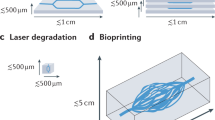
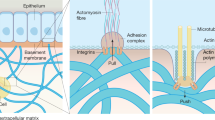
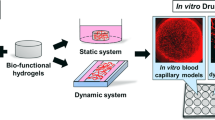
Abstract
Mechanical stimuli from the extracellular matrix (ECM) modulate vascular differentiation, morphogenesis and dysfunction of the vasculature. With innovation in measurements, we can better characterize vascular microenvironment mechanics in health and disease. Recent advances in material sciences and stem cell biology enable us to accurately recapitulate the complex and dynamic ECM mechanical microenvironment for in vitro studies. These biomimetic approaches help us understand the signaling pathways in disease pathologies, identify therapeutic targets, build tissue replacement and activate tissue regeneration. This Review analyzes how ECM mechanics regulate vascular homeostasis and dysfunction. We highlight approaches to examine ECM mechanics at tissue and cellular levels, focusing on how mechanical interactions between cells and the ECM regulate vascular phenotype, especially under certain pathological conditions. Finally, we explore the development of biomaterials to emulate, measure and alter the physical microenvironment of pathological ECM to understand cell–ECM mechanical interactions toward the development of therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rogers, W. J. et al. Age-associated changes in regional aortic pulse wave velocity. J. Am. Coll. Cardiol. 38, 1123–1129 (2001).
Vatner, S. F. et al. Vascular stiffness in aging and disease. Front. Physiol. 12, 762437 (2021).
Jaganathan, S. K., Supriyanto, E., Murugesan, S., Balaji, A. & Asokan, M. K. Biomaterials in cardiovascular research: applications and clinical implications. Biomed. Res. Int. 2014, 459465 (2014).
Attwell, D., Mishra, A., Hall, C. N., O’Farrell, F. M. & Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. 36, 451–455 (2016).
Kruger-Genge, A., Blocki, A., Franke, R. P. & Jung, F. Vascular endothelial cell biology: an update. Int. J. Mol. Sci. 20, 4411 (2019).
Blatchley, M. R. & Gerecht, S. Reconstructing the vascular developmental milieu in vitro. Trends Cell Biol. 30, 15–31 (2020).
Tourovskaia, A., Fauver, M., Kramer, G., Simonson, S. & Neumann, T. Tissue-engineered microenvironment systems for modeling human vasculature. Exp. Biol. Med. 239, 1264–1271 (2014).
Scarpellino, G., Munaron, L., Cantelmo, A. R. & Fiorio Pla, A. Calcium-permeable channels in tumor vascularization: peculiar sensors of microenvironmental chemical and physical cues. In From Malignant Transformation to Metastasis: Ion Transport in Tumor Biology, 111–137 (Springer, 2020).
Schnellmann, R. et al. Stiffening matrix induces age-mediated microvascular phenotype through increased cell contractility and destabilization of adherens junctions. Adv. Sci. 9, 2201483 (2022). This paper shows that on-demand hydrogel stiffening induces endothelial cell contractility, resulting in compromised vascular networks.
Zimmerman, E., Geiger, B. & Addadi, L. Initial stages of cell–matrix adhesion can be mediated and modulated by cell-surface hyaluronan. Biophys. J. 82, 1848–1857 (2002).
Gupta, V. & Grande-Allen, K. J. Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc. Res. 72, 375–383 (2006).
Jin, K. A microcirculatory theory of aging. Aging Dis. 10, 676–683 (2019).
Li, L. et al. Age-related changes of the cutaneous microcirculation in vivo. Gerontology 52, 142–153 (2006).
Ungvari, Z., Tarantini, S., Donato, A. J., Galvan, V. & Csiszar, A. Mechanisms of vascular aging. Circ. Res. 123, 849–867 (2018).
Birch, H. L. Extracellular matrix and ageing. In Biochemistry and Cell Biology of Ageing: Part I Biomedical Science, 169–190 (Springer, 2018).
Xu, J. & Shi, G. P. Vascular wall extracellular matrix proteins and vascular diseases. Biochim. Biophys. Acta 1842, 2106–2119 (2014).
Leclech, C., Natale, C. F. & Barakat, A. I. The basement membrane as a structured surface—role in vascular health and disease. J. Cell Sci. 133, jcs239889 (2020). This paper details the mechanical, structural and topographical properties of BMs across tissue types.
Moiseeva, E. P. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc. Res. 52, 372–386 (2001).
Stratman, A. N. & Davis, G. E. Endothelial cell–pericyte interactions stimulate basement membrane matrix assembly: influence on vascular tube remodeling, maturation, and stabilization. Microsc. Microanal. 18, 68–80 (2012).
Bonkowski, D., Katyshev, V., Balabanov, R. D., Borisov, A. & Dore-Duffy, P. The CNS microvascular pericyte: pericyte–astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS 8, 8 (2011).
Barallobre-Barreiro, J. et al. Extracellular matrix in vascular disease, part 2/4: JACC Focus Seminar. J. Am. Coll. Cardiol. 75, 2189–2203 (2020).
Camasao, D. B. & Mantovani, D. The mechanical characterization of blood vessels and their substitutes in the continuous quest for physiological-relevant performances. A critical review. Mater. Today Bio 10, 100106 (2021).
Hopper, S. E. et al. Comparative study of human and murine aortic biomechanics and hemodynamics in vascular aging. Front. Physiol. 12, 746796 (2021).
Raines, E. W. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int. J. Exp. Pathol. 81, 173–182 (2000).
Ye, G. J., Nesmith, A. P. & Parker, K. K. The role of mechanotransduction on vascular smooth muscle myocytes’ [corrected] cytoskeleton and contractile function. Anat. Rec. 297, 1758–1769 (2014).
Kim, S. A., Sung, J. Y., Woo, C. H. & Choi, H. C. Laminar shear stress suppresses vascular smooth muscle cell proliferation through nitric oxide–AMPK pathway. Biochem. Biophys. Res. Commun. 490, 1369–1374 (2017).
Leley, S. P., Ciulla, T. A. & Bhatwadekar, A. D. Diabetic retinopathy in the aging population: a perspective of pathogenesis and treatment. Clin. Interv. Aging 16, 1367–1378 (2021).
Scioli, M. G., Bielli, A., Arcuri, G., Ferlosio, A. & Orlandi, A. Ageing and microvasculature. Vasc. Cell 6, 19 (2014).
Pober, J. S. & Sessa, W. C. Inflammation and the blood microvascular system. Cold Spring Harb. Perspect. Biol. 7, a016345 (2014).
Mitchell, G. F. Arterial stiffness and hypertension: chicken or egg? Hypertension 64, 210–214 (2014).
Kohn, J. C., Lampi, M. C. & Reinhart-King, C. A. Age-related vascular stiffening: causes and consequences. Front. Genet. 6, 112 (2015).
Ferland-McCollough, D., Slater, S., Richard, J., Reni, C. & Mangialardi, G. Pericytes, an overlooked player in vascular pathobiology. Pharmacol. Ther. 171, 30–42 (2017).
Vilela-Martin, J., Cosenso-Martin, L. & Valente, F. Influence of antihypertensive treatment on MMP-9 levels in controlled hypertensive individuals. J. Hypertens. 36, e46 (2018).
Valente, F. M. et al. Plasma levels of matrix metalloproteinase-9 are elevated in individuals with hypertensive crisis. BMC Cardiovasc. Disord. 20, 132 (2020).
Yabluchanskiy, A. et al. Cardiac aging is initiated by matrix metalloproteinase-9-mediated endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 306, H1398–H1407 (2014).
Craighead, D. H., Wang, H., Santhanam, L. & Alexander, L. M. Acute lysyl oxidase inhibition alters microvascular function in normotensive but not hypertensive men and women. Am. J. Physiol. Heart Circ. Physiol. 314, H424–H433 (2018). This paper shows that sensitivity to LOXL2 inhibition changes depending on normotensive versus hypertensive conditions.
Steppan, J. et al. Tissue transglutaminase modulates vascular stiffness and function through crosslinking-dependent and crosslinking-independent functions. J. Am. Heart Assoc. 6, e004161 (2017).
Gimbrone, M. A. Jr. & Garcia-Cardena, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 118, 620–636 (2016).
Yamin, R. & Morgan, K. G. Deciphering actin cytoskeletal function in the contractile vascular smooth muscle cell. J. Physiol. 590, 4145–4154 (2012).
Romer, L. H., Birukov, K. G. & Garcia, J. G. Focal adhesions: paradigm for a signaling nexus. Circ. Res. 98, 606–616 (2006).
Jain, M. & Chauhan, A. K. Role of integrins in modulating smooth muscle cell plasticity and vascular remodeling: from expression to therapeutic implications. Cells 11, 646 (2022).
Tsimbouri, P. M. Adult stem cell responses to nanostimuli. J. Funct. Biomater. 6, 598–622 (2015).
Chen, Q. W., Edvinsson, L. & Xu, C. B. Role of ERK/MAPK in endothelin receptor signaling in human aortic smooth muscle cells. BMC Cell Biol. 10, 52 (2009).
Shimokawa, H., Sunamura, S. & Satoh, K. RhoA/Rho-kinase in the cardiovascular system. Circ. Res. 118, 352–366 (2016).
Gu, Y. & Gu, C. Physiological and pathological functions of mechanosensitive ion channels. Mol. Neurobiol. 50, 339–347 (2014).
Lai, A. et al. Mechanosensing by Piezo1 and its implications for physiology and various pathologies. Biol. Rev. Camb. Philos. Soc. 97, 604–614 (2022).
Martinac, B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. 117, 2449–2460 (2004).
Takai, Y., Sasaki, T. & Matozaki, T. Small GTP-binding proteins. Physiol. Rev. 81, 153–208 (2001).
Strassheim, D. et al. RhoGTPase in vascular disease. Cells 8, 551 (2019).
Chapados, R. et al. ROCK controls matrix synthesis in vascular smooth muscle cells: coupling vasoconstriction to vascular remodeling. Circ. Res. 99, 837–844 (2006).
Huveneers, S., Daemen, M. J. & Hordijk, P. L. Between Rho(k) and a hard place: the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ. Res. 116, 895–908 (2015).
Piera-Velazquez, S. & Jimenez, S. A. Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol. Rev. 99, 1281–1324 (2019). This paper summarizes the pathobiology of endothelial-to-mesenchymal transition.
Filosa, J. A., Yao, X. & Rath, G. TRPV4 and the regulation of vascular tone. J. Cardiovasc. Pharmacol. 61, 113–119 (2013).
Jackson, W. F. Ion channels and vascular tone. Hypertension 35, 173–178 (2000).
Liu, L. et al. Role of transient receptor potential vanilloid 4 in vascular function. Front. Mol. Biosci. 8, 677661 (2021).
Mack, C. P., Somlyo, A. V., Hautmann, M., Somlyo, A. P. & Owens, G. K. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J. Biol. Chem. 276, 341–347 (2001).
Goncharov, N. V., Nadeev, A. D., Jenkins, R. O. & Avdonin, P. V. Markers and biomarkers of endothelium: when something is rotten in the state. Oxid. Med. Cell. Longev. 2017, 9759735 (2017).
Shu, X. et al. Endothelial nitric oxide synthase in the microcirculation. Cell. Mol. Life Sci. 72, 4561–4575 (2015).
Petsophonsakul, P. et al. Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 39, 1351–1368 (2019).
Zhao, Y. et al. Role of PI3K in the progression and regression of atherosclerosis. Front. Pharmacol. 12, 632378 (2021).
Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605–635 (2017).
Ponticos, M. & Smith, B. D. Extracellular matrix synthesis in vascular disease: hypertension, and atherosclerosis. J. Biomed. Res. 28, 25–39 (2014).
Boopathy, G. T. K. & Hong, W. Role of Hippo pathway–YAP/TAZ signaling in angiogenesis. Front. Cell Dev. Biol. 7, 49 (2019).
DeLoach, S. S. & Townsend, R. R. Vascular stiffness: its measurement and significance for epidemiologic and outcome studies. Clin. J. Am. Soc. Nephrol. 3, 184–192 (2008).
Najjar, S. S. et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J. Am. Coll. Cardiol. 51, 1377–1383 (2008).
Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur. Heart J. 31, 2338–2350 (2010).
Ferruzzi, J., Di Achille, P., Tellides, G. & Humphrey, J. D. Combining in vivo and in vitro biomechanical data reveals key roles of perivascular tethering in central artery function. PloS ONE 13, e0201379 (2018).
Linka, K., Cavinato, C., Humphrey, J. D. & Cyron, C. J. Predicting and understanding arterial elasticity from key microstructural features by bidirectional deep learning. Acta Biomater. 147, 63–72 (2022).
Shahid, M. & Buys, E. S. Assessing murine resistance artery function using pressure myography. J. Vis. Exp. https://doi.org/10.3791/50328 (2013).
Martin, S. et al. Polymer hydrogel particles as biocompatible AFM probes to study CD44/hyaluronic acid interactions on cells. Polymer 102, 342–349 (2016).
Radmacher, M. Studying the mechanics of cellular processes by atomic force microscopy. Methods Cell Biol. 83, 347–372 (2007).
Engler, A. J., Rehfeldt, F., Sen, S. & Discher, D. E. Microtissue elasticity: measurements by atomic force microscopy and its influence on cell differentiation. Methods Cell Biol. 83, 521–545 (2007).
Müller, D. J., Helenius, J., Alsteens, D. & Dufrêne, Y. F. Force probing surfaces of living cells to molecular resolution. Nat. Chem. Biol. 5, 383–390 (2009).
Polacheck, W. J. & Chen, C. S. Measuring cell-generated forces: a guide to the available tools. Nat. Methods 13, 415–423 (2016).
Mao, Y. et al. In vivo nanomechanical imaging of blood-vessel tissues directly in living mammals using atomic force microscopy. Appl. Phys. Lett. 95, 013704 (2009).
Meng, H., Chowdhury, T. T. & Gavara, N. The mechanical interplay between differentiating mesenchymal stem cells and gelatin-based substrates measured by atomic force microscopy. Front. Cell Dev. Biol. 9, 1639 (2021).
Sharma, S. & Gimzewski, J. K. Application of AFM to the nanomechanics of cancer. MRS Advances 1, 1817–1827 (2016).
Rape, A. D., Zibinsky, M., Murthy, N. & Kumar, S. A synthetic hydrogel for the high-throughput study of cell–ECM interactions. Nat. Commun. 6, 8129 (2015).
Norman, M. D., Ferreira, S. A., Jowett, G. M., Bozec, L. & Gentleman, E. Measuring the elastic modulus of soft culture surfaces and three-dimensional hydrogels using atomic force microscopy. Nat. Protoc. 16, 2418–2449 (2021).
Viji Babu, P. K., Rianna, C., Mirastschijski, U. & Radmacher, M. Nano-mechanical mapping of interdependent cell and ECM mechanics by AFM force spectroscopy. Sci. Rep. 9, 12317 (2019).
Maynard, S. A. et al. Nanoscale molecular quantification of stem cell–hydrogel interactions. ACS Nano 14, 17321–17332 (2020). This paper shows approaches to quantify cell–hydrogel interaction at a molecule level.
Puckert, C. et al. Molecular interactions and forces of adhesion between single human neural stem cells and gelatin methacrylate hydrogels of varying stiffness. Acta Biomater. 106, 156–169 (2020).
Henderson, E., Haydon, P. & Sakaguchi, D. Actin filament dynamics in living glial cells imaged by atomic force microscopy. Science 257, 1944–1946 (1992).
Taubenberger, A. V., Hutmacher, D. W. & Muller, D. J. Single-cell force spectroscopy, an emerging tool to quantify cell adhesion to biomaterials. Tissue Eng. Part B Rev. 20, 40–55 (2014).
Chièze, L. et al. Quantitative characterization of single-cell adhesion properties by atomic force microscopy using protein‐functionalized microbeads. J. Mol. Recognit. 32, e2767 (2019).
Dao, L., Gonnermann, C. & Franz, C. M. Investigating differential cell–matrix adhesion by directly comparative single-cell force spectroscopy. J. Mol. Recognit. 26, 578–589 (2013).
Rubies, C. et al. Long-term strenuous exercise promotes vascular injury by selectively damaging the tunica media: experimental evidence. JACC Basic Transl. Sci. 7, 681–693 (2022).
He, X. et al. A new role for host annexin A2 in establishing bacterial adhesion to vascular endothelial cells: lines of evidence from atomic force microscopy and an in vivo study. Lab. Invest. 99, 1650–1660 (2019).
Dudiki, T. et al. Microglia control vascular architecture via a TGFβ1 dependent paracrine mechanism linked to tissue mechanics. Nat. Commun. 11, 986 (2020).
Achner, L. et al. AFM-based nanoindentation indicates an impaired cortical stiffness in the AAV-PCSK9DY atherosclerosis mouse model. Pflugers Arch. 474, 993–1002 (2022).
Rickel, A., Sanyour, H. & Hong, Z. Pleiotropic effect of statin on vascular smooth muscle cell mechanics and migration. Biophys. J. 121, 263A (2022).
Chan, X. Y. et al. HIF2A gain-of-function mutation modulates the stiffness of smooth muscle cells and compromises vascular mechanics. iScience 24, 102246 (2021).
Chtcheglova, L. A., Wildling, L., Waschke, J., Drenckhahn, D. & Hinterdorfer, P. AFM functional imaging on vascular endothelial cells. J. Mol. Recognit. 23, 589–596 (2010).
Trache, A. & Meininger, G. A. Atomic force-multi-optical imaging integrated microscope for monitoring molecular dynamics in live cells. J. Biomed. Opt. 10, 064023 (2005).
Harjumäki, R. et al. AFM force spectroscopy reveals the role of integrins and their activation in cell–biomaterial interactions. ACS Appl. Bio Mater. 3, 1406–1417 (2020).
Bayer, R. J. Mechanical Wear Fundamentals and Testing, Revised and Expanded (CRC, 2004).
Song, M. J. et al. Mapping the mechanome of live stem cells using a novel method to measure local strain fields in situ at the fluid–cell interface. PLoS ONE 7, e43601 (2012).
Hall, M. S. et al. Toward single cell traction microscopy within 3D collagen matrices. Exp. Cell Res. 319, 2396–2408 (2013).
Kubow, K. E. et al. Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat. Commun. 6, 8026 (2015).
Gómez-González, M., Latorre, E., Arroyo, M. & Trepat, X. Measuring mechanical stress in living tissues. Nat. Rev. Phys. 2, 300–317 (2020).
Kaylan, K. B., Kourouklis, A. P. & Underhill, G. H. A high-throughput cell microarray platform for correlative analysis of cell differentiation and traction forces. J. Vis. Exp. https://doi.org/10.3791/55362 (2017).
Shakiba, D. et al. The balance between actomyosin contractility and microtubule polymerization regulates hierarchical protrusions that govern efficient fibroblast–collagen interactions. ACS Nano 14, 7868–7879 (2020).
Hur, S. S. et al. Traction force microscopy for understanding cellular mechanotransduction. BMB Rep. 53, 74–81 (2020).
Uroz, M. et al. Traction forces at the cytokinetic ring regulate cell division and polyploidy in the migrating zebrafish epicardium. Nat. Mater. 18, 1015–1023 (2019).
Wei, Z., Schnellmann, R., Pruitt, H. C. & Gerecht, S. Hydrogel network dynamics regulate vascular morphogenesis. Cell Stem Cell 27, 798–812 (2020). This paper shows that viscoelastic dynamic hydrogels facilitate vascular morphogenesis in vitro and angiogenesis in vivo.
Yuan, H. et al. Synthetic fibrous hydrogels as a platform to decipher cell–matrix mechanical interactions. Proc. Natl Acad. Sci. USA 120, e2216934120 (2023). This paper uses live-cell rheology and TFM to better understand the intricate interactions between cells and the matrix within polyisocyanide hydrogels.
Huebsch, N. et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 9, 518–526 (2010).
Lin, Y., Leartprapun, N., Luo, J. C. & Adie, S. G. Light-sheet photonic force optical coherence elastography for high-throughput quantitative 3D micromechanical imaging. Nat. Commun. 13, 3465 (2022).
Mulligan, J. A., Bordeleau, F., Reinhart-King, C. A. & Adie, S. G. Measurement of dynamic cell-induced 3D displacement fields in vitro for traction force optical coherence microscopy. Biomed. Opt. Express 8, 1152–1171 (2017).
Álvarez-González, B. et al. Two-layer elastographic 3-D traction force microscopy. Sci. Rep. 7, 39315 (2017).
Staunton, J. R., So, W. Y., Paul, C. D. & Tanner, K. High-frequency microrheology in 3D reveals mismatch between cytoskeletal and extracellular matrix mechanics. Proc. Natl Acad. Sci. USA 116, 14448–14455 (2019).
Hagiwara, M., Maruyama, H., Akiyama, M., Koh, I. & Arai, F. Weakening of resistance force by cell–ECM interactions regulate cell migration directionality and pattern formation. Commun. Biol. 4, 808 (2021).
Nikolić, M., Scarcelli, G. & Tanner, K. Multimodal microscale mechanical mapping of cancer cells in complex microenvironments. Biophys. J. 121, 3586–3599 (2022). This paper uses optical tweezers and Brillouin microscopy to measure the mechanical interactions between cells and their surrounding ECM as well as the mechanical properties of the microenvironment in which the cells reside.
Landau, S. et al. Tissue-level mechanosensitivity: predicting and controlling the orientation of 3D vascular networks. Nano Lett. 18, 7698–7708 (2018).
Guo, S. et al. Stimulating extracellular vesicles production from engineered tissues by mechanical forces. Nano Lett. 21, 2497–2504 (2021).
Rosenfeld, D. et al. Morphogenesis of 3D vascular networks is regulated by tensile forces. Proc. Natl Acad. Sci. USA 113, 3215–3220 (2016). This paper demonstrates that tensile forces guide angiogenesis and blood vessel structure.
Lai, A. et al. Piezo1 response to shear stress is controlled by the components of the extracellular matrix. ACS Appl. Mater. Interfaces 14, 40559–40568 (2022).
Yarbrough, D. & Gerecht, S. Engineering smooth muscle to understand extracellular matrix remodeling and vascular disease. Bioengineering 9, 449 (2022).
Song, H.-H. G., Rumma, R. T., Ozaki, C. K., Edelman, E. R. & Chen, C. S. Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell 22, 340–354 (2018).
Wang, Y., Kankala, R. K., Ou, C., Chen, A. & Yang, Z. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioact. Mater. 9, 198–220 (2022).
Baker, B. M., Trappmann, B., Stapleton, S. C., Toro, E. & Chen, C. S. Microfluidics embedded within extracellular matrix to define vascular architectures and pattern diffusive gradients. Lab Chip 13, 3246–3252 (2013).
Xu, W. et al. A double-network poly (Nɛ-acryloyl l-lysine)/hyaluronic acid hydrogel as a mimic of the breast tumor microenvironment. Acta Biomater. 33, 131–141 (2016).
Zhang, G. et al. Mechano-regulation of vascular network formation without branches in 3D bioprinted cell-laden hydrogel constructs. Biotechnol. Bioeng. 118, 3787–3798 (2021).
Wu, C. et al. Injectable conductive and angiogenic hydrogels for chronic diabetic wound treatment. J. Control. Release 344, 249–260 (2022).
Yuan, Y., Fan, D., Shen, S. & Ma, X. An M2 macrophage-polarized anti-inflammatory hydrogel combined with mild heat stimulation for regulating chronic inflammation and impaired angiogenesis of diabetic wounds. Chem. Eng. J. 433, 133859 (2022).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020).
Guimarães, C. F., Gasperini, L., Marques, A. P. & Reis, R. L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 5, 351–370 (2020).
Cohen, N. P., Foster, R. J. & Mow, V. C. Composition and dynamics of articular cartilage: structure, function, and maintaining healthy state. J. Orthop. Sports Phys. Ther. 28, 203–215 (1998).
Carvalho, E. M. & Kumar, S. Lose the stress: viscoelastic materials for cell engineering. Acta Biomater. 163, 146–157 (2022).
Ma, Y. et al. Viscoelastic cell microenvironment: hydrogel‐based strategy for recapitulating dynamic ECM mechanics. Adv. Funct. Mater. 31, 2100848 (2021).
Galli, M., Comley, K. S., Shean, T. A. & Oyen, M. L. Viscoelastic and poroelastic mechanical characterization of hydrated gels. J. Mater. Res. 24, 973–979 (2009).
Rubiano, A. et al. Viscoelastic properties of human pancreatic tumors and in vitro constructs to mimic mechanical properties. Acta Biomater. 67, 331–340 (2018).
van Hoorn, H., Kurniawan, N. A., Koenderink, G. H. & Iannuzzi, D. Local dynamic mechanical analysis for heterogeneous soft matter using ferrule-top indentation. Soft Matter 12, 3066–3073 (2016).
Chaudhuri, O. et al. Substrate stress relaxation regulates cell spreading. Nat. Commun. 6, 6365 (2015).
Hobson, E. C. et al. Resonant acoustic rheometry for non-contact characterization of viscoelastic biomaterials. Biomaterials 269, 120676 (2021).
Aliabouzar, M. et al. Spatiotemporal control of micromechanics and microstructure in acoustically-responsive scaffolds using acoustic droplet vaporization. Soft Matter 16, 6501–6513 (2020).
Wong, L. et al. Substrate stiffness directs diverging vascular fates. Acta Biomater. 96, 321–329 (2019).
Smith, Q. et al. Compliant substratum guides endothelial commitment from human pluripotent stem cells. Sci. Adv. 3, e1602883 (2017).
Xie, S.-A. et al. Matrix stiffness determines the phenotype of vascular smooth muscle cell in vitro and in vivo: role of DNA methyltransferase 1. Biomaterials 155, 203–216 (2018).
Alderfer, L., Russo, E., Archilla, A., Coe, B. & Hanjaya-Putra, D. Matrix stiffness primes lymphatic tube formation directed by vascular endothelial growth factor-C. FASEB J. 35, e21498 (2021).
Hanjaya‐Putra, D. et al. Vascular endothelial growth factor and substrate mechanics regulate in vitro tubulogenesis of endothelial progenitor cells. J. Cell. Mol. Med. 14, 2436–2447 (2010).
Bordeleau, F. et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl Acad. Sci. USA 114, 492–497 (2017).
Wei, Y. et al. Programmable DNA hydrogels as artificial extracellular matrix. Small 18, 2107640 (2022).
Juliar, B. A. et al. Cell-mediated matrix stiffening accompanies capillary morphogenesis in ultra-soft amorphous hydrogels. Biomaterials 230, 119634 (2020).
Xu, R., Boudreau, A. & Bissell, M. J. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 28, 167–176 (2009).
Zamani, M. et al. Single-cell transcriptomic census of endothelial changes induced by matrix stiffness and the association with atherosclerosis. Adv. Funct. Mater. 32, 2203069 (2022).
Zhou, S.-w et al. The substrate stiffness at physiological range significantly modulates vascular cell behavior. Colloids Surf. B Biointerfaces 214, 112483 (2022).
Blatchley, M. R., Hall, F., Wang, S., Pruitt, H. C. & Gerecht, S. Hypoxia and matrix viscoelasticity sequentially regulate endothelial progenitor cluster-based vasculogenesis. Sci. Adv. 5, eaau7518 (2019). This paper uncovers the mechanism by which hypoxia, co-jointly with matrix viscoelasticity, guides cluster-based vasculogenesis using an oxygen-controllable hydrogel.
Lewis, D. M., Blatchley, M. R., Park, K. M. & Gerecht, S. O2-controllable hydrogels for studying cellular responses to hypoxic gradients in three dimensions in vitro and in vivo. Nat. Protoc. 12, 1620–1638 (2017).
Blatchley, M. R. et al. Discretizing three-dimensional oxygen gradients to modulate and investigate cellular processes. Adv. Sci. 8, 2100190 (2021).
Indana, D., Agarwal, P., Bhutani, N. & Chaudhuri, O. Viscoelasticity and adhesion signaling in biomaterials control human pluripotent stem cell morphogenesis in 3D culture. Adv. Mater. 33, 2101966 (2021).
Chaudhuri, O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016).
Adebowale, K. et al. Enhanced substrate stress relaxation promotes filopodia-mediated cell migration. Nat. Mater. 20, 1290–1299 (2021).
Hong, Y. et al. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 10, 2060 (2019).
Abduljauwad, S. N. & Ahmed, H.-U.-R. Enhancing cancer cell adhesion with clay nanoparticles for countering metastasis. Sci. Rep. 9, 5935 (2019).
Wang, X. et al. Dynamic photoelectrical regulation of ECM protein and cellular behaviors. Bioact. Mater. 22, 168–179 (2023).
Bella, J. & Humphries, M. J. Cα-H···O=C hydrogen bonds contribute to the specificity of RGD cell–adhesion interactions. BMC Struct. Biol. 5, 4 (2005).
Zhang, K. et al. Tough hydrogel bioadhesives for sutureless wound sealing, hemostasis and biointerfaces. Adv. Funct. Mater. 32, 2111465 (2022).
Li, X. et al. Nanofiber–hydrogel composite-mediated angiogenesis for soft tissue reconstruction. Sci. Transl. Med. 11, eaau6210 (2019).
Wang, D. Tuning Interfacial Biomolecule Interactions with Massively Parallel Nanopore Arrays. PhD Thesis, Colorado State Univ. (2021).
Park, S. E., Georgescu, A., Oh, J. M., Kwon, K. W. & Huh, D. Polydopamine-based interfacial engineering of extracellular matrix hydrogels for the construction and long-term maintenance of living three-dimensional tissues. ACS Appl. Mater. Interfaces 11, 23919–23925 (2019).
Jeon, O., Kim, T.-H. & Alsberg, E. Reversible dynamic mechanics of hydrogels for regulation of cellular behavior. Acta Biomater. 136, 88–98 (2021).
Wang, Q. et al. A novel hydrogel-based combination therapy for effective neuroregeneration after spinal cord injury. Chem. Eng. J. 415, 128964 (2021).
Zhu, C. et al. Novel enzymatic crosslinked hydrogels that mimic extracellular matrix for skin wound healing. J. Mater. Sci. 53, 5909–5928 (2018).
Machour, M., Szklanny, A. A. & Levenberg, S. Fabrication of engineered vascular flaps using 3D printing technologies. J. Vis. Exp. https://doi.org/10.3791/63920 (2022).
Rodríguez-Soto, M. A. et al. Small diameter cell-free tissue-engineered vascular grafts: biomaterials and manufacture techniques to reach suitable mechanical properties. Polymers 14, 3440 (2022).
Wang, Z. et al. Rapid vascularization of tissue-engineered vascular grafts in vivo by endothelial cells in co-culture with smooth muscle cells. J. Mater. Sci. Mater. Med. 23, 1109–1117 (2012).
Gaharwar, A. K., Singh, I. & Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 5, 686–705 (2020).
Thomsen, M. S., Routhe, L. J. & Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 37, 3300–3317 (2017).
de Oliveira, R. C. & Wilson, S. E. Descemet’s membrane development, structure, function and regeneration. Exp. Eye Res. 197, 108090 (2020).
Allen, P., Melero‐Martin, J. & Bischoff, J. Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks. J. Tissue Eng. Regen. Med. 5, e74–e86 (2011).
Rhodes, J. M. & Simons, M. The extracellular matrix and blood vessel formation: not just a scaffold. J. Cell. Mol. Med. 11, 176–205 (2007).
Marchand, M., Monnot, C., Muller, L. and Germain, S., 2019, May. Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. In Seminars in Cell & Developmental Biology (Vol. 89, pp. 147-156). Academic Press.
Duca, L. et al. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc. Res. 110, 298–308 (2016).
Brooke, B. S., Bayes-Genis, A. & Li, D. Y. New insights into elastin and vascular disease. Trends Cardiovasc. Med. 13, 176–181 (2003).
Grenier, S., Sandig, M. & Mequanint, K. Polyurethane biomaterials for fabricating 3D porous scaffolds and supporting vascular cells. J. Biomed. Mater. Res. A 82, 802–809 (2007).
Goonoo, N., Bhaw-Luximon, A., Bowlin, G. L. & Jhurry, D. An assessment of biopolymer-and synthetic polymer-based scaffolds for bone and vascular tissue engineering. Polym. Int. 62, 523–533 (2013).
Xu, C., Inai, R., Kotaki, M. & Ramakrishna, S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 10, 1160–1168 (2004).
Ji, C. & McCulloch, C. A. TRPV4 integrates matrix mechanosensing with Ca2+ signaling to regulate extracellular matrix remodeling. FEBS J. 288, 5867–5887 (2021).
Givens, C. & Tzima, E. Endothelial mechanosignaling: does one sensor fit all? Antioxid. Redox Signal. 25, 373–388 (2016).
Gordon, E., Schimmel, L. & Frye, M. The importance of mechanical forces for in vitro endothelial cell biology. Front. Physiol. 11, 684 (2020).
Wagenseil, J. E. & Mecham, R. P. Elastin in large artery stiffness and hypertension. J. Cardiovasc. Transl. Res. 5, 264–273 (2012).
Matsuzaki, Y., John, K., Shoji, T. & Shinoka, T. The evolution of tissue engineered vascular graft technologies: from preclinical trials to advancing patient care. Appl. Sci. 9, 1274 (2019).
Acknowledgements
T.B. is a Ford Foundation Predoctoral Fellow. Several studies described from the Gerecht laboratory were funded by a grant from the Air Force Office of Scientific Research (FA9550-20-1-0356 to S.G.).
Author information
Authors and Affiliations
Contributions
D.W. and S.G. prepared the manuscript outline. D.W. and T.B. drafted the tables and figures. D.W., T.B., L.S. and S.G. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Sara Baratchi, Ngan Huang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, D., Brady, T., Santhanam, L. et al. The extracellular matrix mechanics in the vasculature. Nat Cardiovasc Res 2, 718–732 (2023). https://doi.org/10.1038/s44161-023-00311-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-023-00311-0