Abstract
The mammalian neonatal heart can regenerate for 1 week after birth, after which, the majority of cardiomyocytes exit the cell cycle. Recent studies demonstrated that calcineurin mediates cell-cycle arrest of postnatal cardiomyocytes, partly through induction of nuclear translocation of the transcription factor Hoxb13 (a cofactor of Meis1). Here we show that inducible cardiomyocyte-specific deletion of calcineurin B1 in adult cardiomyocytes markedly decreases cardiomyocyte size and promotes mitotic entry, resulting in increased total cardiomyocyte number and improved left ventricular (LV) systolic function after myocardial infarction (MI). Similarly, pharmacological inhibition of calcineurin activity using FK506 promotes cardiomyocyte proliferation in vivo and increases cardiomyocyte number; however, FK506 administration after MI in mice failed to improve LV systolic function, possibly due to inhibition of vasculogenesis and blunting of the post-MI inflammatory response. Collectively, our results demonstrate that loss of calcineurin activity in adult cardiomyocytes promotes cell cycle entry; however, the effects of the calcineurin inhibitor FK506 on other cell types preclude a significant improvement of LV systolic function after MI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analyzed are included in the main article and associated files. Source data are provided with this paper.
References
Ziaeian, B. & Fonarow, G. C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 13, 368–378 (2016).
Virani, S. S. et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141, e139–e596 (2020).
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011).
Porrello, E. R. et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl Acad. Sci. USA 110, 187–192 (2013).
Mahmoud, A. I. et al. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature 497, 249–253 (2013).
Lam, N. T. & Sadek, H. A. Neonatal heart regeneration. Circulation 138, 412–423 (2018).
Soonpaa, M. H., Kim, K. K., Pajak, L., Franklin, M. & Field, L. J. Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. Heart Circ. Physiol. 271, H2183–H2189 (1996).
Rothermel, B. A. et al. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc. Natl Acad. Sci. USA 98, 3328–3333 (2001).
Schaeffer, P. J. et al. Impaired contractile function and calcium handling in hearts of cardiac-specific calcineurin b1-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 297, H1263–H1273 (2009).
Molkentin, J. D. et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93, 215–228 (1998).
Parra, V. & Rothermel, B. A. Calcineurin signaling in the heart: the importance of time and place. J. Mol. Cellular Cardiol. 103, 121–136 (2017).
Nguyen, N. U. N. et al. A calcineurin–Hoxb13 axis regulates growth mode of mammalian cardiomyocytes. Nature 582, 271–276 (2020).
Liu, J. et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66, 807–815 (1991).
O’Keefe, S. J., Tamura, J. I., Kincaid, R. L., Tocci, M. J. & O’Neill, E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature 357, 692–694 (1992).
Clipstone, N. A. & Crabtree, G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 357, 695–697 (1992).
Neilson, J. R., Winslow, M. M., Hur, E. M. & Crabtree, G. R. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity 20, 255–266 (2004).
Ali, S. R. et al. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc. Natl Acad. Sci. USA 111, 8850–8855 (2014).
Starzl, T. et al. FK 506 for liver, kidney, and pancreas transplantation. Lancet 334, 1000–1004 (1989).
Tong, L. et al. Tacrolimus inhibits insulin release and promotes apoptosis of Min6 cells through the inhibition of the PI3K/Akt/mTOR pathway. Mol. Med. Rep. 24, 658 (2021).
Rodriguez-Rodriguez, A. E. et al. Inhibition of the mTOR pathway: a new mechanism of β cell toxicity induced by tacrolimus. Am. J. Transplant. 19, 3240–3249 (2019).
Li, S. et al. Mechanism of eccentric cardiomyocyte hypertrophy secondary to severe mitral regurgitation. Circulation 141, 1787–1799 (2020).
Wang, T., Donahoe, P. K. & Zervos, A. S. Specific interaction of type i receptors of the TGF-β; family with the immunophilin FKBP-12. Science 265, 674–676 (1994).
Aurora, A. B. et al. Macrophages are required for neonatal heart regeneration. J. Clin. Investig. 124, 1382–1392 (2014).
Setkowicz, Z., Caryk, M., Szafraniec, M., Żmudzińska, A. & Janeczko, K. Tacrolimus (FK506) and cyclosporin A reduce macrophage recruitment to the rat brain injured at perinatal and early postnatal periods. Neurol. Res. 31, 1060–1067 (2009).
Hisatomi, K. et al. Changes in the mononuclear cell subpopulations of rat cardiac transplant recipients administered FK506 for the treatment of ongoing rejection. Surg. Today 25, 145–150 (1995).
Eguchi, R. et al. FK506 induces endothelial dysfunction through attenuation of Akt and ERK1/2 independently of calcineurin inhibition and the caspase pathway. Cell. Signalling 25, 1731–1738 (2013).
Renier, N. et al. Mapping of brain activity by automated volume analysis of immediate early genes. Cell 165, 1789–1802 (2016).
Rios Coronado, P. E. & Red-Horse, K. Enhancing cardiovascular research with whole-organ imaging. Curr. Opin. Hematol. 28, 214–220 (2021).
Maillet, M. et al. Heart-specific deletion of CnB1 reveals multiple mechanisms whereby calcineurin regulates cardiac growth and function. J. Biol. Chem. 285, 6716–6724 (2010).
Jiao, K. et al. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 17, 2362–2367 (2003).
Lambert, J. M., Lopez, E. F. & Lindsey, M. L. Macrophage roles following myocardial infarction. Int. J. Cardiol. 130, 147–158 (2008).
Lai, S.-L. et al. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. eLife 6, e25605 (2017).
Kang, Y. J. et al. Calcineurin negatively regulates TLR-mediated activation pathways. J. Immunol. 179, 4598–4607 (2007).
Hofmann, U. et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125, 1652–1663 (2012).
Chiasson, V. L. et al. Endothelial cell transforming growth factor-β; receptor activation causes tacrolimus-induced renal arteriolar hyalinosis. Kidney Int. 82, 857–866 (2012).
Su, L. et al. Tacrolimus (FK506) prevents early retinal neovascularization in streptozotocin-induced diabetic mice. Int. Immunopharmacol. 14, 606–612 (2012).
Siamakpour-Reihani, S. et al. The role of calcineurin/NFAT in SFRP2 induced angiogenesis—a rationale for breast cancer treatment with the calcineurin inhibitor tacrolimus. PLoS ONE 6, e20412 (2011).
Morishita, T. et al. Higher peak tacrolimus concentrations after allogeneic hematopoietic stem cell transplantation increase the risk of endothelial cell damage complications. Biol. Blood Marrow Transplant. 24, 2509–2516 (2018).
Kujawski, S. et al. Calcineurin regulates coordinated outgrowth of zebrafish regenerating fins. Dev. Cell 28, 573–587 (2014).
Francavilla, A. et al. Augmentation of rat liver regeneration by FK 506 compared with cyclosporin. Lancet 334, 1248–1249 (1989).
Francavilla, A. et al. Studies on mechanisms of augmentation of liver regeneration by cyclosporine and FK 506. Hepatology 14, 140–143 (1991).
Tanaka, N., Yamamoto, H., Tatemoto, A., Urabe, T. & Orita, K. Regulation of liver regeneration by interleukin-2 and its inhibitors: cyclosporine A and FK 506. Int. J. Immunopharmacol. 15, 211–218 (1993).
Gold, B. G. FK506 and the role of immunophilins in nerve regeneration. Mol. Neurobiol. 15, 285–306 (1997).
Nakada, Y. et al. Hypoxia induces heart regeneration in adult mice. Nature 541, 222–227 (2017).
Nascimento, D. S. et al. MIQuant–semi-automation of infarct size assessment in models of cardiac ischemic injury. PLoS ONE 6, e25045 (2011).
Cardoso, A. C. et al. Mitochondrial substrate utilization regulates cardiomyocyte cell-cycle progression. Nat. Metab. 2, 167–178 (2020).
Anbazhakan, S. et al. Blood flow modeling reveals improved collateral artery performance during mammalian heart regeneration. Preprint at bioRxiv https://doi.org/10.1101/2021.09.17.460699 (2021).
Acknowledgements
H.A.S. was supported by NIH R01 HL137415-02, NIH R01 HL147276-01, NIH R01 HL149137-01, Hamon Center for Regenerative Science and Medicine and Leducq Foundation (Redox Regulation of Cardiomyocyte Renewal). N.T.L. was supported by a Haberecht Wildhare-Idea research grant. N.U.N.N. was supported by American Heart Association Career Development Award (856552) and Postdoctoral Fellowship (19POST34450039). I.M.M. was supported by American Heart Association grant 903385 and Alfonso Martin Escudero Foundation Fellowship. We acknowledge the services provided by the institutionally supported Preclinical Pharmacology Core at UTSW.
Author information
Authors and Affiliations
Contributions
N.U.N.N. and N.T.L. conducted immunohistochemistry and cardiomyocyte isolation studies and interpreted results. N.U.N.N., C.C.H., S.L. and D.C.C. performed mouse surgeries. N.U.N.N., S.L. and C.C.H conducted echocardiography experiments and interpreted results. S.T., N.T.L and N.U.N.N. managed mouse colonies and injections. N.T.L. conducted BrdU-labeling, pump insertion and MADM-related experiments and interpreted results. N.U.N.N. conducted western blotting. N.T.L., M.S.A., X.W. and N.S.W. conducted FK506 pharmacokinetics. P.E.R.C., N.U.N.N., N.T.L. and C.C.H. performed vasculature study. N.T.L. and I.M.M. performed confocal imaging. N.U.N.N. and N.T.L. designed and conducted experiments, interpreted results and contributed to manuscript preparation. F.X., W.M.E., B.A.R. and K.R. interpreted results. H.A.S. designed the experiments, conceived the project and contributed to manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Enrique Lara-Pezzi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
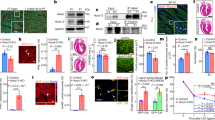
Extended Data Fig. 1 Ejection fraction and histology of CnB-iKO mice following gene deletion.
Following gene deletion of CnB-iKO, hearts were assessed for: (a) Hoxb13 staining in control and CnB-iKO adult hearts at 2 weeks after gene deletion showing that Hoxb13 is not localized to myocardial nuclei in CnB-iKO hearts. Scale bars, 10 µm. (b) Ejection fraction was assessed up to 24 weeks after tamoxifen injections. (c) Heart weight at 2 weeks after gene deletion. (d) Body weight at 2 weeks after gene deletion. (e) Heart weight at 24 weeks after gene deletion; (f) Body weight at 24 weeks after gene deletion. (g) Heart weight/body weight at 24 weeks after gene deletion. (h) Hematoxylin and eosin stain (upper panels) and Masson trichrome staining (lower panels) at 2 weeks after gene deletion. Scale bars, 1 mm Data in a and h were independently repeated two times with similar results. Data are mean ± s.e.m.; unpaired two-sided t-test. *P < 0.05, **P < 0.01 (f). Sample numbers, n = 3 for each group (b, e, f, g), n = 12 for each group (c, d).
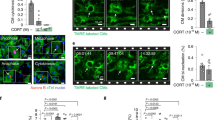
Extended Data Fig. 2 Cardiomyocyte mitosis is increased after MI and CnB gene deletion.
(a-b) At 1-week post MI, tamoxifen was administered to induce cardiomyocyte-specific deletion of CnB in CnB-iKO hearts and assessed at 4-weeks post MI: (a) schematic; (b) Immunostaining of hearts for cTnT (red) and pH3 (green), and quantification of mitotic cardiomyocytes for control and CnB-iKO hearts. (c-i) At 1-week post MI, tamoxifen was administered to induce cardiomyocyte-specific deletion of CnB in CnB-iKO hearts and assessed till 12-weeks post MI: (c) Heart weight; (d) Body weight; (e) Left ventricular end-diastolic anterior wall thickness (LVAW, d); (f) Left ventricular posterior wall end diastole (LVPW, d); (g) Left ventricular internal diameter end diastole (LVID, d); (h) Left ventricular internal diameter end systole (LVID, s); (i) Heart rate. Data are mean ± s.e.m.; unpaired two-sided t-test. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars, 10 μm (b). Sample numbers, n = 7 for each group (c-i), n = 12 for each group (c, d), n = 3 for control and n = 5 for CnB-iKO (b).
Extended Data Fig. 3 Subcutaneous injections of FK506 (3 mg/kg) twice a day does not promote cardiomyocyte proliferation in CD1 mice.
(a) CD-1 mice were subcutaneously injected twice a day with FK506 (3 mg/kg) from 8-10 weeks of age. (b) Immunostaining of hearts for cTnT (red) and pH3 (green), and quantification of mitotic cardiomyocytes for control (DMSO) and FK506 (3 mg/kg). Scale bar, 10 µm
Extended Data Fig. 4 FK506 increases cardiomyocyte mitosis after MI.
(a-b) From 1-week post MI, FK506 was administered and assessed at 2-weeks post MI in CD1 mice: (a) schematic; (b) Immunostaining of hearts for cTnT (red) and pH3 (green), and quantification of mitotic cardiomyocytes for DMSO control and FK506-treated hearts. (c-d) 11-weeks post MI CD1 mouse hearts) for (c) Heart weight and (d) Body weight. (e-j): Serial echocardiographic parameters of injury for DMSO control and FK506-treated CD1 mice for: (e) changes in EF relative to 1-week post MI; (f) Left ventricular end-diastolic anterior wall thickness (LVAW, d), (g) Left ventricular posterior wall end diastole (LVPW, d); (h) Left ventricular internal diameter end diastole (LVID, d); (i) Left ventricular internal diameter end systole (LVID, s); (j) Heart rate. (k-u) From 1-week post MI, FK506 was administered and assessed at 2-weeks post MI in C57Bl6N mice (l-n) 11-weeks post MI for (l) Heart weight/Body weight (m) Heart weight and (n) Body weight. (o-u): Serial echocardiographic parameters of injury for DMSO control and FK506 treated C57Bl6N mice for: (o) Ejection fraction (p) changes in EF relative to 1-week post MI; (q) Left ventricular end-diastolic anterior wall thickness (LVAW, d), (r) Left ventricular posterior wall end diastole (LVPW, d); (s) Left ventricular internal diameter end diastole (LVID, d); (t) Left ventricular internal diameter end systole (LVID, s); (u) Heart rate. Data are mean ± s.e.m.; unpaired two-sided t-test. *P < 0.05. Scale bars, 10 μm (b). Sample numbers, n = 6 for each group (l-u), n = 4 for DMSO-treated and n = 5 for FK506-treated hearts (b-j).
Extended Data Fig. 5 Pharmacokinetics of FK506 delivered by subcutaneous injection and osmotic pump.
Pharmacokinetics (PK) of FK506 by subcutaneous injection (6 mg/kg, twice/day) (a) FK506 chemical structure. PK profile of FK506 in plasma (b) Graph, (c) Table and (d) parameters. PK profile of FK506 in heart (e) Graph, (f) Table and (g) parameters. Stability assay of FK506 delivered by osmotic pump for: (h) 7 days and (i) 14 days. Pharmacokinetics profile of FK506 (osmotic pump) in: (j) plasma and blood, and (k) heart and kidney. Data are mean ± s.e.m.; unpaired two-sided t-test. (h, i) *P < 0.05, **P < 0.01. Sample numbers, n = 3 for each group (b, e, h, i), n = 5 for each group (j, k). Note that a One-way ANOVA was performed for the cross comparison of treatment conditions (h, i).
Extended Data Fig. 6 Characterization of FK506-treated C57Bl6N mice using pump following MI.
(a-h) Delivery of FK506 treatment by osmotic pump from 1-7 weeks post MI was similar to DMSO vehicle control treatment for: (a) The heart weight/body weight; (b) Heart weight. (c) Body weight. (d-h) Serial echocardiography of injury DMSO Ctrl and FK506-treated mice for: (d) diastolic anterior wall thickness; (e) diastolic posterior wall thickness; (f) heart rate; (g) diastolic left ventricular internal dimension; (h) systolic left ventricular internal dimension. (i-p) Delivery of FK506 treatment by osmotic pump from 4-8 weeks post MI was similar to DMSO vehicle control treatment for: (i) The heart weight/body weight; (j) Heart weight. (k) Body weight. (l-p) Serial echocardiography of injury DMSO Ctrl and FK506-treated mice for: (l) diastolic anterior wall thickness; (m) diastolic posterior wall thickness; (n) heart rate; (o) diastolic left ventricular internal dimension; (p) systolic left ventricular internal dimension. Data are mean ± s.e.m.; unpaired two-sided t-test. Sample numbers, n = 4 for DMSO-treated and n = 5 for FK506-treated hearts (a-h), n = 3 for DMSO-treated and n = 4 for FK506-treated hearts (i-p).
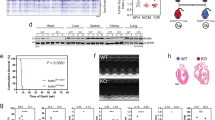
Extended Data Fig. 7 Western blot analysis of Osmotic pump delivery of FK506.
Western blot was performed on heart samples harvested from C57Bl6N mice treated with osmotic pump delivery of DMSO vehicle control or FK506 (2.88 mg/kg/day) for 2 weeks. (a) Western blot analysis of hypertrophy-related proteins: Rcan1.4, calcineurin B, calcineurin A, p-mTOR, and mTOR; and cell cycle-related proteins: p15/16 and pH3-Ser10. (b-g) Densitometry of DMSO versus FK506 treated hearts (n = 3 for each group): (b) Rcan1.4/GAPDH; (c) CnA-1/GAPDH; (d) CnB/GAPDH; (e) p15/GAPDH; (f) pH3-Ser10/GAPDH; (g) p-mTOR/mTOR. Data are mean ± s.e.m.; unpaired two-sided t-test. *P < 0.05. Sample numbers, n = 3 for each group.
Extended Data Fig. 8 Osmotic pump delivery of Rapamycin does not promote cardiomyocyte proliferation in adult heart.
(a) Schematic of subcutaneously installed 14-day osmotic pump delivering Rapamycin (1.8 mg/kg/day) or DMSO vehicle control for 14 days from 9-11 weeks of age in CD-1 mice. (b) Immunostaining of hearts for cTnT (red) and pH3 (green), and quantification of mitotic cardiomyocytes (n = 4 for each group). The level of pH3+ cardiomyocytes observed in two of the rapamycin-treated sample was very low when compared to Fig. 3b. (c) Heart weight/Body weight of mice on DMSO (n = 5) and Rapamycin (n = 5). (b, c) Data are mean ± s.e.m.; unpaired two-sided t-test. Scale bar = 10μm.
Supplementary information
Supplementary Data 1
Supplementary dataset of all echocardiography raw data.
Source data
Source Data Fig. 1
Source data used to generate the graphs in Fig. 1a–m.
Source Data Fig. 1
Unprocessed western blots
Source Data Fig. 2
Source data used to generate the graphs in Fig. 2a–l.
Source Data Fig. 3
Source data used to generate the graphs in Fig. 3a–n.
Source Data Fig. 4
Source data used to generate the graphs in Fig. 4a–j.
Source Data Extended Data Fig. 1
Source data used to generate the graphs in Extended Data Fig. 1a–h.
Source Data Extended Data Fig. 2
Source data used to generate the graphs in Extended Data Fig. 2a–i.
Source Data Extended Data Fig. 3
Source data used to generate the graphs in Extended Data Fig. 3a,b.
Source Data Extended Data Fig. 4
Source data used to generate the graphs in Extended Data Fig. 4a–u.
Source Data Extended Data Fig. 5
Source data used to generate the graphs in Extended Data Fig. 5a–k.
Source Data Extended Data Fig. 6
Source data used to generate the graphs in Extended Data Fig. 6a–p.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 7
Source data used to generate the graphs in Extended Data Fig. 7a–g.
Source Data Extended Data Fig. 8
Source data used to generate the graphs in Extended Data Fig. 8a–c.
Rights and permissions
About this article
Cite this article
Lam, N.T., Nguyen, N.U.N., Ahmed, M.S. et al. Targeting calcineurin induces cardiomyocyte proliferation in adult mice. Nat Cardiovasc Res 1, 679–688 (2022). https://doi.org/10.1038/s44161-022-00098-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-022-00098-6
This article is cited by
-
Identification of FDA-approved drugs that induce heart regeneration in mammals
Nature Cardiovascular Research (2024)
-
Cardiomyocyte proliferation by calcineurin inhibition
Nature Cardiovascular Research (2022)