Abstract
Cone beam computed tomography (CBCT) is widely used in medical and dental imaging. Compared to a multidetector CT, it provides volumetric images with high isotropic resolution at a reduced radiation dose, cost and footprint without the need for patient translation. The current CBCT has several intrinsic limitations including reduced soft tissue contrast, inaccurate quantification of X-ray attenuation, image distortions and artefacts, which have limited its clinical applications primarily to imaging hard tissues and made quantitative analysis challenging. Here we report a multisource CBCT (ms-CBCT) which overcomes the shortcomings of the conventional CBCT by using multiple narrowly collimated and rapidly scanning X-ray beams from a carbon nanotube field emission source array. Phantom imaging studies show that, the ms-CBCT increases the accuracy of the Hounsfield unit values by 60%, eliminates the cone beam artefacts, extends the axial coverage, and improves the soft tissue contrast-to-noise ratio by 30–50%, compared to the CBCT configuration.
Similar content being viewed by others
Introduction
Cone beam computed tomography (CBCT) has widespread applications in dentistry for clinical tasks including implant and orthodontic treatment planning and evaluation of endodontic conditions of the jaws and teeth1,2; and in medical imaging including image-guided radiation therapy, intraoperative surgical guidance, and ENT, extremity and breast imaging3,4,5,6. Compared to the multidetector CT (MDCT), the current standard for diagnostic imaging7, CBCT provides volumetric images with a high isotropic resolution at a relatively low cost, low radiation dose and small footprint, and eliminates the need for patient translation. The large imaging volume of CBCT, however, produces strong scattered radiation that reduces the soft tissue contrast and leads to large errors in quantification of X-ray attenuation8,9,10. The large X-ray cone angle also results in image distortion and artefacts due to insufficient sampling and data truncation which reduce the effective axial coverage. These inherent limitations have restricted the clinical applications of CBCT primarily to imaging hard tissues, and hindered quantitative analyses that are now routinely performed by MDCT, such as accurate determination of the bone mineral density, tissue differentiation, and dose calculation in radiation therapy11,12,13,14.
To improve the performance of CBCT, various approaches have been proposed and investigated extensively15,16,17,18,19. While progresses have been made, the abovementioned issues still remain. Several volumetric CT architectures were designed to address the root cause of these limitations, the large imaging volume, by utilizing multiple X-ray sources. The inverse geometry CT20,21 with a narrowly collimated scanning beam and a small area detector and the tetrahedron-beam CT22 with orthogonally placed linear source array and detector is effective in rejecting scatter, but still have the off-axis geometry and the X-ray photon fluxes required are difficult to achieve with the current source technologies. An extremity CBCT with three axially placed X-ray tubes each covering the entire field of view (FOV) showed reduced cone beam artefact compared to a regular CBCT23. Numerical and experimental simulations demonstrated that the performance of the CBCT could be improved if the single wide cone angle X-ray tube was replaced with multiple collimated sources/beams positioned along the axial axis of the scanner24,25. In an experimental simulation study using a single collimated X-ray tube, we showed the multisource CBCT (ms-CBCT) design reduced the scatter-primary-ratio (SPR), increased the uniformity and accuracy of the CT Hounsfield Unit (HU) values, and improved soft tissue contrast for maxillofacial imaging. The ms-CBCT design, however, puts high demands on the X-ray generator, detector, and reconstruction algorithm. An operational ms-CBCT scanner has not been realized till now.
Here, we report a prototype ms-CBCT scanner designed for 3D maxillofacial imaging. The scanner replaces the single wide cone-angle X-ray tube used in the conventional CBCT with a carbon nanotube (CNT) based field emission X-ray source array26,27 which generates multiple narrowly collimated and rapidly scanning X-ray beams from multiple closely packed focal spots (“sources”). A residual scatter reduction algorithm enabled by the ms-CBCT data acquisition scheme is implemented to further enhance the image quality. The prototype ms-CBCT was evaluated and compared to the same scanner operating in the conventional CBCT configuration (referred to as “N1 ms-CBCT”) using only one wide cone angle X-ray beam under otherwise the same imaging conditions, the clinical CBCT Accuitomo 170 (Morita, Japan, referred to as “CBCT M”) and the clinical CBCT CS9300 (Carestream Dental, US, referred to as “CBCT C”) at the same kVp and similar imaging dose.
Results
Prototype multisource CBCT (ms-CBCT) scanner
The prototype scanner consists of a CNT X-ray source array with multiple focal spots (“sources”) aligned along the axial direction of the system and a conventional flat panel detector (FPD), with a system geometry similar to that of a typical dental CBCT, as illustrated in Fig. 1. The radiation from each source is confined to a narrow cone angle by an external multisource collimator to expose only a small section of the object in the axial direction and the full detector width. The FPD was shifted laterally by 70 mm to provide an FOV of 187 mm × 100 mm at the rotation center to cover the entire maxillofacial region of a patient without data truncation.
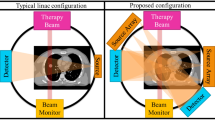
a A schematic of MDCT. It images only a small section of the object in the axial direction per rotation. The patient needs to be translated for volumetric imaging, and the orange dashed arrow marks the translation direction. b With a wider X-ray beam cone angle, the region of interest (ROI) is imaged in one gantry rotation in CBCT. c The ms-CBCT replaces the single X-ray tube with an X-ray source array generating multiple narrowly collimated and rapidly scanning X-ray beams, each illuminating a section of the object and collectively covering the entire ROI. (MDCT multidetector computed tomography, CBCT cone beam computed tomography, ms-CBCT multisource cone beam computed tomography).
The CNT X-ray source array consists of eight gated CNT field emission cathodes and eight corresponding focal spots on an extended tungsten (W) anode with an inter-source spacing of 12 mm, as shown in Fig. 2a. The X-ray exposure and exposure sequence are programmed using an electronic control system (ECS) which automatically adjusts the voltages applied between the individual gate and cathode to achieve and maintain the output. For volumetric CT imaging, the collimated sources are electronically and sequentially scanned across the object with each source activated \({N}_{{{{{{\rm{view}}}}}}}\) times (\({N}_{{{{{{\rm{view}}}}}}}\) = 300–500) as the gantry with the array and the FPD rotates by 360°. Each exposure is recorded by one detector frame to collect a total of 8 × Nview number of projection images.
a The photograph of the CNT field emission X-ray source array with the external multisource collimator. b The radiation fields formed by the eight collimated beams were recorded at 200 mm away from the focal line using a flat panel detector. c The X-ray dose rate profiles were measured at the detector surface using a dose meter (Raysafe X2, Unfors Raysafe AB, Sweden), with the source operating at 90 kVp, 15 mA anode current and 6.5 ms exposure per source with 0.3 mm Cu filtration.
X-ray beam profile and dose rate
Figure 2b shows the radiation fields formed by the individual X-ray beams of the CNT X-ray source array after collimation at 200 mm away from the focal line. Each beam was confined to a rectangular field with a measured cone beam angle of \(2.4^\circ\), which is comparable to that used in a 64-slice MDCT but smaller than the ~\(10^\circ\) cone angle needed to cover the same FOV when the same scanner is operated in the conventional CBCT geometry using only one X-ray source (the “N1 ms-CBCT” configuration). The dose profile measured at the detector surface 615 mm away from the source focal line is shown in Fig. 2c. The average dose rate of the eight sources was 1299 μGy s−1 at the exposure condition used in this study. The X-ray pulses show faster rising and damping times (less than 0.5 ms) than those from a typical clinical CBCT with a thermionic X-ray generator. The fast response time minimizes the error in dose calculation and exposure to patients. The dose and dose rate remained constant over the entire ms-CBCT scan of hundreds of exposures per source.
Quality of the reconstructed phantom images
The image quality and accuracy of the ms-CBCT scanner were investigated by imaging a Contrast phantom, a Defrise phantom, and a RANDO phantom. For comparison, the same phantoms were also imaged using the ms-CBCT scanner operating in the conventional CBCT configuration by using only one source (N1 ms-CBCT) using otherwise the same exposure parameters, the state-of-the-art clinical CBCT M (Accuitomo 170, Morita, Japan) and CBCT C (CS9300 Carestream Dental, US) under similar conditions.
The spatial nonuniformity of the SolidWater CT HU values was quantified by measuring the standard deviations of the mean HU values of the nine ROIs placed on the central axial image of the Contrast phantom. As shown in Fig. 3a, the nonuniformity decreased from 38.0 HU for the clinical CBCT M and 38.4 HU for the N1 ms-CBCT to 9.1 HU for the ms-CBCT, representing a 76% decrease in the nonuniformity. The mean HU value for the water equivalent SolidWater measured from the ms-CBCT is 5.8 HU, close to the nominal value of 0. In contrast, the clinical CBCT M and the N1 CBCT show large errors for the same material, by 129 HU and 20 HU, respectively.
a The boxplot shows the statistical distribution of the SolidWater mean HU values of 9 regions of interest (ROI) marked by yellow solid boxes in the central axial images from the different system configurations. The \(\sigma\) is the standard deviation of the HU mean values of 9 ROIs. b, c The sagittal views of the Contrast phantom measured using the N1 ms-CBCT and ms-CBCT under otherwise the same conditions, respectively. The image distortion caused by data truncation was essentially removed in the ms-CBCT. (Image window: [−1000 HU, 1000 HU], CBCT cone beam computed tomography, ms-CBCT multisource cone beam computed tomography).
Figure 3b, c shows the sagittal images of the Contrast phantom from the N1 ms-CBCT and ms-CBCT, respectively. In the N1 ms-CBCT configuration (conventional CBCT), the top and bottom sections of the image are distorted, which is caused by data truncation due to insufficient detector coverage in the axial direction. In contrast, with the same FPD, this distortion is essentially removed in the ms-CBCT, as shown in Fig. 3c. By reducing the X-ray beam cone angle and therefore the truncation error, the ms-CBCT expands the effective axial FOV from 70 mm in the N1 configuration to 95 mm under otherwise the same geometry.
Figure 4 presents sagittal images and corresponding line profiles of the Defrise phantom scanned using the clinical CBCT M, N1 ms-CBCT and ms-CBCT, respectively. Severe cone beam artefacts in the form of image distortion and blur were observed in the images obtained using the CBCT configuration, due to incomplete sampling in the off-center axial planes23. These image blurs and distortions were eliminated in the reconstructed image from the ms-CBCT because of the reduced X-ray beam cone angle. The top surface of the phantom was placed at the upper edge of the effective FOV for the N1 ms-CBCT. Because of the reduced axial coverage from data truncation, the holder plate and the first disc of the Defrise phantom were outside of the FOV in N1 ms-CBCT but were clearly visualized by the ms-CBCT.
a–c Sagittal slices of the reconstructed CT images of a homemade Defrise phantom imaged using the clinical CBCT M, N1 ms-CBCT and ms-CBCT. d–f Corresponding Hounsfield unit (HU) values line profiles measured within the regions of interest marked by yellow solid boxes along the vertical direction. Severe cone beam artefacts were observed in the image from the clinical CBCT scanner, which were essentially eliminated in the image from the ms-CBCT. (Image window: [−1000 HU, 300 HU], CBCT cone beam computed tomography, ms-CBCT multisource cone beam computed tomography).
Figure 5 shows the central axial images of the Contrast phantom imaged using the clinical CBCT M (Fig. 5a), N1 ms-CBCT (Fig. 5b) and ms-CBCT (Fig. 5c). Visually the image from the ms-CBCT contains fewer artefacts and has a more homogeneous SolidWater background and enhanced contrast compared to the CBCT images. The images from the CBCT M and N1 ms-CBCT appear darker in the center than the periphery, which is the cupping artefact caused by the inhomogeneous distribution of scatter within the scanned volume. Furthermore, the streak and shading artefacts observed around the high-attenuation ceramic resulted from a combination of photon starvation and scatter-induced distortions. As anticipated, the ms-CBCT image exhibited mitigation of these artefacts because of the substantial scatter reduction.
a–c The axial images of the Contrast phantom were scanned using the clinical CBCT M, N1 ms-CBCT and ms-CBCT, respectively. The yellow solid boxes were selected to measure the mean Hounsfield unit (HU) values and standard deviations of different materials for the HU accuracy evaluation. The two closest yellow dashed boxes around each insert were selected to measure the mean HU value and standard deviation of their corresponding SolidWater background for contrast and contrast-to-noise ratio calculations. (Image window: [−1000 HU, 1000 HU], CBCT cone beam computed tomography, ms-CBCT multisource cone beam computed tomography, LDPE low-density polyethylene).
Quantitative analysis was performed to compare the contrast and contrast-to-noise ratio (CNR) using the data from the N1 ms-CBCT and ms-CBCT as the two used the same exposure parameters and reconstruction algorithm except the N1 ms-CBCT has the conventional CBCT configuration. As shown in Fig. 5b, four 10 mm × 10 mm solid squares were selected to measure the mean HU value of four different inserts, and two 10 mm × 10 mm dashed squares near each insert were used to measure the mean HU value and standard deviation of the SolidWater background associated with each insert respectively. This method of selecting ROIs could effectively decouple the impact of the cupping effect by ensuring the measurements of the inserts and their corresponding SolidWater background were taken at the same radius. The contrast was calculated as the absolute HU difference between the insert and its background, and the CNR was calculated as the contrast of the insert divided by the standard deviation of its SolidWater background. The results of all four inserts were summarized in Table 1. Compared to N1 ms-CBCT, ms-CBCT increased the contrast by ~20%, and enhanced the CNR by about 30–50%.
Accuracy of the CT HU value
The mean HU values and the standard deviations of various inserts and the SolidWater of the Contrast phantom were measured by averaging over the solid ROIs shown in Fig. 5b. The results are listed in Table 2. The results from the same Contrast phantom measured using a clinical MDCT (SOMATOM Force, Siemens, Germany) were included as the reference. As shown, the HU values derived from the N1 ms-CBCT and the clinical CBCT M both deviate from the MDCT reference, consistent with previous studies of CBCT28,29,30. The deviations are reduced in results from the ms-CBCT. The root-mean-square error (RMSE) of the HU values for the five materials is reduced from 385 HU in N1 ms-CBCT and 316 HU in the clinical CBCT M to 145 HU in the ms-CBCT.
Image quality and accuracy improvement for maxillofacial imaging
Figure 6 shows the axial images of the RANDO phantom scanned by the CBCT M, CBCT C, and ms-CBCT. The severe shading and streak artefacts around the bones and teeth shown in clinical CBCTs were reduced in the ms-CBCT due to the substantial scatter reduction.
To compare the HU values measured from the mandible and the maxilla bones of the RANDO phantom, the reconstructed volumes from all used scanners were first registered under the rigid body condition using an open-source software 3D Slicer31 to ensure the same ROIs were selected. The cortical and cancellous bones of the mandible and a section of the maxilla were then segmented using the threshold method. Figure 7a is the sagittal image of the RANDO phantom scanned by the ms-CBCT. The discontinuities in the image were caused by the air gaps and misalignments between different slabs of the phantom. The registration and segmentation were conducted using one slab of the head phantom. Figure 7b shows the segmented cortical bones of the mandible. Each sagittal slice (0.3 mm in thickness) of the segmented image was used as one ROI, resulting in a total of 131 ROIs in the mandible and 251 ROIs in the maxilla. The averaged HU numbers for each ROI were obtained and statistical analysis was performed. The results for the three different types of bones, which have a non-normal data distribution, are presented in a Bland-Altman (BA) plot of the difference versus the mean HU value in Fig. 7c–e.
a The sagittal view of the RANDO phantom scanned by the ms-CBCT. b Segmented cortical bones of the mandible. c–e BA plots of the difference between averaged Hounsfield unit (HU) values of the registered regions of interest from the MDCT and the clinical CBCT M, the clinical CBCT C and ms-CBCT, respectively. The median difference, 2.5th and 97.5th percentile were used for non-normally distributed data and calculated based on all 382 data points. Note: The discontinuities shown in Fig. 7a were caused by air gaps and misalignments between different slabs of the RANDO phantom (Image window: [−1000 HU, 3000 HU], ms-CBCT: multisource cone beam computed tomography, MDCT: multidetector computed tomography, CBCT: cone beam computed tomography).
The median difference in the HU values between the MDCT and the clinical CBCT M was found to be −166.7 HU, and the 2.5th and 97.5th percentile lines were −375.3 HU and 179.0 HU. The results of the clinical CBCT C showed a median difference 354.4 HU, and the 2.5th and 97.5th percentile lines were −40.4 HU and 673.7 HU. The agreement with the MDCT was improved for the ms-CBCT. The median difference was found to be 43.7 HU, and the 2.5th and 97.5th percentile lines were −106.0 and 213.6 HU.
Discussion
The results presented here demonstrate the ms-CBCT design improves the image quality and accuracy compared to the conventional CBCT. The imaging dose used by the ms-CBCT is in the range of that by clinical CBCTs for the same object, which varies with the manufacturer, model and imaging protocol32,33. At 11.8 dGy cm2 DAP, the dose of the ms-CBCT with the detector offset geometry was calculated to be 6.3 mGy at the rotation center, similar to the 9.0 mGy dose for the clinical CBCT C based on the 19.8 dGy cm2 DAP.
One limitation of the current prototype is the increased scanning time, which is determined by:
Where the \({\Delta t}_{{{{{{\rm{readout}}}}}}}\) is the detector readout time and \({\triangle t}_{{{{{{\rm{exposure}}}}}}}\) is the exposure time per X-ray pulse. For the imaging protocol used in this study and the readout speed of the present FPD, \({T}_{{{{{{\rm{scan}}}}}}}\) is 52 s. The longer than desired imaging time is partially caused by the inefficiency of photon utilization in the offset geometry using a narrow FPD. One way to mitigate this issue is to increase the detector width to cover the entire FOV without the offset while keeping other parameters the same. This will reduce the scanning time to 26 s without compromising the performance, which is in the time range of the current clinical CBCT32,33. Further reduction of the scanning time can be achieved by using an FPD with a faster readout speed, optimization of the number of projection views and the imaging dose.
In conclusion, the results of this study demonstrate that the ms-CBCT design with a spatially distributed X-ray source array improves the image quality and the diagnostic accuracy of volumetric CT. The scanner is made possible by the CNT field emission X-ray source array technology which generates multiple X-ray beams that can be readily programmed and rapidly scanned with the resolution, consistency, stability and dose rate required for maxillofacial imaging. Phantom imaging studies show it reduces the spatial nonuniformity and RMSE of the CT HU values by respectively 75% and 60%, essentially eliminates the cone beam artefacts, increases the effective axial coverage, and improves CNR of different materials by 30% ~ 50%. It increases the accuracy and reliability of measuring the mandible and maxilla bones compared to the state-of-the-art clinical CBCT. These improvements have the potential to bring the diagnostic accuracy of CBCT to a level comparable or close to that of an MDCT, without changing its important attributes including volumetric imaging at low radiation, low cost, and small footprint. It can potentially expand the clinical applications of CBCT from primarily imaging hard tissue to clinical tasks commonly performed by the more costly and bulky MDCT, including quantitative analysis.
Methods
CNT X-ray source array
The CNT X-ray source array (NuRay Technology Co., Ltd., Changzhou, China) was specifically designed for this scanner and consists of eight gated CNT field emission cathodes and eight corresponding focal spots on an extended W anode with an inter-source spacing of 12 mm. The entire assembly is enclosed in a stainless housing with a 1.7 mm thick Al window which also serves as the inherent filtration. The averaged focal spot size, measured by the standard pin-hole method, is 0.88 mm (width) × 1.10 mm (height) at the 15% maximum intensity with a standard deviation of 0.05 mm (width) × 0.04 mm (height) for the eight spots34. The spot size is comparable to that commonly used in a clinical dental CBCT32.
Flat panel detector (FPD)
The FPD (Xineos-1511 from Teledyne DALSA in Waterloo, CA) is a CMOS flat panel detector with a CsI scintillator designed for dental CBCT. It has an active area of 147.3 mm (width) × 113.7 mm (height) and a pixel pitch of 99 μm. The FPD was operated in the 2 × 2 binning mode with a readout time \({t}_{{{{{{\rm{readout}}}}}}}\) of 11.6 ms per frame.
Adjacent scatter ratio subtraction
The projection images are processed using an adjacent scatter ratio subtraction (ASRS) method specifically for the ms-CBCT geometry. It utilizes the scatter \({I}_{{{{{{\rm{s}}}}}}}^{{\prime} }\) recorded on the adjacent director rows not exposed by the primary beam \({I}_{{{{{{\rm{p}}}}}}}\) to estimate and remove the residual scatter \({I}_{{{{{{\rm{s}}}}}}}\) in each projection image. To minimize contamination from the primary beam, 50 detector rows centered 50 mm away from the center of the illuminated area are averaged to obtain the \({I}_{{{{{{\rm{s}}}}}}}^{{\prime} }\) as a function of the detector column number. The adjacent scatter ratio \(r\), defined as \({I}_{{{{{{\rm{s}}}}}}}^{{\prime} }/({I}_{{{{{{\rm{p}}}}}}}+{I}_{{{{{{\rm{s}}}}}}})\), is calculated for each column. A spline function is used to fit the raw data to obtain the smoothed ratio \({r}^{{\prime} }\). The primary photons \({I}_{{{{{{\rm{p}}}}}}}\) in the exposed region are estimated by applying \(({I}_{{{{{{\rm{p}}}}}}}+{I}_{{{{{{\rm{s}}}}}}})(1-{r}^{{\prime} })\). The same \({r}^{{\prime} }\) function is applied to for all detector rows in the directly illuminated area to subtract the residual scatter. This approach was found to be effective in further reducing the scatter radiation in our feasibility study35.
Image reconstruction and calibration
For N1 ms-CBCT and ms-CBCT, the processed projections from all sources are used for volumetric CT reconstruction using the 3D Simultaneous Iterative Reconstruction Technique algorithm based on the ASTRA Toolbox36. The reconstruction is performed using a single system matrix, including the source/detector rays from all sources. The total variation (TV) algorithm is incorporated for image noise reduction based on the Tigre Toolbox37,38. The CT numbers of all reconstructions are calibrated according to Gammex CT ACR 464 phantom testing instruction39. The images from the clinical CBCT M and MDCT were reconstructed by the manufacturer-specific software packages.
Phantoms
The Defrise phantom consists of a stack of acrylic discs of 160 mm diameter × 4 mm thickness with a 4 mm air gap. The homemade cylindrical Contrast phantom consists of a 16 cm diameter water equivalent plastic material (SolidWater, Sun Nuclear Co, Melbourne FL) with four wells filled with acrylic, low-density polyethylene, air, and Macor (machinable ceramic) sandwiched between uniform SolidWater discs of the same diameter. The RANDO phantom is an anthropomorphic adult skull and tissue equivalent head phantom (RANDO, Nuclear Associates, NY).
DAP calculation
The dose area product (DAP) of the ms-CBCT was calculated using the experimentally measured dose rate at the detector surface and the detector area illuminated by the primary beam per exposure, which is the detector width (147.3 mm) × full width half maximum of the primary beam in the axial direction (32.5 mm), following Eq. (2).
where \(D\) is the dose rate, \(\triangle {t}_{{{{{{\rm{exposure}}}}}}}\) is the exposure time per X-ray pulse, \(A\) is the area of the detector segment illuminated by the primary photons per exposure, \({N}_{{{{{{\rm{view}}}}}}}\) is the number of views and \({N}_{{{{{{\rm{source}}}}}}}\) is the number of sources, which are 360 and 8, respectively in this study.
Scanner parameters
Four scanners were used in this study, including the clinical CBCT M (Accuitomo 170, Morita, Japan), the clinical CBCT C (CS9300, Carestream Dental, US), the benchtop ms-CBCT and the clinical MDCT (SOMATOM Force, Siemens, Germany). Their geometry and exposure parameters are summarized in Table 3 below. The N1 ms-CBCT is the ms-CBCT scanner operating in the conventional CBCT configuration, using only one of its sources and the same exposure parameters. The CBCT C malfunctioned during this study, only results from the RANDO phantom are available from this instrument.
Statistics and reproducibility
To measure the HU spatial nonuniformity, we selected nine regions of interest (ROIs) consisting of 20 × 20 pixels each. The mean value for all pixels within each ROI was calculated, and the standard deviation of these nine mean values was then determined and presented in the boxplot Fig. 3a. For measuring the CNR and HU accuracy, we selected 4 ROIs with 20 × 20 pixels, as well as one larger ROI consisting of 80 × 80 pixels. The mean HU value for each ROI was determined, and the standard deviation of all pixels within corresponding ROIs was calculated and recorded in Table 2. To compare the agreement with the MDCT for mandible and maxilla bone measurements, the MDCT reconstructed volume was used as the reference to create Bland-Altman plots. Prior to analysis, image registration and segmentation were conducted to exclude orientation differences. The sagittal views for the mandible and maxilla consisted of 131 and 251 slices. The mean HU value for each slice of two scanners was used as the horizontal axis. The difference in each slice between two scanners was used as the vertical axis. The data for the three different types of bones do not follow the normal distribution. A variation of the BA plot for non-normal distribution data was used40,41,42. The middle line represents the median difference between two scanners. The lower and upper lines represent the boundary of the 2.5th and 97.5th percentile.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Maxillofacial Cone Beam Computed Tomography (eds William C Scarfe & Christos Angelopoulos) (Springer, Cham, 2018).
Gaêta-Araujo, H. et al. Cone beam computed tomography in dentomaxillofacial radiology: a two-decade overview. Dentomaxillofac. Radiol. 49, 20200145 (2020).
Siewerdsen, J. H. Cone-beam C.T. systems. in Computed Tomography (eds E. Samei & N. J. Pelc) (Springer, Cham, 2020).
Grégoire, V. et al. Image guidance in radiation therapy for better cure of cancer. Mol. Oncol. 14, 1470–1491 (2020).
Hodez, C., Griffaton-Taillandier, C. & Bensimon, I. Cone-beam imaging: applications in ENT. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 128, 65–78 (2011).
Zhu, Y. et al. Dedicated breast CT: state of the art-Part I. Historical evolution and technical aspects. Eur. Radiol. 32, 1579–1589 (2022).
Hsieh, J. & Flohr, T. Computed tomography recent history and future perspectives. J. Med. Imaging 8, 052109 (2021).
Siewerdsen, J. H. & Jaffray, D. A. Cone-beam computed tomography with a flat-panel imager: magnitude and effects of X-ray scatter. Med. Phys. 28, 220–231 (2000).
Kiljunen, T., Kaasalainen, T., Suomalainen, A. & Kortesniemi, M. Dental cone beam CT: a review. Phys. Med. 31, 844–860 (2015).
Posiewnik, M. & Piotrowski, T. A review of cone-beam CT applications for adaptive radiotherapy of prostate cancer. Phys. Med. 59, 13–21 (2019).
Lenchik, L., Weaver, A. A., Ward, R. J., Boone, J. M. & Boutin, R. D. Opportunistic screening for osteoporosis using computed tomography: state of the art and argument for paradigm shift. Curr. Rheumatol. Rep. 20, 74 (2018).
Gupta, S., Martinson, J. R., Ricaurte, D., Scalea, T. M. & Morrison, J. J. Cone-beam computed tomography for trauma. J. Trauma Acute Care Surg. 89, e34–e40 (2020).
Giacometti, V., Hounsell, A. R. & McGarry, C. K. A review of dose calculation approaches with cone beam CT in photon and proton therapy. Phys. Med. 76, 243–276 (2020).
De Los Santos, J. et al. Image guided radiation therapy (IGRT) technologies for radiation therapy localization and delivery. Int. J. Radiat. Oncol. Biol. Phys. 87, 33–45 (2013).
Tang, X., Krupinski, E. A., Xie, H. & Stillman, A. E. On the data acquisition, image reconstruction, cone beam artifacts, and their suppression in axial MDCT and CBCT—a review. Med. Phys. 45, e761–e782 (2018).
Fahrig, R., Jaffray, D. A., Sechopoulos, I. & Webster Stayman, J. Flat-panel conebeam CT in the clinic: history and current state. J. Med. Imaging 8, 052115 (2021).
Yong, T. H. et al. QCBCT-NET for direct measurement of bone mineral density from quantitative cone-beam CT: a human skull phantom study. Sci. Rep. 11, 15083 (2021).
Rührnschopf, E. P. & Klingenbeck, K. A general framework and review of scatter correction methods in X-ray cone-beam computerized tomography. Part 1: scatter compensation approaches. Med. Phys. 38, 4296–4311 (2011).
Hatamikia, S. et al. Source-detector trajectory optimization in cone-beam computed tomography: a comprehensive review on today’s state-of-the-art. Phys. Med. Biol. 67, 16TR03 (2022).
Schmidt, T. G. et al. A prototype table-top inverse-geometry volumetic CT system. Med. Phys. 33, 1867–1878 (2006).
Neculaes, V. B. et al. Multisource inverse-geometry CT. Part II. X-ray source design and prototype. Med. Phys. 43, 4617–4627 (2016).
Zhang, T., Schulze, D., Xu, X. & Kim, J. Tetrahedron beam computed tomography(TBCT): a new design of volumetric CT system. Phys. Med. Biol. 54, 3365 (2009).
Gang, G. J. et al. Image quality and dose for a multisource cone-beam CT extremity scanner. Med. Phys. 45, 144–155 (2018).
Yin, Z., de Man, B. & Pack, J. 3D analytic cone-beam reconstruction for multiaxial CT acquisitions. Int. J. Biomed. Imaging 2009, 538389 (2009).
Becker, A. E., Hernandez, A. M., Schwoebel, P. R. & Boone, J. M. Cone beam CT multisource configurations: evaluating image quality, scatter, and dose using phantom imaging and Monte Carlo simulations. Phys. Med. Biol. 65, 235032 (2020).
Zhang, J. et al. Stationary scanning X-ray source based on carbon nanotube field emitters. Appl. Phys. Lett. 86, 184104 (2005).
Inscoe, C., Lee, Y., Lu, J. & Zhou, O. Development of CNT X-ray technology for medical and dental imaging. In Nanostructured Carbon Electron Emitters and Applications (ed Y. Saito) (Jenny Stanford Publishing Pte Ltd, Singapore, 2020).
Niu, T. et al. Shading correction for on-board cone-beam CT in radiation therapy using planning MDCT images. Med. Phys. 37, 5395–5406 (2010).
Wang, J., Mao, W. & Solberg, T. Scatter correction for cone-beam computed tomography using moving blocker strips: a preliminary study. Med. Phys. 37, 5792–5800 (2010).
Cui, H., Jiang, X., Tang, W., Lu, H. M. & Yang, Y. A practical and robust method for beam blocker-based cone beam CT scatter correction. Phys. Med. Biol. 68, 045006 (2023).
Fedorov, A. et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 30, 1323–1341 (2012).
Ludlow, J. B. et al. Effective dose of dental CBCT-a meta analysis of published data and additional data for nine CBCT units. Dentomaxillofac. Radiol. 44, 20140197 (2015).
Kaasalainen, T., Ekholm, M., Siiskonen, T. & Kortesniemi, M. Dental cone beam CT: an updated review. Phys. Med. 88, 193–217 (2021).
Li, B. et al. Medical Imaging 2023: Physics of Medical Imaging (2023).
Xu, S. et al. Evaluation of the feasibility of a multisource CBCT for maxillofacial imaging. Phys. Med. Biol. 68, 175012 (2023).
van Aarle, W. et al. The ASTRA Toolbox: a platform for advanced algorithm development in electron tomography. Ultramicroscopy 157, 35–47 (2015).
Sidky, E. Y. & Pan, X. Image reconstruction in circular cone-beam computed tomography by constrained, total-variation minimization. Phys. Med. Biol. 53, 4777–4807 (2008).
Biguri, A., Dosanjh, M., Hancock, S. & Soleimani, M. TIGRE: a MATLAB-GPU toolbox for CBCT image reconstruction. Biomed. Phys. Eng. Express 2, 055010 (2016).
Phantom Testing: CT (Revised 11-9-2022), https://accreditationsupport.acr.org/support/solutions/articles/11000056197-phantom-testing-ct-revised-11-9-2022
Estimating nonparametric limits of agreement in non-normally distributed data, https://analyse-it.com/docs/tutorials/bland-altman/estimating-loa-percentile
Chu, A. H. et al. Comparison of wrist-worn Fitbit Flex and waist-worn ActiGraph for measuring steps in free-living adults. PLoS One 12, e0172535 (2017).
Gialamas, A. et al. Assessing agreement between point of care and laboratory results for lipid testing from a clinical perspective. Clin. Biochem. 43, 515–518 (2010).
CT ACR 464 Phantom Datasheet, https://www.sunnuclear.com/uploads/documents/datasheets/Diagnostic/CTACRPhantom_121520.pdf
Siewerdsen, J. H., Waese, A. M., Moseley, D. J., Richard, S. & Jaffray, D. A. Spektr: a computational tool for X-ray spectral analysis and imaging system optimization. Med. Phys. 31, 3057–3067 (2004).
Acknowledgements
The work was partially supported by NIH under Grant R56DE030962-01 and NC Biotechnology Center award 2021-TRG-6725. We thank Negar Mehrabi for technical assistance. We thank Dr. Enrique Platin for the helpful discussions. We thank Amber Dodson, Rose Saulsbury, Emily Rogers, Courtney Wrenn, Chanetha McCabe and Jane Isley at the Oral Radiology Department and Dr. Adam Kimple and Dana St Pierre at the ENT Clinic of UNC for assistance.
Author information
Authors and Affiliations
Contributions
S.X. contributed to the experimental setup, participated in the data acquisition, performed image reconstructions, measured HU uniformity and accuracy and wrote the original draft. Y.H. participated in the data acquisition and analyzed the agreement with MDCT for bone measurements. B.L. contributed to the experimental setup, participated in the data acquisition and characterized the CNT source array. C.R.I. contributed to the experimental setup, characterized the CNT source array and supervised the whole work. D.A.T. supervised the whole work. Y.Z.L. supervised the whole work. J.L. conceived the concept of the ms-CBCT and supervised the whole work. O.Z. conceived the concept of the ms-CBCT, supervised the whole work and wrote the original draft. All authors were involved in revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: O.Z. has equity ownership and serves on the board of directors of XinVisio, LLC., to which the technologies used or evaluated in this project have been licensed, and NuRay Co., Ltd, which manufactures the X-ray sources used in this study. J.L. has equity ownership in XinVisio, LLC. and NuRay Co. Ltd. O.Z., J.L., C.R.I., Y.Z.L., and B.L. are co-inventors of licensed technology evaluated in this study. All activities have been approved by the institutional COI committee. All other authors declare no competing interests.
Peer review
Peer review information
Communications Engineering thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: [Mengying Su].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, S., Hu, Y., Li, B. et al. Volumetric computed tomography with carbon nanotube X-ray source array for improved image quality and accuracy. Commun Eng 2, 71 (2023). https://doi.org/10.1038/s44172-023-00123-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44172-023-00123-x
This article is cited by
-
Improving the accuracy of bone mineral density using a multisource CBCT
Scientific Reports (2024)