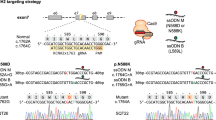
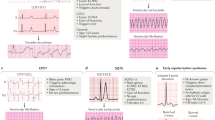
Abstract
Inherited arrhythmias are a heterogeneous group of conditions that confer risk of sudden death. Many inherited arrhythmias have been linked to pathogenic genetic variants that result in ion channel dysfunction, although current genetic testing panels fail to identify variants in many patients, potentially secondary to their underlying substrates being oligogenic or polygenic. Here we review the current state of knowledge surrounding the cellular mechanisms of inherited arrhythmias generated from stem cell models with a focus on integrating genetic and mechanistic data. The utility and limitations of human induced pluripotent stem cell models in disease modeling and drug development are also explored with a particular focus on examples of pharmacogenetics and precision medicine. We submit that progress in understanding inherited arrhythmias is likely to be made by using human induced pluripotent stem cells to model probable polygenic cases as well as to interrogate the diverse and potentially complex molecular networks implicated by genome-wide association studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mazzanti, A., Maragna, R. & Priori, S. G. Genetic causes of sudden cardiac death in the young. Curr. Opin. Cardiol. 32, 253–261 (2017).
Moretti, A. et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 363, 1397–1409 (2010).
Bellin, M. et al. Isogenic human pluripotent stem cell pairs reveal the role of a KCNH2 mutation in long-QT syndrome. EMBO J. 32, 3161–3175 (2013).
Mesquita, F. C. P. et al. R534C mutation in hERG causes a trafficking defect in iPSC-derived cardiomyocytes from patients with type 2 long QT syndrome. Sci. Rep. 9, 19203 (2019).
Feng, L. et al. Long QT syndrome KCNH2 variant induces hERG1a/1b subunit imbalance in patient-specific induced pluripotent stem cell-derived cardiomyocytes. Circ. Arrhythm. Electrophysiol. 14, e009343 (2021).
Mehta, A. et al. Re-trafficking of hERG reverses long QT syndrome 2 phenotype in human iPS-derived cardiomyocytes. Cardiovasc. Res. 102, 497–506 (2014).
Lee, Y. K. et al. MTMR4 SNVs modulate ion channel degradation and clinical severity in congenital long QT syndrome: insights in the mechanism of action of protective modifier genes. Cardiovasc. Res. 117, 767–779 (2021).
Arking, D. E. et al. Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nat. Genet. 46, 826–836 (2014).
Lahrouchi, N. et al. Transethnic genome-wide association study provides insights in the genetic architecture and heritability of long QT syndrome. Circulation 142, 324–338 (2020).
Arking, D. E. et al. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat. Genet. 38, 644–651 (2006).
Ronchi, C. et al. NOS1AP polymorphisms reduce NOS1 activity and interact with prolonged repolarization in arrhythmogenesis. Cardiovasc. Res. 117, 472–483 (2021).
Nishio, Y. et al. D85N, a KCNE1 polymorphism, is a disease-causing gene variant in long QT syndrome. J. Am. Coll. Cardiol. 54, 812–819 (2009).
Kolder, I. et al. Analysis for genetic modifiers of disease severity in patients with long-QT syndrome type 2. Circ Cardiovasc. Genet. 8, 447–456 (2015).
Roberts, J. D. et al. An international multicenter evaluation of type 5 long QT syndrome: a low penetrant primary arrhythmic condition. Circulation 141, 429–439 (2020).
Adler, A. et al. An international, multicentered, evidence-based reappraisal of genes reported to cause congenital long QT syndrome. Circulation 141, 418–428 (2020).
Kim, M. et al. Development of a patient-specific p.D85N-potassium voltage-gated channel subfamily E member 1-induced pluripotent stem cell-derived cardiomyocyte model for drug-induced long QT syndrome. Circ. Genom. Precis. Med. 14, e003234 (2021).
Wada, Y. et al. Common ancestry-specific ion channel variants predispose to drug-induced arrhythmias. Circulation 145, 299–308 (2022).
Sala, L. et al. A new hERG allosteric modulator rescues genetic and drug-induced long-QT syndrome phenotypes in cardiomyocytes from isogenic pairs of patient induced pluripotent stem cells. EMBO Mol. Med. 8, 1065–1081 (2016).
Mehta, A. et al. Identification of a targeted and testable antiarrhythmic therapy for long-QT syndrome type 2 using a patient-specific cellular model. Eur. Heart J. 39, 1446–1455 (2018).
Priori, S. G. et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 10, 1932–1963 (2013).
O’Hare, B. J. et al. Promise and potential peril with lumacaftor for the trafficking defective type 2 long-QT syndrome-causative variants, p.G604S, p.N633S, and p.R685P, using patient-specific re-engineered cardiomyocytes. Circ. Genom. Precis. Med. 13, 466–475 (2020). This study showed that rescuing trafficking defects in long QT syndrome can worsen the arrhythmic phenotype depending on the variant.
Garg, P. et al. Genome editing of induced pluripotent stem cells to decipher cardiac channelopathy variant. J. Am. Coll. Cardiol. 72, 62–75 (2018).
Perry, M. D. et al. Pharmacological activation of IKr in models of long QT type 2 risks overcorrection of repolarization. Cardiovasc Res 116, 1434–1445 (2020). This study showed that a small-molecule activator of Kv11.1 could overcorrect prolonged repolarization in an hiPS cell model of long QT, resulting in a pro-arrhythmic effect.
McKeithan, W. L. et al. Reengineering an antiarrhythmic drug using patient hiPSC cardiomyocytes to improve therapeutic potential and reduce toxicity. Cell Stem Cell 27, 813–821 (2020). McKeithan et al. use hiPS cell-derived cardiomyocytes to re-engineer mexiletine generating a molecule with improved efficacy and reduced toxicity.
Colatsky, T. et al. The Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative - update on progress. J. Pharmacol. Toxicol. Methods 81, 15–20 (2016).
Strauss, D. G. et al. Comprehensive In vitro Proarrhythmia Assay (CiPA) Update from a Cardiac Safety Research Consortium / Health and Environmental Sciences Institute / FDA meeting. Ther. Innov. Regul. Sci. 53, 519–525 (2019).
Ando, H. et al. A new paradigm for drug-induced torsadogenic risk assessment using human iPS cell-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 84, 111–127 (2017).
Goversen, B., van der Heyden, M. A. G., van Veen, T. A. B. & de Boer, T. P. The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: special focus on IK1. Pharmacol. Ther. 183, 127–136 (2018).
Li, M. et al. Overexpression of KCNJ2 in induced pluripotent stem cell-derived cardiomyocytes for the assessment of QT-prolonging drugs. J. Pharmacol. Sci. 134, 75–85 (2017).
Goversen, B. et al. A hybrid model for safety pharmacology on an automated patch clamp platform: using dynamic clamp to join iPSC-derived cardiomyocytes and simulations of Ik1 ion channels in real-time. Front. Physiol. 8, 1094 (2017).
Vaidyanathan, R. et al. IK1-enhanced human-induced pluripotent stem cell-derived cardiomyocytes: an improved cardiomyocyte model to investigate inherited arrhythmia syndromes. Am. J. Physiol. Heart Circ. Physiol. 310, H1611–H1621 (2016).
Hosseini, S. M. et al. Reappraisal of reported genes for sudden arrhythmic death: evidence-based evaluation of gene validity for brugada syndrome. Circulation 138, 1195–1205 (2018).
Barc, J. et al. Genome-wide association analyses identify new Brugada syndrome risk loci and highlight a new mechanism of sodium channel regulation in disease susceptibility. Nat. Genet. 54, 232–239 (2022).
Liang, P. et al. Patient-specific and genome-edited induced pluripotent stem cell-derived cardiomyocytes elucidate single-cell phenotype of Brugada syndrome. J. Am. Coll. Cardiol. 68, 2086–2096 (2016).
Perez-Hernandez, M. et al. Brugada syndrome trafficking-defective Nav1.5 channels can trap cardiac Kir2.1/2.2 channels. JCI Insight 3, e96291 (2018).
Lu, A. et al. Inhibition of Wnt/β-catenin signaling upregulates Nav1.5 channels in Brugada syndrome iPSC-derived cardiomyocytes. Physiol Rep. 11, e15696 (2023).
Cai, D. et al. Patient-specific iPSC-derived cardiomyocytes reveal aberrant activation of Wnt/beta-catenin signaling in SCN5A-related Brugada syndrome. Stem Cell Res. Ther. 14, 241 (2023). Refs. 36 and 37 show that Wnt signaling is probably involved in the pathophysiology of BrS.
Sun, Y. et al. Patient-specific iPSC-derived cardiomyocytes reveal variable phenotypic severity of Brugada syndrome. EBioMedicine 95, 104741 (2023).
Glatter, K. A. et al. Effectiveness of sotalol treatment in symptomatic Brugada syndrome. Am. J. Cardiol. 93, 1320–1322 (2004).
Bezzina, C. R. et al. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat. Genet. 45, 1044–1049 (2013).
El-Battrawy, I. et al. A cellular model of Brugada syndrome with SCN10A variants using human-induced pluripotent stem cell-derived cardiomyocytes. Europace 21, 1410–1421 (2019).
Man, J. C. K. et al. Variant intronic enhancer controls SCN10A-short expression and heart conduction. Circulation 144, 229–242 (2021).
van den Boogaard, M. et al. A common genetic variant within SCN10A modulates cardiac SCN5A expression. J. Clin. Invest. 124, 1844–1852 (2014).
Arnolds, D. E. et al. TBX5 drives Scn5a expression to regulate cardiac conduction system function. J. Clin. Invest. 122, 2509–2518 (2012).
Nieto-Marin, P. et al. Tbx5 variants disrupt Nav1.5 function differently in patients diagnosed with Brugada or long QT syndrome. Cardiovasc. Res. https://doi.org/10.1093/cvr/cvab045 (2021).
Bersell, K. R. et al. Transcriptional dysregulation underlies both monogenic arrhythmia syndrome and common modifiers of cardiac repolarization. Circulation https://doi.org/10.1161/CIRCULATIONAHA.122.062193 (2022).
Li, Y. et al. Novel insights in the pathomechanism of Brugada syndrome and fever-related type 1 ECG changes in a preclinical study using human-induced pluripotent stem cell-derived cardiomyocytes. Clin. Transl. Med. 13, e1130 (2023).
McCormick, J. J., Dokladny, K., Moseley, P. L. & Kenny, G. P. Autophagy and heat: a potential role for heat therapy to improve autophagic function in health and disease. J. Appl. Physiol. 130, 1–9 (2021).
Stamenkovic, M. et al. Comparative analysis of cell death mechanisms induced by lysosomal autophagy inhibitors. Eur. J. Pharmacol. 859, 172540 (2019).
Cerrone, M. et al. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation 129, 1092–1103 (2014).
Veerman, C. C. et al. hiPSC-derived cardiomyocytes from Brugada syndrome patients without identified mutations do not exhibit clear cellular electrophysiological abnormalities. Sci. Rep. 6, 30967 (2016).
Catalano, O. et al. Magnetic resonance investigations in Brugada syndrome reveal unexpectedly high rate of structural abnormalities. Eur. Heart J. 30, 2241–2248 (2009).
Gussak, I. et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology 94, 99–102 (2000).
Walsh, R. et al. Evaluation of gene validity for CPVT and short QT syndrome in sudden arrhythmic death. Eur. Heart J. 43, 1500–1510 (2022).
McPate, M. J., Duncan, R. S., Hancox, J. C. & Witchel, H. J. Pharmacology of the short QT syndrome N588K-hERG K+ channel mutation: differential impact on selected class I and class III antiarrhythmic drugs. Br. J. Pharmacol. 155, 957–966 (2008).
El-Battrawy, I. et al. Modeling short QT syndrome using human-induced pluripotent stem cell-derived cardiomyocytes. J. Am. Heart Assoc. 7, e007394 (2018).
Shinnawi, R. et al. Modeling reentry in the short QT syndrome with human-induced pluripotent stem cell-derived cardiac cell sheets. J. Am. Coll. Cardiol. 73, 2310–2324 (2019).
Guo, F. et al. Patient-specific and gene-corrected induced pluripotent stem cell-derived cardiomyocytes elucidate single-cell phenotype of short QT syndrome. Circ. Res. 124, 66–78 (2019).
van der Werf, C. et al. Implantable cardioverter-defibrillators in previously undiagnosed patients with catecholaminergic polymorphic ventricular tachycardia resuscitated from sudden cardiac arrest. Eur. Heart J. 40, 2953–2961 (2019).
Itzhaki, I. et al. Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. J. Am. Coll. Cardiol. 60, 990–1000 (2012).
Jung, C. B. et al. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol. Med. 4, 180–191 (2012).
Kujala, K. et al. Cell model of catecholaminergic polymorphic ventricular tachycardia reveals early and delayed afterdepolarizations. PLoS ONE 7, e44660 (2012).
Lodola, F. et al. Adeno-associated virus-mediated CASQ2 delivery rescues phenotypic alterations in a patient-specific model of recessive catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis. 7, e2393 (2016).
Dobrev, D. & Wehrens, X. H. Role of RyR2 phosphorylation in heart failure and arrhythmias: controversies around ryanodine receptor phosphorylation in cardiac disease. Circ. Res. 114, 1311–1319 (2014).
Park, S. J. et al. Insights into the pathogenesis of catecholaminergic polymorphic ventricular tachycardia from engineered human heart tissue. Circulation 140, 390–404 (2019). Park et al. explored the molecular mechanism of CPVT and showed that whereas individual cardiomyocytes did exhibit abnormal calcium handling at baseline, a tissue model only displayed reentry in response to high-frequency pacing or adrenergic stimulus, in keeping with the clinical phenotype.
Bezzerides, V. J. et al. Gene therapy for catecholaminergic polymorphic ventricular tachycardia by inhibition of Ca2+/calmodulin-dependent kinase II. Circulation 140, 405–419 (2019).
Uchinoumi, H. et al. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ. Res. 106, 1413–1424 (2010).
Penttinen, K. et al. Antiarrhythmic effects of dantrolene in patients with catecholaminergic polymorphic ventricular tachycardia and replication of the responses using iPSC models. PLoS ONE 10, e0125366 (2015).
Word, T. A. et al. Efficacy of RyR2 inhibitor EL20 in induced pluripotent stem cell-derived cardiomyocytes from a patient with catecholaminergic polymorphic ventricular tachycardia. J. Cell. Mol. Med. 25, 6115–6124 (2021).
Di Pasquale, E. et al. CaMKII inhibition rectifies arrhythmic phenotype in a patient-specific model of catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis. 4, e843 (2013).
Schweitzer, M. K. et al. Suppression of arrhythmia by enhancing mitochondrial Ca2+ uptake in catecholaminergic ventricular tachycardia models. JACC Basic Transl. Sci. 2, 737–747 (2017).
Sander, P. et al. Approved drugs ezetimibe and disulfiram enhance mitochondrial Ca2+ uptake and suppress cardiac arrhythmogenesis. Br. J. Pharmacol. 178, 4518–4532 (2021).
Blackwell, D. J. et al. The Purkinje-myocardial junction is the anatomic origin of ventricular arrhythmia in CPVT. JCI Insight 7, e151893 (2022).
Roselli, C. et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat. Genet. 50, 1225–1233 (2018).
Benaglio, P. et al. Allele-specific NKX2-5 binding underlies multiple genetic associations with human electrocardiographic traits. Nat. Genet. 51, 1506–1517 (2019).
Ellinor, P. T. et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat. Genet. 44, 670–675 (2012).
Gudbjartsson, D. F. et al. Large-scale whole-genome sequencing of the Icelandic population. Nat. Genet. 47, 435–444 (2015).
Orr, N. et al. A mutation in the atrial-specific myosin light chain gene (MYL4) causes familial atrial fibrillation. Nat. Commun. 7, 11303 (2016).
Ghazizadeh, Z. et al. Metastable atrial state underlies the primary genetic substrate for MYL4 mutation-associated atrial fibrillation. Circulation 141, 301–312 (2020).
Hong, L. et al. Human induced pluripotent stem cell-derived atrial cardiomyocytes carrying an SCN5A mutation identify nitric oxide signaling as a mediator of atrial fibrillation. Stem Cell Reports 16, 1542–1554 (2021).
Benzoni, P. et al. Human iPSC modelling of a familial form of atrial fibrillation reveals a gain of function of If and ICaL in patient-derived cardiomyocytes. Cardiovasc. Res. 116, 1147–1160 (2020).
Garg, P. et al. Human induced pluripotent stem cell-derived cardiomyocytes as models for cardiac channelopathies: a primer for non-electrophysiologists. Circ. Res. 123, 224–243 (2018).
Ma, J. et al. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am. J. Physiol. Heart Circ. Physiol. 301, H2006–H2017 (2011).
Veerman, C. C. et al. Switch from fetal to adult SCN5A isoform in human induced pluripotent stem cell-derived cardiomyocytes unmasks the cellular phenotype of a conduction disease-causing mutation. J. Am. Heart Assoc. 6, e005135 (2017).
Kim, J. J. et al. Mechanism of automaticity in cardiomyocytes derived from human induced pluripotent stem cells. J. Mol. Cell. Cardiol. 81, 81–93 (2015).
Satin, J. et al. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J. Physiol. 559, 479–496 (2004).
Hong, T. & Shaw, R. M. Cardiac T-tubule microanatomy and function. Physiol. Rev. 97, 227–252 (2017).
Vuckovic, S. et al. Characterization of cardiac metabolism in iPSC-derived cardiomyocytes: lessons from maturation and disease modeling. Stem Cell Res. Ther. 13, 332 (2022).
Mason, F. E., Pronto, J. R. D., Alhussini, K., Maack, C. & Voigt, N. Cellular and mitochondrial mechanisms of atrial fibrillation. Basic Res. Cardiol. 115, 72 (2020).
Tanaka, M. et al. Elevated oxidative stress is associated with ventricular fibrillation episodes in patients with Brugada-type electrocardiogram without SCN5A mutation. Cardiovasc. Pathol. 20, e37–e42 (2011).
Karbassi, E. et al. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 17, 341–359 (2020).
Campostrini, G. et al. Maturation of hiPSC-derived cardiomyocytes promotes adult alternative splicing of SCN5A and reveals changes in sodium current associated with cardiac arrhythmia. Cardiovasc. Res. 119, 167–182 (2023).
Morrissey, J., Mesquita, F. C. P., Hochman-Mendez, C. & Taylor, D. A. Whole heart engineering: advances and challenges. Cells Tissues Organs 211, 395–405 (2022).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Itzhaki, I. et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 471, 225–229 (2011).
Nakajima, T. et al. Novel mechanism of HERG current suppression in LQT2: shift in voltage dependence of HERG inactivation. Circ. Res. 83, 415–422 (1998).
Salvage, S. C. et al. Ion channel gating in cardiac ryanodine receptors from the arrhythmic RyR2-P2328S mouse. J. Cell Sci. 132, jcs229039 (2019).
Giustetto, C. et al. Long-term follow-up of patients with short QT syndrome. J. Am. Coll. Cardiol. 58, 587–595 (2011).
Zeppenfeld, K. et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 43, 3997–4126 (2022).
Hindricks, G. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42, 373–498 (2021).
Author information
Authors and Affiliations
Contributions
T.R. and J.D.R. performed manuscript conception and writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Fadi Akar, Milena Bellin, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ryan, T., Roberts, J.D. Stem cell models of inherited arrhythmias. Nat Cardiovasc Res 3, 420–430 (2024). https://doi.org/10.1038/s44161-024-00451-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-024-00451-x