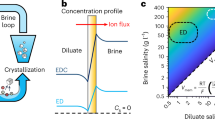
Abstract
Current desalination methods, with high energy/cost demands and large volumes of brine discharged to the environment, are not sustainable. Here we propose a sustainable electrodialysis that enables direct brine valorization with efficient high-water-recovery desalination via zinc–iodine redox reactions. In a single process comprising electrodialysis and two chemical reactions in brine streams, we achieve seawater desalination with a remarkable water recovery of 90.09% without compromising other metrics (salt-removal ratio <98.29%, electric energy consumption of <2.18 kWh m−3). Such performance advantage is attributable to (1) high solubility of zinc–iodine-based ‘water-in-salt’ electrolytes mitigating the osmotic pressure, achieving high water recovery even for high concentration feed water (98–82% for 0.1–1.5 M) with minimal energy burdens, (2) zinc–iodine redox potential lowering electric energy demand and (3) electroconvection in the overlimiting regime enhancing desalination speed. Also, profitable ZnCl2/NaI are electrosynthesized in brine, enabling direct valorization of desalination brines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text or the Supplementary Information. Source data are provided with this paper.
References
Urban, J. J. Emerging scientific and engineering opportunities within the water–energy nexus. Joule 1, 665–688 (2017).
Park, S. & Kwak, R. Microscale electrodeionization: in situ concentration profiling and flow visualization. Water Res. 170, 115310 (2020).
Kim, J., Kim, S. & Kwak, R. Controlling ion transport with pattern structures on ion exchange membranes in electrodialysis. Desalination 499, 114801 (2021).
Elimelech, M. & Phillip, W. A. The future of seawater desalination: energy, technology, and the environment. Science 333, 712–717 (2011).
Mauter, M. S. & Fiske, P. S. Desalination for a circular water economy. Energy Environ. Sci. 13, 3180–3184 (2020).
Jones, E., Qadir, M., van Vliet, M. T. H., Smakhtin, V. & Kang, S.-M. The state of desalination and brine production: a global outlook. Sci. Total Environ. 657, 1343–1356 (2019).
Darre, N. C. & Toor, G. S. Desalination of water: a review. Curr. Pollut. Rep. 4, 104–111 (2018).
Xu, N. et al. Synergistic tandem solar electricity–water generators. Joule 4, 347–358 (2020).
Zhang, L. et al. Passive, high-efficiency thermally-localized solar desalination. Energy Environ. Sci. 14, 1771–1793 (2021).
Wang, J. et al. Continuous desalination with a metal-free redox-mediator. J. Mater. Chem. A 7, 13941–13947 (2019).
Desai, D. et al. Electrochemical desalination of seawater and hypersaline brines with coupled electricity storage. ACS Energy Lett. 3, 375–379 (2018).
Suss, M. E. & Presser, V. Water desalination with energy storage electrode materials. Joule 2, 10–15 (2018).
Abu Khalla, S. & Suss, M. E. Desalination via chemical energy: an electrodialysis cell driven by spontaneous electrode reactions. Desalination 467, 257–262 (2019).
Srimuk, P., Wang, L., Budak, Ö. & Presser, V. High-performance ion removal via zinc–air desalination. Electrochem. Commun. 115, 106713 (2020).
Bhat, Z. M. et al. An electrochemical neutralization cell for spontaneous water desalination. Joule 4, 1730–1742 (2020).
Zhang, Y., Wang, L. & Presser, V. Electrocatalytic fuel cell desalination for continuous energy and freshwater generation. Cell Rep. Phys. Sci. 2, 100416 (2021).
Mavukkandy, M. O., Chabib, C. M., Mustafa, I., Al Ghaferi, A. & AlMarzooqi, F. Brine management in desalination industry: from waste to resources generation. Desalination 472, 114187 (2019).
Panagopoulos, A., Haralambous, K.-J. & Loizidou, M. Desalination brine disposal methods and treatment technologies—a review. Sci. Total Environ. 693, 133545 (2019).
Zhang, X., Zhao, W., Zhang, Y. & Jegatheesan, V. A review of resource recovery from seawater desalination brine. Rev. Environ. Sci. Bio/Technol. 20, 333–361 (2021).
Charisiadis, C. Brine Zero Liquid Discharge (ZLD) Fundamentals and Design (Lenntech, 2018).
Tong, T. & Elimelech, M. The global rise of zero liquid discharge for wastewater management: drivers, technologies, and future directions. Environ. Sci. Technol. 50, 6846–6855 (2016).
Probstein, R. F. Physicochemical Hydrodynamics: An Introduction (John Wiley & Sons, 2005).
Nikonenko, V. V. et al. Desalination at overlimiting currents: state-of-the-art and perspectives. Desalination 342, 85–106 (2014).
Kang, S. & Kwak, R. Pattern formation of three-dimensional electroconvection on a charge selective surface. Phys. Rev. Lett. 124, 154502 (2020).
Kwak, R., Lim, K. M. & Han, J. Shear flow of an electrically charged fluid by ion concentration polarization: scaling laws for electroconvective vortices. Phys. Rev. Lett. 110, 114501 (2013).
Kwak, R. & Han, J. Sheltering the perturbed vortical layer of electroconvection under shear flow. J. Fluid Mech. 813, 799–823 (2017).
Dai, J. et al. Dual-zinc electrode electrochemical desalination. ChemSusChem 13, 2792–2798 (2020).
Dong, X. et al. Environmentally-friendly aqueous Li (or Na)-ion battery with fast electrode kinetics and super-long life. Sci. Adv. 2, e1501038 (2016).
da Silva, D. A. et al. Effect of conductivity, viscosity, and density of water-in-salt electrolytes on the electrochemical behavior of supercapacitors: molecular dynamics simulations and in situ characterization studies. Mater. Adv. 3, 611–623 (2022).
Luo, Y., Jiang, W., Yu, H., MacKerell, A. D. & Roux, B. Simulation study of ion pairing in concentrated aqueous salt solutions with a polarizable force field. Faraday Discuss. 160, 135–149 (2013).
Luo, Y. & Roux, B. Simulation of osmotic pressure in concentrated aqueous salt solutions.J. Phys. Chem. Lett. 1, 183–189 (2010).
Nayar, K. G., Fernandes, J., McGovern, R. K., Al-Anzi, B. S. & Lienhard, J. H. Cost and energy needs of RO-ED-crystallizer systems for zero brine discharge seawater desalination. Desalination 457, 115–132 (2019).
Kwak, R., Guan, G., Peng, W. K. & Han, J. Microscale electrodialysis: concentration profiling and vortex visualization. Desalination 308, 138–146 (2013).
Larchet, C., Dammak, L., Auclair, B., Parchikov, S. & Nikonenko, V. A simplified procedure for ion-exchange membrane characterisation. New J. Chem. 28, 1260–1267 (2004).
Zhao, R., Biesheuvel, P. & Van der Wal, A. Energy consumption and constant current operation in membrane capacitive deionization. Energy Environ. Sci. 5, 9520–9527 (2012).
Vermaas, D. A., Saakes, M. & Nijmeijer, K. Doubled power density from salinity gradients at reduced intermembrane distance. Environ. Sci. Technol. 45, 7089–7095 (2011).
Chehayeb, K. M. & Lienhard, J. H. On the electrical operation of batch electrodialysis for reduced energy consumption. Environ. Sci. Water Res. Tech. 5, 1172–1182 (2019).
Al-Karaghouli, A., Kazmerski, L. L. J. R. & Reviews, S. E. Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renew. Sustain. Energy Rev. 24, 343–356 (2013).
Ahdab, Y. D., Schücking, G., Rehman, D. & Lienhard, J. H. Cost effectiveness of conventionally and solar powered monovalent selective electrodialysis for seawater desalination in greenhouses. Appl. Energy 301, 117425 (2021).
Wetterau, G. Desalination of Seawater: M61 Vol. 61 (American Water Works Association, 2011).
McGovern, R. K., Weiner, A. M., Sun, L., Chambers, C. G. & Zubair, S. M. On the cost of electrodialysis for the desalination of high salinity feeds. Appl. Energy 136, 649–661 (2014).
Acknowledgements
This work was supported by Samsung Research Funding and Incubation Center of Samsung Electronics (SRFC-MA1901-08), Individual Basic Science and Engineering Research Program from the National Research Foundation of Korea (NRF-2022R1A2C4001521) and National R&D Program from the National Research Foundation of Korea (NRF-2021K1A4A7A02102628).
Author information
Authors and Affiliations
Contributions
J.L., M.K. and R.K. conceived and designed the experiment. J.L. and M.K. performed the experiment. R.K. directed the project. All authors analysed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors declare the following competing financial interest(s): patents related to this work have been filed (application number 10-2020-0166881 (Korea) and application number 17/111,145 (United States)).
Peer review
Peer review information
Nature Water thanks John Lienhard and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Texts 1–7, Figs. 1–9, Tables 1–3 and references.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lim, J., Kim, M. & Kwak, R. Zinc–iodine redox reaction enables direct brine valorization with efficient high-water-recovery desalination. Nat Water 2, 475–484 (2024). https://doi.org/10.1038/s44221-024-00238-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44221-024-00238-1