytes and vascular stability. Exp. Cell Res. 312, 623–629 (2006).
Levenberg, S. et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 23, 879–884 (2005).
Perry, L., Flugelman, M. Y. & Levenberg, S. Elderly patient-derived endothelial cells for vascularization of engineered muscle. Mol. Ther. 25, 935–948 (2017).
Perry, L., Landau, S., Flugelman, M. Y. & Levenberg, S. Genetically engineered human muscle transplant enhances murine host neovascularization and myogenesis. Commun. Biol. 1, 161 (2018).
Kulesza, A. et al. The mutual interactions between mesenchymal stem cells and myoblasts in an autologous co-culture model. PLoS ONE 11, e0161693 (2016).
Huttala, O. et al. Development of versatile human in vitro vascularized adipose tissue model with serum-free angiogenesis and natural adipogenesis induction. Basic Clin. Pharmacol. Toxicol. 123, 62–71 (2018).
Tremolada, C., Palmieri, G. & Ricordi, C. Adipocyte transplantation and stem cells: plastic surgery meets regenerative medicine. Cell Transpl. 19, 1217–1223 (2010).
Kang, J. H., Gimble, J. M. & Kaplan, D. L. In vitro 3D model for human vascularized adipose tissue. Tissue Eng. A 15, 2227–2236 (2009).
Volz, A. C., Huber, B., Schwandt, A. M. & Kluger, P. J. EGF and hydrocortisone as critical factors for the co-culture of adipogenic differentiated ASCs and endothelial cells. Differentiation 95, 21–30 (2017).
Volz, A. C., Hack, L., Atzinger, F. B. & Kluger, P. J. Completely defined co-culture of adipogenic differentiated ASCs and microvascular endothelial cells. ALTEX 35, 464–476 (2018).
Michael Sorrell, J., Baber, M. A., Traktuev, D. O., March, K. L. & Caplan, A. I. The creation of an in vitro adipose tissue that contains a vascular–adipocyte complex. Biomaterials 32, 9667–9676 (2011).
Xue, W. et al. 3D bioprinted white adipose model for in vitro study of cancer-associated cachexia induced adipose tissue remodeling. Biofabrication 14, ac6c4b (2022).
Choi, J. H. et al. Adipose tissue engineering for soft tissue regeneration. Tissue Eng. B 16, 413–426 (2010).
Hausman, G. J., Basu, U., Du, M., Fernyhough-Culver, M. & Dodson, M. V. Intermuscular and intramuscular adipose tissues: bad vs. good adipose tissues. Adipocyte 3, 242–255 (2014).
Shaw, C. S., Clark, J. & Wagenmakers, A. J. M. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu. Rev. Nutr. 30, 13–34 (2010).
Addison, O., Marcus, R. L., Lastayo, P. C. & Ryan, A. S. Intermuscular fat: a review of the consequences and causes. Int. J. Endocrinol. 2014, 34–36 (2014).
Leal, L. G., Lopes, M. A. & Batista, M. L. Physical exercise-induced myokines and muscle–adipose tissue crosstalk: a review of current knowledge and the implications for health and metabolic diseases. Front. Physiol. https://doi.org/10.3389/fphys.2018.01307 (2018).
Patrick, C. W. Tissue engineering strategies for adipose tissue repair. Anat. Rec. 263, 361–366 (2001).
Jo, B., Morimoto, Y. & Takeuchi, S. Skeletal muscle–adipose cocultured tissue fabricated using cell-laden microfibers and a hydrogel sheet. Biotechnol. Bioeng. 119, 636–643 (2022).
Pandurangan, M. & Hwang, I. Application of cell co-culture system to study fat and muscle cells. Appl. Microbiol. Biotechnol. 98, 7359–7364 (2014).
Seo, K., Suzuki, T., Kobayashi, K. & Nishimura, T. Adipocytes suppress differentiation of muscle cells in a co-culture system. Anim. Sci. J. 90, 423–434 (2019).
Pellegrinelli, V., Clément, K., Butler-Browne, G. S. & Lacasa, D. Human adipocytes induce inflammation and atrophy in muscle cells during obesity.Diabetes 64, 3121–3134 (2015).
Specht, L. An Analysis of Culture Medium Costs and Production Volumes for Cultivated Meat, 1–30 (Good Food Institute, 2020).
Church, R. L. Procollagen and collagen produced by normal bovine corneal stroma fibroblasts in cell culture. Investig. Ophthalmol. Vis. Sci. 19, 192–202 (1980).
Berthod, F., Hayek, D., Damour, O. & Collombel, C. Collagen synthesis by fibroblasts cultured within a collagen sponge. Biomaterials 14, 749–754 (1993).
Haraguchi, Y., Okamoto, Y. & Shimizu, T. A circular cell culture system using microalgae and mammalian myoblasts for the production of sustainable cultured meat. Arch. Microbiol. 204, 615 (2022).
Haraguchi, Y. et al. Thicker three-dimensional tissue from a ‘symbiotic recycling system’ combining mammalian cells and algae. Sci. Rep. 7, 41594 (2017).
Yue, Y., Zhang, L., Zhang, X., Li, X. & Yu, H. De novo lipogenesis and desaturation of fatty acids during adipogenesis in bovine adipose-derived mesenchymal stem cells. Vitr. Cell. Dev. Biol. Anim. 54, 23–31 (2018).
Li, C. H. et al. The production of fat-containing cultured meat by stacking aligned muscle layers and adipose layers formed from gelatin-soymilk scaffold. Front. Bioeng. Biotechnol.https://doi.org/10.3389/fbioe.2022.875069 (2022).
Baldwin, J. et al. In vitro pre-vascularisation of tissue-engineered constructs a co-culture perspective. Vasc. Cell 6, 13 (2014).
Vis, M. A. M., Ito, K. & Hofmann, S. Impact of culture medium on cellular interactions in in vitro co-culture systems. Front. Bioeng. Biotechnol. https://doi.org/10.3389/fbioe.2020.00911 (2020).
Ghezelayagh, Z. et al. Improved differentiation of hESC-derived pancreatic progenitors by using human fetal pancreatic mesenchymal cells in a micro‐scalable three-dimensional co-culture system. Stem Cell Rev. Rep. 18, 360–377 (2022).
Kay Sinclair, S. S. & Burg, K. J. L. Effect of osteoclast co-culture on the differentiation of human mesenchymal stem cells grown on bone graft granules. J. Biomater. Sci. Polym. Ed. 22, 789–808 (2011).
Acknowledgements
The authors acknowledge J. Zavin for graphical illustrations and thank Y. Posen for editorial support.
Author information
Authors and Affiliations
Contributions
S.D., A.T., D.S. and A.M.-S. conducted the literature survey, collated relevant information, and wrote the paper with N.L. and S.L.
Corresponding author
Ethics declarations
Competing interests
The following authors are affiliated with Aleph Farms: S.L. is a chief scientific advisor, N.L. is a chief technology officer and A.M.-S. is a senior director, cell line and media development, R&D. The other authors do not have any conflicts of interest related to this article.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Joshua Flack, Lesley Chow, Paula Camacho Sierra and Masatoshi Suzuki for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
David, S., Tsukerman, A., Safina, D. et al. Co-culture approaches for cultivated meat production. Nat Rev Bioeng 1, 817–831 (2023). https://doi.org/10.1038/s44222-023-00077-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44222-023-00077-x