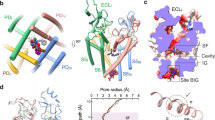
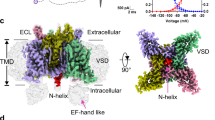
Abstract
Voltage-gated sodium (NaV) channels generate the upstroke of the cardiac action potential by activating rapidly in response to depolarization and conducting Na+ inward across the membrane1,2. NaV1.5 is the predominant NaV channel in the heart3,4. It is the molecular target for class I antiarrhythmic drugs (AADs), which often have unwanted side effects, including arrhythmias5,6. In contrast, the atypical AAD ranolazine is effective in treatment of atrial arrhythmias and angina pectoris, but with less proarrhythmia than traditional AADs7,8,9,10,11. Structures of NaV channels from prokaryotes12, skeletal muscle13, nerve14 and heart15 have been determined with AADs bound within the pore to physically block Na+ conductance12,15,16,17,18,19. Here we use electrophysiology and cryogenic electron microscopy to define the interaction of ranolazine with NaV1.5 at high resolution. We reveal ranolazine’s binding pose and elucidate distinct molecular interactions that might underlie its mechanism of action and high therapeutic index relative to traditional class I AADs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the finding in this study are included in the main article and associated files. Structural data are available from the Protein Data Bank (PDB) under EMDB entry ID EMD-28887 and PDB entries ID 8F6P & 6UZ3. Source data are provided with this paper.
References
Hall, A. E., Hutter, O. F. & Noble, D. Current–voltage relations of Purkinje fibres in sodium-deficient solutions. J. Physiol. 166, 2225–2240 (1963).
McAllister, R. E., Noble, D. & Tsien, R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J. Physiol. 251, 1–59 (1975).
Fozzard, H. A. & Hanck, D. A. Structure and function of voltage-dependent sodium channels: comparison of brain II and cardiac isoforms. Physiol. Rev. 76, 887–926 (1996).
Catterall, W. A. & Lenaeus, M. J. in Cardiac Electrophysiology: From Cell to Bedside (eds Jalife, J. et al.) Ch. 1 (Elsevier, 2022).
Sampson, K. J. & Kass, R. K. in Goodman & Gilman’s Pharmacological Basis of Therapeutics 12th edn (eds Brunton, L. et al.) 815–848 (McGraw-Hill Education/Medical, 2011).
Catterall, W. A., Lenaeus, M. J. & Gamal El-Din, T. M. Structure and pharmacology of voltage-gated sodium and calcium channels. Annu. Rev. Pharmacol. Toxicol. 60, 133–154 (2020).
Chaitman, B. R. et al. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J. Am. Coll. Cardiol. 43, 1375–1382 (2004).
Koren, M. J., Crager, M. R. & Sweeney, M. Long-term safety of a novel antianginal agent in patients with severe chronic stable angina: the Ranolazine Open Label Experience (ROLE). J. Am. Coll. Cardiol. 49, 1027–1034 (2007).
Reiffel, J. A. et al. The HARMONY Trial: combined ranolazine and dronedarone in the management of paroxysmal atrial fibrillation: mechanistic and therapeutic synergism. Circ. Arrhythm. Electrophysiol. 8, 1048–1056 (2015).
Miles, R. H., Passman, R. & Murdock, D. K. Comparison of effectiveness and safety of ranolazine versus amiodarone for preventing atrial fibrillation after coronary artery bypass grafting. Am. J. Cardiol. 108, 673–676 (2011).
Fragakis, N. et al. Comparison of effectiveness of ranolazine plus amiodarone versus amiodarone alone for conversion of recent-onset atrial fibrillation. Am. J. Cardiol. 110, 673–677 (2012).
Payandeh, J., Scheuer, T., Zheng, N. & Catterall, W. A. The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358 (2011).
Pan, X. et al. Structure of the human voltage-gated sodium channel Nav1.4 in complex with beta1. Science https://doi.org/10.1126/science.aau2486 (2018).
Shen, H., Liu, D., Wu, K., Lei, J. & Yan, N. Structures of human Nav1.7 channel in complex with auxiliary subunits and animal toxins. Science 363, 1303–1308 (2019).
Jiang, D. et al. Structure of the cardiac sodium channel. Cell 180, 122–134.e110 (2020).
Gamal El-Din, T. M., Lenaeus, M. J., Zheng, N. & Catterall, W. A. Fenestrations control resting-state block of a voltage-gated sodium channel. Proc. Natl Acad. Sci. USA 115, 13111–13116 (2018).
Lenaeus, M. J. et al. Structures of closed and open states of a voltage-gated sodium channel. Proc. Natl Acad. Sci. USA 114, E3051–E3060 (2017).
Jiang, D. et al. Open-state structure and pore gating mechanism of the cardiac sodium channel. Cell 184, 5151–5162.e5111 (2021).
Li, Z. et al. Structural basis for pore blockade of the human cardiac sodium channel Nav1.5. Angew. Chem. Int. Ed. Engl. 60, 11474–11480 (2021).
Catterall, W. A. The molecular basis of neuronal excitability. Science 223, 653–661 (1984).
Numa, S. & Noda, M. Molecular structure of sodium channels. Ann. NY Acad. Sci. 479, 338–355 (1986).
Catterall, W. A. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26, 13–25 (2000).
West, J. W. et al. A cluster of hydrophobic amino acid residues required for fast Na+ channel inactivation. Proc. Natl Acad. Sci. USA 89, 10910–10914 (1992).
Antzelevitch, C. et al. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation 110, 904–910 (2004).
Rayner-Hartley, E. & Sedlak, T. Ranolazine: a contemporary review. J. Am. Heart Assoc. 5, e003196 (2016).
Lei, M., Wu, L., Terrar, D. A. & Huang, C. L. Modernized classification of cardiac antiarrhythmic drugs. Circulation 138, 1879–1896 (2018).
Makielski, J. C. Late sodium current: a mechanism for angina, heart failure, and arrhythmia. Trends Cardiovasc. Med. 26, 115–122 (2016).
Fredj, S., Sampson, K. J., Liu, H. & Kass, R. S. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br. J. Pharmacol. 148, 16–24 (2006).
Wang, C., Chung, B. C., Yan, H., Lee, S. Y. & Pitt, G. S. Crystal structure of the ternary complex of a NaV C-terminal domain, a fibroblast growth factor homologous factor, and calmodulin. Structure 20, 1167–1176 (2012).
Ragsdale, D. S., McPhee, J. C., Scheuer, T. & Catterall, W. A. Molecular determinants of state-dependent block of sodium channels by local anesthetics. Science 265, 1724–1728 (1994).
Ragsdale, D. R., McPhee, J. C., Scheuer, T. & Catterall, W. A. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc. Natl Acad. Sci. USA 93, 9270–9275 (1996).
Low, B. & Babutt, R. The pi helix—a hydrogen bonded configuration of the polypeptide chain. J. Am. Chem. Soc. 74, 5806–5907 (1952).
Huang, G. et al. High-resolution structures of human Na. Cell Rep. 39, 110735 (2022).
Zubcevic, L. & Lee, S. Y. The role of π-helices in TRP channel gating. Curr. Opin. Struct. Biol. 58, 314–323 (2019).
Fodje, M. N. & Al-Karadaghi, S. Occurrence, conformational features and amino acid propensities for the pi-helix. Protein Eng. 15, 353–358 (2002).
Choudhury, K., Kasimova, M. A., McComas, S., Howard, R. J. & Delemotte, L. An open state of a voltage-gated sodium channel involving a π-helix and conserved pore-facing asparagine. Biophys. J. 121, 11–22 (2022).
Huang, H., Priori, S. G., Napolitano, C., O’Leary, M. E. & Chahine, M. Y1767C, a novel SCN5A mutation, induces a persistent Na+ current and potentiates ranolazine inhibition of Nav1.5 channels. Am. J. Physiol. Heart Circ. Physiol. 300, H288–H299 (2011).
Sunami, A., Dudley, S. C. & Fozzard, H. A. Sodium channel selectivity filter regulates antiarrhythmic drug binding. Proc. Natl Acad. Sci. USA 94, 14126–14131 (1997).
Sunami, A., Glaaser, I. W. & Fozzard, H. A. A critical residue for isoform difference in tetrodotoxin affinity is a molecular determinant of the external access path for local anesthetics in the cardiac sodium channel. Proc. Natl Acad. Sci. USA 97, 2326–2331 (2000).
Sasaki, K. et al. Unexpected mexiletine responses of a mutant cardiac Na+ channel implicate the selectivity filter as a structural determinant of antiarrhythmic drug access. Mol. Pharmacol. 66, 330–336 (2004).
Pless, S. A., Galpin, J. D., Frankel, A. & Ahern, C. A. Molecular basis for class Ib anti-arrhythmic inhibition of cardiac sodium channels. Nat. Commun. 2, 351 (2011).
Echt, D. S. et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 324, 781–788 (1991).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Acknowledgements
This work was funded by National Institutes of Health Research grants K08 HL145630 (M.L.) and R01 HL112808-09 (W.A.C.) and by the Howard Hughes Medical Institue (N.Z.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors thank J. Quispe (Cryo-EM Facility, University of Washington) for support with cryo-EM data collection and J. Li (Pharmacology, University of Washington) for technical and editorial support.
Author information
Authors and Affiliations
Contributions
M.L, T.M.G.E.-D., L.T., N.Z. and W.A.C. designed the experiments; M.L. and L.T. carried out the cryo-EM experiments; T.M.G.E.-D. carried out the electrophysiology experiments; and M.L. carried out the molecular model building, refinement and comparison with previous data. All authors contributed to writing and revising the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended Data
Extended Data Fig. 1 The purification of rNav1.5c and preparation of samples for cryo-EM.
a, The size-exclusion chromatography profile is shown for the final step of purification of the rNav1.5c, FGF12B, calmodulin and ranolazine complex with red lines indicating the sample moved forward for cryo-EM analysis. This experiment was only performed once. b, Sample micrograph collected during cryo-EM data collection and representative 2D classifications as determined by Relion. Information on cryo-EM data collection and data processing is shown in Extended Data Fig. 2.
Extended Data Fig. 2 Cryo-EM data processing and 3D reconstruction.
a, Flowchart for EM data processing. b, Gold-standard FSC curve for the 3D reconstruction of rNav1.5c, FGF12B, calmodulin and ranolazine complex.
Extended Data Fig. 3 3D reconstruction of rNav1.5c, FGF12B, calmodulin, and ranolazine complex color coded for map resolution.
EM density is shown from below rNav1.5c as if one is inside the cell and looking outward at the plasma membrane. The map is colored by resolution with scale shown (blue = high resolution, red = lower). A close-up of the ranolazine site is shown, highlighting the quality of EM density at ranolazine’s binding site. A red circle is shown to identify the EM density corresponding to ranolazine.
Extended Data Fig. 4 Functional characterization of the Nav1.5c mutant Q372A and its effects on ranolazine binding.
a. G-V curves of Nav1.5c WT and Q372A mutant derived from I-V relationships. The voltages for half maximal activation and slopes are: WT V1/2 = -73 ± 0.8 mV, K = 5.6 ± 0.6, Q372A V1/2 = -69 ± 0.3 mV, K = 5.5 ± 0.3. Steady-state inactivation of Nav1.5c WT and Q372A mutant. Two pulses were applied: a 500-ms conditioning pulse to the indicated potentials followed by 50 ms test pulse to 0 mV. Nav1.5c WT Vh = -110 ± 0.4, K = 7.8 ± 0.5, Q372A Vh = -104 ± 0.6, K = 7.6 ± 0.5. WT activation and steady-state inactivation curves N = 4 distinct cells, Q372A GV and SSI curves N = 4 distinct cells. b. Dose-response curve for the tonic block of Q372A compared to Nav1.5c WT. Each concentration is an average of three different cells and each cell is used only once for each concentration. Error bars indicates s.e.m.
Extended Data Fig. 5 α-π transition of DIV S6 and π-helix dependent binding of ranolazine.
a, An overlay of DIV-S6 helices from the current structure (light green with pink π-helix highlighted), the apo structure of rNav1.5c (pdb 6uz3, grey), and the open structure of rNav1.5c (pdb 7fbs, cyan). The π-helical portion of DIV-S6 (pink) creates additional drug binding surface in the central cavity of rNav1.5c. b, A close-up of the ranolazine binding site with sticks shown for important residues F1762, V1765, and V1766. Coloring is as in part a and the side chain position of V1766 is shown for both the apo structure and the ranolazine-bound structure, with a black arrow highlighting the distance between these alpha carbon positions (2.7 Å). Yellow dashed lines show the strengthened van der waals contacts in the π-helical form of DIV-S6 found in the ranolazine structure (distance is 3.3 Å). c, A bubble diagram showing the local differences between π-helical (top) and α-helical (bottom) forms of DIV-S6. Residues are shown as bubbles with coloring dependent on the nearest approach of the side chain to the bound drug according to the color scale shown in the figure. The structure and the dimethylbenzyl moiety of ranolazine is shown for reference. d, Stick diagrams of ranolazine and the class IB AADs lidocaine, mexilitine, tocainide, and etidocaine are shown with a red line highlighting each molecule’s dimethylbenzyl moiety.
Extended Data Fig. 6 Effect of α-π transition of DIV S6 on the DIV-DI fenestration.
a. Cartoon models of DIV-S6 (green) and DI-S6 (pale yellow) as in earlier figures. Sticks are shown for DIV-S6 residue Y1769 (green, in π helix conformation), F403 (pale yellow) and the non-lidocaine portion of ranolazine that interacts with F403 by π-teeing (dashed line). Transparent sticks are shown in blue for the apo (α-helix) conformations of Y1769 and F403, illustrating the clash between the side chain of π-Y1769 and apo F403. b, Surface illustration of DIV/DI fenestration in α and π forms of DIV-S6. Domains are colored as in prior figures - DI pale yellow, DIII, pink, DIV, green, DIII/DIV linker (IFM) orange.
Supplementary information
Source data
Source Data Fig. 3
The effects of mutations on ranolazine binding as measured by electrophysiology. This is a table of individual measurements used to create the data displayed in Fig. 3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lenaeus, M., Gamal El-Din, T.M., Tonggu, L. et al. Structural basis for inhibition of the cardiac sodium channel by the atypical antiarrhythmic drug ranolazine. Nat Cardiovasc Res 2, 587–594 (2023). https://doi.org/10.1038/s44161-023-00271-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-023-00271-5
This article is cited by
-
Dual receptor-sites reveal the structural basis for hyperactivation of sodium channels by poison-dart toxin batrachotoxin
Nature Communications (2024)
-
New insights on cardiac Na channel block by an atypical anti-arrhythmic drug
Nature Cardiovascular Research (2023)