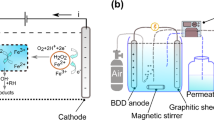
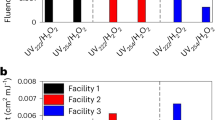
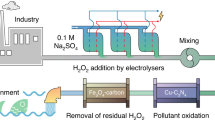
Abstract
Oxidation and reduction reactions govern a wide range of biotic and abiotic processes. Among these are advanced oxidation processes (AOPs) and advanced reduction processes (ARPs) that have been developed to remove contaminants and pathogens from water. While these AOPs and ARPs can be very effective, they do not take full advantage of the synergy between oxidation and reduction. In this Review we summarize the chemistry of state-of-the-art UV-based AOPs and ARPs and compare them with a novel alternative process that involves UV activation of low-molecular-weight diketones. Contaminant removal by this process involves a synergistic combination of oxidation and reduction, the benefits of which could lead to a shift in water treatment technologies from discrete AOPs and ARPs to combined oxidation–reduction processes. Beyond applications for water treatment, the proposed advanced oxidation–reduction processes may also be helpful for the design and application of electron transfer in a range of other fields.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Larsen, T., Hoffmann, S., Lüthi, C., Truffer, B. & Maurer, M. Emerging solutions to the water challenges of an urbanizing world. Science 352, 928–933 (2016).
Shannon, M. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008).
Antonopoulou, M., Evgenidou, E., Lambropoulou, D. & Konstantinou, I. A review on advanced oxidation processes for the removal of taste and odor compounds from aqueous media. Water Res. 53, 215–234 (2014).
von Gunten, U. Oxidation processes in water treatment: are we on track? Environ. Sci. Technol. 52, 5062–5075 (2018). This paper addresses the challenges of oxidative abatement of organic micropollutants in water treatment, emphasizing the questions that need to be considered, and proposes the use of multidisciplinary knowledge-based systems to assess the benefits of oxidation processes.
Marron, E. L., Mitch, W. A., von Gunten, U. & Sedlak, D. L. A tale of two treatments: the multiple barrier approach to removing chemical contaminants during potable water reuse. Acc. Chem. Res. 52, 615–622 (2019).
Lim, S., Shi, J. L., von Gunten, U. & McCurry, D. L. Ozonation of organic compounds in water and wastewater: a critical review. Water Res. 213, 118053–118085 (2022).
Guo, K. H., Wu, Z. H., Chen, C. Y. & Fang, J. Y. UV/chlorine process: an efficient advanced oxidation process with multiple radicals and functions in water treatment. Acc. Chem. Res. 55, 286–297 (2022).
Ao, X. W. et al. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: a review. Water Res. 188, 116479–116501 (2021).
Miklos, D. B. et al. Evaluation of advanced oxidation processes for water and wastewater treatment–a critical review. Water Res. 139, 118–131 (2018). This paper provides an overview of established and emerging AOPs, compares their energy efficiency based on electrical energy per order values, and highlights the influence of operational conditions on the electrical energy per order, offering recommendations for the use and upscaling of AOPs.
Chu, C. H., Ryberg, E. C., Loeb, S. K., Suh, M. J. & Kim, J. H. Water disinfection in rural areas demands unconventional solar technologies. Acc. Chem. Res. 52, 1187–1195 (2019).
Parvulescu, V. I., Epron, F., Garcia, H. & Granger, P. Recent progress and prospects in catalytic water treatment. Chem. Rev. 122, 2981–3121 (2021).
Marin, M. L., Santos-Juanes, L., Arques, A., Amat, A. M. & Miranda, M. A. Organic photocatalysts for the oxidation of pollutants and model compounds. Chem. Rev. 112, 1710–1750 (2012).
Shang, Y. N., Xu, X., Gao, B. Y., Wang, S. B. & Duan, X. G. Single-atom catalysis in advanced oxidation processes for environmental remediation. Chem. Soc. Rev. 50, 5281–5322 (2021).
Zhang, Y. J. et al. Distinguishing homogeneous advanced oxidation processes in bulk water from heterogeneous surface reactions in organic oxidation. Proc. Natl Acad. Sci. USA 120, e2302407120 (2023). This paper provides a fundamental understanding of catalytic organic oxidation processes at the solid–water interface, revealing the prevalence of radical-based AOPs in bulk water and differing reaction pathways on solid catalyst surfaces, which can guide the design of heterogeneous nanocatalysts.
Escher, B. I. & Fenner, K. Recent advances in environmental risk assessment of transformation products. Environ. Sci. Technol. 45, 3835–3847 (2011).
Prasse, C., Ford, B., Nomura, D. K. & Sedlak, D. L. Unexpected transformation of dissolved phenols to toxic dicarbonyls by hydroxyl radicals and UV light. Proc. Natl Acad. Sci. USA 115, 2311–2316 (2018).
Wang, Y. Y. et al. Reactive nitrogen species mediated inactivation of pathogenic microorganisms during UVA photolysis of nitrite at surface water levels. Environ. Sci. Technol. 56, 12542–12552 (2022).
Lei, Y., Lei, X., Westerhoff, P., Zhang, X. R. & Yang, X. Reactivity of chlorine radicals (Cl• and Cl2•–) with dissolved organic matter and the formation of chlorinated byproducts. Environ. Sci. Technol. 55, 689–699 (2021).
Liu, T. C. et al. Unexpected role of nitrite in promoting transformation of sulfonamide antibiotics by peracetic acid: reactive nitrogen species contribution and harmful disinfection byproduct formation potential. Environ. Sci. Technol. 56, 1300–1309 (2022).
Koubek, E. Oxidation of refractory organics in aqueous waste streams by hydrogen peroxide and ultraviolet light. US patent 4,012,321 (1977).
Glaze, W. H., Kang, J. W. & Chapin, D. H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. Eng. 9, 335–352 (1987).
Patel, M. et al. Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem. Rev. 119, 3510–3673 (2019).
Yang, W. B., Zhou, H. D. & Cicek, N. Treatment of organic micropollutants in water and wastewater by UV-based processes: a literature review. Crit. Rev. Environ. Sci. Technol. 44, 1443–1476 (2014).
Yang, J. L., Zhu, M. S. & Dionysiou, D. D. What is the role of light in persulfate-based advanced oxidation for water treatment? Water Res. 189, 116627–116641 (2021).
Baxendale, J. H. & Wilson, J. A. The photolysis of hydrogen peroxide at high light intensities. Trans. Faraday Soc. 53, 344–356 (1957).
Guan, Y. H., Ma, J., Li, X. C., Fang, J. Y. & Chen, L. W. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system. Environ. Sci. Technol. 45, 9308–9314 (2011).
Comninellis, C. et al. Advanced oxidation processes for water treatment: advances and trends for R&D. J. Chem. Technol. Biotechnol. 83, 769–776 (2008).
Oller, I., Malato, S. & Sánchez-Pérez, J. Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Sci. Total Environ. 409, 4141–4166 (2011).
Bolton, J. R., Bircher, K. G., Tumas, W. & Tolman, C. A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric-and solar-driven systems. Pure Appl. Chem. 73, 627–637 (2001).
Lin, C. C., Smith, F. R., Ichikawa, N., Baba, T. & Itow, M. Decomposition of hydrogen peroxide in aqueous solutions at elevated temperatures. Int. J. Chem. Kinet. 23, 971–987 (1991).
Lu, H., Wang, X. Y., Li, X. Q. & Zhang, X. Y. Study on the disinfection efficiency of the combined process of ultraviolet and sodium hypochlorite on the secondary effluent of the sewage treatment plant. Processes 10, 1622–1639 (2022).
Hubbs, S. A., Amundsen, D. & Olthius, P. Use of chlorine dioxide, chloramines, and short‐term free chlorination as alternative disinfectants. J. AWWA 73, 97–101 (1981).
Roberson, J. A. What’s next after 40 years of drinking water regulations? Environ. Sci. Technol. 45, 154–160 (2011).
Yu, Y. C. et al. Microbial cleavage of C–F bonds in two C6 per- and polyfluorinated compounds via reductive defluorination. Environ. Sci. Technol. 54, 14393–14402 (2020).
Xu, G. F. et al. Dehalogenation of polybrominated diphenyl ethers and polychlorinated biphenyls catalyzed by a reductive dehalogenase in Dehalococcoides mccartyi strain MB. Environ. Sci. Technol. 56, 4039–4049 (2022).
Bäckström, H. The chain-reaction theory of negative catalysis1. J. Am. Chem. Soc. 49, 1460–1472 (1927).
Gehringer, P. & Eschweiler, H. Ozone/electron beam process for water treatment: design, limitations and economic considerations. Ozone Sci. Eng. 21, 523–538 (1999).
Yoon, S. H., Abdel-Wahab, A. & Batchelor, B. Advanced reduction processes for hazardous waste treatment. Qatar Found. Annu. Res. Forum Proc. 1, EVP16 (2011).
Vellanki, B. P., Batchelor, B. & Abdel-Wahab, A. Advanced reduction processes: a new class of treatment processes. Environ. Eng. Sci. 30, 264–271 (2013).
Zhao, Y. M. et al. Hydrated electron based photochemical processes for water treatment. Water Res. 225, 119212–119227 (2022). This review summarizes the optimal process conditions, reduction mechanisms and possible by-products of UV-based ARPs.
Gu, Y. R., Liu, T. Z., Zhang, Q. & Dong, W. Y. Efficient decomposition of perfluorooctanoic acid by a high photon flux UV/sulfite process: kinetics and associated toxicity. Chem. Eng. J. 326, 1125–1133 (2017).
Yang, L. et al. UV/SO32− based advanced reduction processes of aqueous contaminants: current status and prospects. Chem. Eng. J. 397, 125412–125421 (2020).
Brandt, C. & van Eldik, R. Transition metal-catalyzed oxidation of sulfur(IV) oxides. Atmospheric-relevant processes and mechanisms. Chem. Rev. 95, 119–190 (1995).
Vellanki, B. P. & Batchelor, B. Perchlorate reduction by the sulfite/ultraviolet light advanced reduction process. J. Hazard. Mater. 262, 348–356 (2013).
Fischer, M. & Warneck, P. Photodecomposition and photooxidation of hydrogen sulfite in aqueous solution. J. Phys. Chem. 100, 15111–15117 (1996).
Lu, L. et al. Wastewater treatment for carbon capture and utilization. Nat. Sustain. 1, 750–758 (2018).
Kou, J. H. et al. Selectivity enhancement in heterogeneous photocatalytic transformations. Chem. Rev. 117, 1445–1514 (2017).
Xiao, J. D. et al. Visible-light photocatalytic ozonation using graphitic C3N4 catalysts: a hydroxyl radical manufacturer for wastewater treatment. Acc. Chem. Res. 53, 1024–1033 (2020).
Tian, H. T., Gao, J., Li, H., Boyd, S. A. & Gu, C. Complete defluorination of perfluorinated compounds by hydrated electrons generated from 3-indole-acetic-acid in organomodified montmorillonite. Sci Rep. 6, 1–9 (2016).
Sun, Z. Y. et al. UV/nitrilotriacetic acid process as a novel strategy for efficient photoreductive degradation of perfluorooctanesulfonate. Environ. Sci. Technol. 52, 2953–2962 (2018).
Chen, G. D., Hanukovich, S., Chebeir, M., Christopher, P. & Liu, H. Z. Nitrate removal via a formate radical-induced photochemical process. Environ. Sci. Technol. 53, 316–324 (2018).
Kugler, A. et al. Reductive defluorination of perfluorooctanesulfonic acid (PFOS) by hydrated electrons generated upon UV irradiation of 3-indole-acetic-acid in 12-aminolauric-modified montmorillonite. Water Res. 200, 117221–117229 (2021).
Kong, L. H., Zhao, J. M., Hu, X. Y., Zhu, F. & Peng, X. J. Reductive removal and recovery of As(V) and As(III) from strongly acidic wastewater by a UV/formic acid process. Environ. Sci. Technol. 56, 9732–9743 (2022).
Wang, M. S., Liu, X. T., Pan, B. C. & Zhang, S. J. Photodegradation of Acid Orange 7 in a UV/acetylacetone process. Chemosphere 93, 2877–2882 (2013).
Zhang, S. J. et al. Diketone-mediated photochemical processes for target-selective degradation of dye pollutants. Environ. Sci. Technol. Lett. 1, 167–171 (2014). This article proposed UV/diketones as a new type of AOP for water treatment.
Liu, X. T., Song, X. J., Zhang, S. J., Wang, M. S. & Pan, B. C. Non-hydroxyl radical mediated photochemical processes for dye degradation. Phys. Chem. Chem. Phys. 16, 7571–7577 (2014).
Song, X. J., Wu, B. D. & Zhang, S. J. Decoloration of alizarin red (an anthraquinone dye) with the UV/acetylacetone process. Acta Chim. Sinica 72, 461–466 (2014).
Wu, B. D., Zhang, S. J., Li, X. C., Liu, X. T. & Pan, B. C. Iron in non-hydroxyl radical mediated photochemical processes for dye degradation: catalyst or inhibitor? Chemosphere 131, 55–62 (2015).
Wu, B. D., Yin, R., Zhang, G. Y., Yu, C. & Zhang, S. J. Effects of water chemistry on decolorization in three photochemical processes: pro and cons of the UV/AA process. Water Res. 105, 568–574 (2016).
Wu, B. D., Zhang, G. Y. & Zhang, S. J. Fate and implication of acetylacetone in photochemical processes for water treatment. Water Res. 101, 233–240 (2016). This article describes the possible environmental fate of AcAc and demonstrates that its photolysis products are bioavailable small molecular acids.
Chen, Z. H. et al. Acetylacetone as an efficient electron shuttle for concerted redox conversion of arsenite and nitrate in the opposite direction. Water Res. 124, 331–340 (2017). This article demonstrates the possibility of AcAc being involved in oxidation and reduction processes as an electron shuttle.
Zhang, G. Y., Wu, B. D. & Zhang, S. J. Effects of acetylacetone on the photoconversion of pharmaceuticals in natural and pure waters. Environ. Pollut. 225, 691–699 (2017).
Yang, M. H. et al. Feasibility of the UV/AA process as a pretreatment approach for bioremediation of dye-laden wastewater. Chemosphere 194, 488–494 (2018).
Chen, Z. H., Jin, J. Y., Song, X. J., Zhang, G. Y. & Zhang, S. J. Redox conversion of arsenite and nitrate in the UV/quinone systems. Environ. Sci. Technol. 52, 10011–10018 (2018).
Jin, J. Y. et al. Effects of acetylacetone on the thermal and photochemical conversion of benzoquinone in aqueous solution. Chemosphere 223, 628–635 (2019).
Jin, J. Y. et al. Enhanced photooxidation of hydroquinone by acetylacetone, a novel photosensitizer and electron shuttle. Environ. Sci. Technol. 53, 11232–11239 (2019). This article investigates the interactions between AcAc and hydroquinone under photochemical conditions, confirming AcAc as a photosensitizer and electron shuttle that might be able to regulate the redox cycle of quinones.
Zhang, L., Wu, B. D., Zhang, G. Y., Gan, Y. H. & Zhang, S. J. Enhanced decomplexation of Cu(II)-EDTA: the role of acetylacetone in Cu-mediated photo-Fenton reactions. Chem. Eng. J. 358, 1218–1226 (2019).
Wu, B. D. et al. Reduction of chromate with UV/diacetyl for the final effluent to be below the discharge limit. J. Hazard. Mater. 389, 121841–121850 (2020).
Wu, B. D. et al. Role of complexation in the photochemical reduction of chromate by acetylacetone. J. Hazard. Mater. 400, 123306–123313 (2020). This study demonstrates the role of complexation in the UV/AcAc process.
Wu, B. D. et al. Key factors in the ligand effects on the photo redox cycling of aqueous iron species. Geochim. Cosmochim. Acta 281, 1–11 (2020).
Zhang, G. Y. & Zhang, S. J. Quantitative structure-activity relationship in the photodegradation of azo dyes. J. Environ. Sci. 90, 41–50 (2020).
Chen, Z. H. et al. Effects of low-molecular-weight organics on the photoreduction of bromate in water. ACS EST Eng. 3, 581–590 (2021).
Zhang, L. et al. Photochemical synthesis of selenium nanospheres of tunable size and colloidal stability with simple diketones. Langmuir 37, 9793–9801 (2021).
Zhang, L. et al. An all-in-one approach for synthesis and functionalization of nano colloidal gold with acetylacetone. Nanotechnology 33, 075605–075616 (2021).
Zheng, H. C. et al. UV-induced redox conversion of tellurite by biacetyl. Environ. Sci. Technol. 55, 16646–16654 (2021).
Zhang, G. Y. et al. Key structural features that determine the selectivity of UV/acetylacetone for the degradation of aromatic pollutants when compared to UV/H2O2. Water Res. 196, 117046–117054 (2021).
Zhang, G. Y., Wu, B. D., Zhang, S. J. & Pan, B. C. Diketone and UV based advanced oxidation/reduction technologies: molecular mechanisms and research advances. Sci. Sin. Chim. 51, 1060–1074 (2021).
Xie, M., Zhang, C. Y., Zheng, H. C., Zhang, G. Y. & Zhang, S. J. Peroxyl radicals from diketones enhanced the indirect photochemical transformation of carbamazepine: kinetics, mechanisms, and products. Water Res. 217, 118424–118432 (2022).
Zhang, L. et al. Diketone-mediated photochemical reduction of selenite to elemental selenium: role of carbon-centered radicals and complexation. Chem. Eng. J. 445, 136831–136840 (2022).
Wei, S. S. et al. Effects of a redox-active diketone on the photochemical transformation of roxarsone: mechanisms and environmental implications. Chemosphere 308, 136326–136335 (2022).
Loeb, S. et al. The technology horizon for photocatalytic water treatment: sunrise or sunset? Environ. Sci. Technol. 53, 2937–2947 (2018).
Geng, Z. et al. Enhanced photocatalytic conversion and selectivity of nitrate reduction to nitrogen over AgCl/TiO2 nanotubes. Dalton Trans. 47, 11104–11112 (2018).
Nagai, K. et al. Antagonistic regulation of the gibberellic acid response during stem growth in rice. Nature 584, 109–114 (2020).
European Food Safety Authority Aliphatic dialcohols, diketones, and hydroxyketones from chemical group 10 (Commission Regulation (EC) No 1565/2000). EFSA J. 166, 1–44 (2004).
Shibamoto, T. Diacetyl: occurrence, analysis, and toxicity. J. Agric. Food Chem. 62, 4048–4053 (2014).
Urbaniak, W. et al. Properties and application of diketones and their derivatives. Chemik 65, 273–277 (2011).
Wang, X., Zhang, Y., Wang, Z. W., Xu, C. H. & Tratnyek, P. G. Advances in metal (loid) oxyanion removal by zerovalent iron: kinetics, pathways, and mechanisms. Chemosphere 280, 130766–130784 (2021).
Zheng, H. C. et al. Unveiling the synergic effect in UV/acetylacetone for redox transformation of toxic oxysalts. Preprint at https://doi.org/10.1021/acsestwater.3c00081 (2023).
Kim, C., Zhou, Q. H., Deng, B. L., Thornton, E. C. & Xu, H. F. Chromium(VI) reduction by hydrogen sulfide in aqueous media: Stoichiometry and kinetics. Environ. Sci. Technol. 35, 2219–2225 (2001).
Barrera-Díaz, C. E., Lugo-Lugo, V. & Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 223, 1–12 (2012).
Xie, B. H. et al. One-step removal of Cr(VI) at alkaline pH by UV/sulfite process: reduction to Cr(III) and in situ Cr(III) precipitation. Chem. Eng. J. 308, 791–797 (2017).
Sun, J., Mao, J., Gong, H. & Lan, Y. Q. Fe(III) photocatalytic reduction of Cr(VI) by low-molecular-weight organic acids with α-OH. J. Hazard. Mater. 168, 1569–1574 (2009).
Marinho, B. A., Cristovao, R. O., Loureiro, J. M., Boaventura, R. A. & Vilar, V. J. Solar photocatalytic reduction of Cr(VI) over Fe(III) in the presence of organic sacrificial agents. Appl. Catal. B 192, 208–219 (2016).
Shah, C. P., Dwivedi, C., Singh, K. K., Kumar, M. & Bajaj, P. N. Riley oxidation: a forgotten name reaction for synthesis of selenium nanoparticles. Mater. Res. Bull. 45, 1213–1217 (2010).
Scaiano, J. C., Stamplecoskie, K. G. & Hallett-Tapley, G. L. Photochemical Norrish type I reaction as a tool for metal nanoparticle synthesis: importance of proton coupled electron transfer. Chem. Commun. 48, 4798–4808 (2012).
Brenninger, C., Jolliffe, J. D. & Bach, T. Chromophore activation of α,β-unsaturated carbonyl compounds and its spplication to enantioselective photochemical reactions. Angew. Chem. Int. Ed. 57, 14338–14349 (2018).
Albini, A. Norrish’ type I and II reactions and their role in the building of photochemical science. Photochem. Photobiol. Sci. 20, 161–181 (2021).
Sugden, T. M. Photochemistry and Reaction Kinetics Ch. 3 (Cambridge Univ. Press, 2010).
Nau, W. M. & Scaiano, J. C. Oxygen quenching of excited aliphatic ketones and diketones. J. Phys. Chem. 100, 11360–11367 (1996).
Neevel, J. G. The Biacetyl-azo Dye System: A Model System to Investigate Oxidative Dye Fading. PhD thesis, Delft Univ. Technology (1992).
Lowrey, A. H., George, C., d’Antonio, P. & Karle, J. Structure of acetylacetone by electron diffraction. J. Am. Chem. Soc. 93, 6399–6403 (1971).
Verma, P. K., Koch, F., Steinbacher, A., Nuernberger, P. & Brixner, T. Ultrafast UV-induced photoisomerization of intramolecularly H-bonded symmetric β-diketones. J. Am. Chem. Soc. 136, 14981–14989 (2014).
Sandler, I., Harper, J. B. & Ho, J. M. Explanation of substituent effects on the enolization of β-diketones and β-ketoesters. J. Chem. Educ. 98, 1043–1048 (2021).
Bhattacherjee, A., Pemmaraju, C. D., Schnorr, K., Attar, A. R. & Leone, S. R. Ultrafast intersystem crossing in acetylacetone via femtosecond X-ray transient absorption at the carbon K-edge. J. Am. Chem. Soc. 139, 16576–16583 (2017).
Kaya, K., Harshbarger, W. R. & Robin, M. B. Triplet states of biacetyl and energy transfer as revealed by opto-acoustic spectroscopy. J. Chem. Phys. 60, 4231–4236 (1974).
Faust, B. C., Powell, K., Rao, C. J. & Anastasio, C. Aqueous-phase photolysis of biacetyl (an α-dicarbonyl compound): a sink for biacetyl, and a source of acetic acid, peroxyacetic acid, hydrogen peroxide, and the highly oxidizing acetylperoxyl radical in aqueous aerosols, fogs, and clouds. Atmos. Environ. 31, 497–510 (1997).
Forbes, M. D. Carbon-Centered Free Radicals and Radical Cations: Structure, Reactivity, and Dynamics Ch. 3 (John Wiley & Sons, 2010).
Gligorovski, S., Strekowski, R., Barbati, S. & Vione, D. Environmental implications of hydroxyl radicals (·OH). Chem. Rev. 115, 13051–13092 (2015).
Wei, S. J. Performance and Structure-Activity Relationship of Acetylacetone and its Structural Analogues for Photochemical Conversion of Metal Oxysalts and Organic Dyes. MSc thesis, Nanjing Univ. (2020).
Mattes, S. L. & Farid, S. Exciplexes and electron transfer reactions. Science 226, 917–921 (1984).
Kuzmin, M. G., Soboleva, I. V. & Dolotova, E. V. Transient exciplex formation electron transfer mechanism. Adv. Phys. Chem. 2011, 1–18 (2011).
Jiang, B. et al. The reduction of Cr(VI) to Cr(III) mediated by environmentally relevant carboxylic acids: state-of-the-art and perspectives. J. Hazard. Mater. 365, 205–226 (2019).
Beck, M. T. Critical evaluation of equilibrium constants in solution. Stability constants of metal complexes. Pure Appl. Chem 49, 127–136 (1977).
Kawaguchi, S. Variety in the coordination modes of β-dicarbonyl compounds in metal complexes. Coord. Chem. Rev. 70, 51–84 (1986).
Sodhi, R. K. & Paul, S. An overview of metal acetylacetonates: developing areas/routes to new materials and applications in organic syntheses. Catal. Surv. Asia 22, 31–62 (2018).
Mofaddel, N., Bar, N., Villemin, D. & Desbène, P. Determination of acidity constants of enolisable compounds by capillary electrophoresis. Anal. Bioanal. Chem. 380, 664–668 (2004).
Fernandes, C. I., Veiga, P. M., Ferreira, J. J. M. & Hughes, M. Green growth versus economic growth: do sustainable technology transfer and innovations lead to an imperfect choice? Bus. Strateg. Environ. 30, 2021–2037 (2021).
Anastas, P. & Eghbali, N. Green chemistry: principles and practice. Chem. Soc. Rev. 39, 301–312 (2010).
Food Additive Status List (US Food and Drug Administration, 2022).
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. EUR-Lex (20 April 2023); https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32008R1272
Singleton, R. & Singleton, D. R. Remembering our forebears: Albert Jan Kluyver and the unity of life. J. Hist. Biol. 50, 169–218 (2017).
van Niel, C. B., Kluyver, A. J. & Derv, H. G. About diacetyl. Biochem. Z. 210, 234–251 (1929).
Ciamician, G. The photochemistry of the future. Science 36, 385–394 (1912).
Yang, F. et al. Performance of UV/acetylacetone process for saline dye wastewater treatment: kinetics and mechanism. J. Hazard. Mater. 406, 124774–124784 (2021).
Zhang, S. J., Jin, J. Y., Wang, X. M., Wang, X. & Zhang, W. T. Application of acetylacetone in inhibiting growth of cyanobacteria. US patent, 146,422,8B2 (2022).
Yilimulati, M. et al. Regulation of photosynthesis in bloom-forming cyanobacteria with the simplest β-diketone. Environ. Sci. Technol. 55, 14173–14184 (2021). This article demonstrates that AcAc can effectively inhibit cyanobacterial growth in a non-oxidative pathway, providing a new approach for controlling harmful blooms.
Yilimulati, M., Zhou, L., Shevela, D. & Zhang, S. J. Acetylacetone interferes with carbon and nitrogen metabolism of Microcystis aeruginosa by cutting off the electron flow to ferredoxin. Environ. Sci. Technol. 56, 9683–9692 (2022).
Castelvecchi, D. & Stoye, E. ‘Elegant’ catalysts that tell left from right scoop chemistry Nobel. Nature 598, 247–248 (2021).
Wacławek, S. et al. Making waves: defining advanced reduction technologies from the perspective of water treatment. Water Res. 212, 118101–118104 (2022).
Campos-Martin, J. M., Blanco-Brieva, G. & Fierro, J. L. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 45, 6962–6984 (2006).
Stolarski, R. S. History of the study of atmospheric ozone. Ozone Sci. Eng. 23, 421–428 (2001).
Koppenol, W. H. The Haber-Weiss cycle—70 years later. Redox Rep. 6, 229–234 (2001).
Crow, S. Peracetic acid sterilization: a timely development for a busy healthcare industry. Infect. Control Hosp. Epidemiol. 13, 111–113 (1992).
Heath, R. L. Breakdown of ozone and formation of hydrogen-peroxide in aqueous-solutions of amine buffers exposed to ozone. Toxicol. Lett. 4, 449–453 (1979).
Peyton, G. R., Huang, F. Y., Burleson, J. L. & Glaze, W. H. Destruction of pollutants in water with ozone in combination with ultraviolet radiation. 1. General principles and oxidation of tetrachloroethylene. Environ. Sci. Technol. 16, 448–453 (1982).
Glaze, W. H. Drinking-water treatment with ozone. Environ. Sci. Technol. 21, 224–230 (1987).
Langlois, G. W., Jones, B. M., Sakaji, R. H. & Daughton, C. G. Quantitation of carbon in oil shale process wastewaters: coulometry coupled with UV-peroxydisulfate and high-temperature oxidation. J. Test. Eval. 14, 5157519 (1984).
Pignatello, J. J. Dark and photoassisted Fe3+-catalyzed degradation of chlorophenoxy herbicides by hydrogen peroxide. Environ. Sci. Technol. 26, 944–951 (1992).
Lubello, C., Caretti, C. & Gori, R. Comparison between PAA/UV and H2O2/UV disinfection for wastewater reuse. Water Sci. Technol. Water Supply 2, 205–212 (2002).
Watts, M. J. & Linden, K. G. Chlorine photolysis and subsequent OH radical production during UV treatment of chlorinated water. Water Res. 41, 2871–2878 (2007).
Huang, L., Dong, W. B. & Hou, H. Q. Investigation of the reactivity of hydrated electron toward perfluorinated carboxylates by laser flash photolysis. Chem. Phys. Lett. 436, 124–128 (2007).
Vecitis, C. D., Park, H., Cheng, J., Mader, B. T. & Hoffmann, M. R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Front. Environ. Sci. Eng. China 3, 129–151 (2009).
Fang, J. Y., Fu, Y. & Shang, C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 48, 1859–1868 (2014).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant numbers 21976083 and 22176087) and the Key Technologies Research and Development Program of the Ministry of Science and Technology of the People’s Republic of China (grant number 2019YFC0408302).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Ran Yin and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, S., Zheng, H. & Tratnyek, P.G. Advanced redox processes for sustainable water treatment. Nat Water 1, 666–681 (2023). https://doi.org/10.1038/s44221-023-00098-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44221-023-00098-1
This article is cited by
-
Tailoring d-band center of high-valent metal-oxo species for pollutant removal via complete polymerization
Nature Communications (2024)