Abstract
Vascular endothelial growth factor (VEGF)-driven increase in vascular permeability is a key feature of many disease states associated with inflammation and ischemic injury, contributing significantly to morbidity and mortality in these settings. Despite its importance, no specific regulators that preferentially control a VEGF-dependent increase in permeability versus its other biological activities have been identified. Here, we report that a proteoglycan, Syndecan-2 (Sdc2), regulates the interaction between a transmembrane phosphatase, DEP1, and VEGFR2 by controlling cell surface levels of DEP1. In the absence of Sdc2 or the presence of an antibody that blocks the Sdc2–DEP1 interaction, increased plasma membrane DEP1 levels promote selective dephosphorylation of the VEGFR2 Y951 site that is involved in permeability control. Either an endothelial-specific Sdc2 deletion or a treatment with an anti-Sdc2 antibody results in a marked reduction in stroke size due to a decrease in intracerebral edema.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and associated files. Source data are provided with this paper.
Change history
09 June 2022
A Correction to this paper has been published: https://doi.org/10.1038/s44161-022-00092-y
References
Bates, D. O. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. 87, 262–271 (2010).
Park-Windhol, C. & D’Amore, P. A. Disorders of vascular permeability. Annu. Rev. Pathol. 11, 251–281 (2016).
Claesson-Welsh, L., Dejana, E. & McDonald, D. M. Permeability of the endothelial barrier: identifying and reconciling controversies. Trends Mol. Med. 27, 314–331 (2021).
van Bruggen, N. et al. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J. Clin. Invest. 104, 1613–1620 (1999).
Croll, S. D. et al. VEGF-mediated inflammation precedes angiogenesis in adult brain. Exp. Neurol. 187, 388–402 (2004).
Barratt, S., Medford, A. R. & Millar, A. B. Vascular endothelial growth factor in acute lung injury and acute respiratory distress syndrome. Respiration 87, 329–342 (2014).
Sharp, C., Millar, A. B. & Medford, A. R. Advances in understanding of the pathogenesis of acute respiratory distress syndrome. Respiration 89, 420–434 (2015).
Orsenigo, F. et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat. Commun. 3, 1208 (2012).
Sun, Z. et al. VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd. J. Exp. Med. 209, 1363–1377 (2012).
Simons, M., Gordon, E. & Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. 17, 611–625 (2016).
Smith, R. O. et al. Vascular permeability in retinopathy is regulated by VEGFR2 Y949 signaling to VE-cadherin.Elife 9, e54056 (2020).
Eliceiri, B. P. et al. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell 4, 915–924 (1999).
Paul, R. et al. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat. Med. 7, 222–227 (2001).
Gavard, J. & Gutkind, J. S. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 8, 1223–1234 (2006).
Elias, B. C. et al. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J. Biol. Chem. 284, 1559–1569 (2009).
Fantin, A. et al. VEGF165-induced vascular permeability requires NRP1 for ABL-mediated SRC family kinase activation. J. Exp. Med. 214, 1049–1064 (2017).
Gitay-Goren, H., Soker, S., Vlodavsky, I. & Neufeld, G. The binding of vascular endothelial growth factor to its receptors is dependent on cell surface-associated heparin-like molecules. J. Biol. Chem. 267, 6093–6098 (1992).
Chiodelli, P., Bugatti, A., Urbinati, C. & Rusnati, M. Heparin/Heparan sulfate proteoglycans glycomic interactome in angiogenesis: biological implications and therapeutical use. Molecules 20, 6342–6388 (2015).
Corti, F. et al. N-terminal syndecan-2 domain selectively enhances 6-O heparan sulfate chains sulfation and promotes VEGFA165-dependent neovascularization. Nat. Commun. 10, 1562 (2019).
Ferrara, N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol. Biol. Cell 21, 687–690 (2010).
Li, X. et al. VEGFR2 pY949 signalling regulates adherens junction integrity and metastatic spread. Nat. Commun. 7, 11017 (2016).
Brash, J. T. Evaluating vascular hyperpermeability-inducing agents in the skin with the Miles assay. J. Vis. Exp.(136), 57524.
Wang, Y. et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 (2010).
Hayashi, M. et al. VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation. Nat. Commun. 4, 1672 (2013).
Grazia Lampugnani, M. et al. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J. Cell Biol. 161, 793–804 (2003).
Fournier, P. et al. The protein tyrosine phosphatase PTPRJ/DEP-1 contributes to the regulation of the Notch-signaling pathway and sprouting angiogenesis. Angiogenesis 23, 145–157 (2020).
Lanahan, A. A. et al. PTP1b is a physiologic regulator of vascular endothelial growth factor signaling in endothelial cells. Circulation 130, 902–909 (2014).
Corti, F. & Simons, M. Modulation of VEGF receptor 2 signaling by protein phosphatases. Pharmacol. Res. 115, 107–123 (2017).
Lanahan, A. A. et al. VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev. Cell 18, 713–724 (2010).
Ostman, A., Yang, Q. & Tonks, N. K. Expression of DEP-1, a receptor-like protein-tyrosine-phosphatase, is enhanced with increasing cell density. Proc. Natl. Acad. Sci. U S A 91, 9680–9684 (1994).
Takahashi, T. et al. Endothelial localization of receptor tyrosine phosphatase, ECRTP/DEP-1, in developing and mature renal vasculature. J. Am. Soc. Nephrol. 10, 2135–2145 (1999).
Adam, A. P. Regulation of endothelial adherens junctions by tyrosine phosphorylation.Mediators Inflamm. 2015, 272858 (2015).
Whiteford, J. R. et al. Syndecan-2 is a novel ligand for the protein tyrosine phosphatase receptor CD148. Mol. Biol. Cell 22, 3609–3624 (2011).
Kofler, N. et al. The Rab-effector protein RABEP2 regulates endosomal trafficking to mediate vascular endothelial growth factor receptor-2 (VEGFR2)-dependent signaling. J. Biol. Chem. 293, 4805–4817 (2018).
Srinivasan, B. & Kolli, A. R. in Blood-Brain Barrier (ed. Barichell, T.) 99–114 (Springer, 2019).
Lange, C., Storkebaum, E., de Almodovar, C. R., Dewerchin, M. & Carmeliet, P. Vascular endothelial growth factor: a neurovascular target in neurological diseases. Nat. Rev. Neurol. 12, 439–454 (2016).
Apte, R. S., Chen, D. S. & Ferrara, N. VEGF in signaling and disease: beyond discovery and development. Cell 176, 1248–1264 (2019).
Simard, J. M., Kent, T. A., Chen, M., Tarasov, K. V. & Gerzanich, V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 6, 258–268 (2007).
Ma, Y., Zechariah, A., Qu, Y. & Hermann, D. M. Effects of vascular endothelial growth factor in ischemic stroke. J. Neurosci. Res. 90, 1873–1882 (2012).
Geiseler, S. J. & Morland, C. The Janus face of VEGF in stroke.Int. J. Mol. Sci. 19, 1362 (2018).
Doyle, K. P. & Buckwalter, M. S. A mouse model of permanent focal ischemia: distal middle cerebral artery occlusion. Methods Mol. Biol. 1135, 103–110 (2014).
Jin, Z. G. et al. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ. Res. 93, 354–363 (2003).
Warren, C. M. A ligand-independent VEGFR2 signaling pathway limits angiogenic responses in diabetes. Sci. Signal. 7, ra1 (2014).
Couchman, J. R., Gopal, S., Lim, H. C., Norgaard, S. & Multhaupt, H. A. Fell-Muir Lecture: Syndecans: from peripheral coreceptors to mainstream regulators of cell behaviour. Int. J. Exp. Pathol. 96, 1–10 (2015).
De Rossi, G. et al. Pathological angiogenesis requires Syndecan-4 for efficient VEGFA-induced VE-Cadherin internalization. Arter. Thromb. Vasc. Biol. 41, 1374–1389 (2021).
Dews, I. C. & Mackenzie, K. R. Transmembrane domains of the syndecan family of growth factor coreceptors display a hierarchy of homotypic and heterotypic interactions. Proc. Natl. Acad. Sci. U S A 104, 20782–20787 (2007).
Ruiz, X. D. et al. Syndecan-2 is a novel target of insulin-like growth factor binding protein-3 and is over-expressed in fibrosis. PLoS One 7, e43049 (2012).
Choi, S. et al. Inflammatory hypoxia induces syndecan-2 expression through IL-1beta-mediated FOXO3a activation in colonic epithelia. FASEB J. 31, 1516–1530 (2017).
Hong, H. et al. Up-regulation of syndecan-2 in proximal colon correlates with acute inflammation. FASEB J. 33, 11381–11395 (2019).
Zhang, Z. G. et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Invest. 106, 829–838 (2000).
Reeson, P. et al. Delayed inhibition of VEGF signaling after stroke attenuates blood-brain barrier breakdown and improves functional recovery in a comorbidity-dependent manner. J. Neurosci. 35, 5128–5143 (2015).
Corti, F., Finetti, F., Ziche, M. & Simons, M. The Syndecan-4/protein kinase C alpha pathway mediates prostaglandin E-2-induced extracellular regulated kinase (ERK) activation in endothelial cells and angiogenesis in vivo. J. Biol. Chem. 288, 12712–12721 (2013).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Carrodus, N. L. et al. Differential labeling of cell-surface and internalized proteins after antibody feeding of live cultured neurons. J. Vis. Exp. e51139 (2014).
Tang, Z. et al. A mouse model of the cornea pocket assay for angiogenesis study. J. Vis. Exp. 54, 3077 (2011).
Ziche, M. & Morbidelli, L. The corneal pocket assay. Methods Mol. Biol. 1214, 15–28 (2015).
Gerriets, T. et al. Noninvasive quantification of brain edema and the space-occupying effect in rat stroke models using magnetic resonance imaging. Stroke 35, 566–571 (2004).
Kovalenko, M. et al. Site-selective dephosphorylation of the platelet-derived growth factor beta-receptor by the receptor-like protein-tyrosine phosphatase DEP-1. J. Biol. Chem. 275, 16219–16226 (2000).
Persson, C., Engstrom, U., Mowbray, S. L. & Ostman, A. Primary sequence determinants responsible for site-selective dephosphorylation of the PDGF beta-receptor by the receptor-like protein tyrosine phosphatase DEP-1. FEBS Lett. 517, 27–31 (2002).
Tarcic, G. et al. An unbiased screen identifies DEP-1 tumor suppressor as a phosphatase controlling EGFR endocytosis. Curr. Biol. 19, 1788–1798 (2009).
Acknowledgements
We thank D. Chen for the technical assistance and helpful discussion regarding the corneal pocket assay and confocal imaging. This work was supported by grants from National Institute of Health (NIH) HL149343 and HL062289 to Michael Simons.
Author information
Authors and Affiliations
Contributions
F.C. designed and performed experiments, analyzed data and wrote manuscript. E.R. designed/performed experiments and analyzed data. R.-M.F. performed experiments and analyzed data. T.D. designed experiments and analyzed data. J.Z., Z.W.Z. and J.M., performed experiments. M.S. designed experiments, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Christiana Ruhrberg, Philipp Berger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
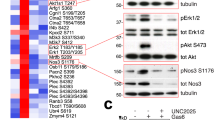
a-b, western blot analysis of VEGFR2 phosphorylation (pVEGFR2) following stimulation with VEGFA165 (50 ng/ml) or VEGFA121 (50 ng/ml) in Control (Ctr Si) vs. Sdc2-depleted (Sdc2 siRNA) HUVEC. c, VEGFA165 -induced Evans blue leakage in the back skin of WT vs Sdc2iECKO mice (n = 5–6) (representative blots of 3 independent experiments). d, evaluation of baseline permeability in various organs measured as Evans blue dye leakage in Wild type (WT) vs Sdc2-/- mice (n = 3–6). e, f western blot analysis of cell surface protein levels in Control (Ctr) vs. Sdc2-depleted (Sdc2 siRNA) HUVEC (n = 3). Data are presented as mean values + /- SEM (standard error of the mean). In all figure panels, each dot represents a biological independent experiment (n). Statistical analysis was performed by one-way ANOVA with Sidak’s multiple comparison test (panels c, d), and by unpaired t-student test (panel f), (N.S. not significant, * P < 0.05, ** P < 0.01, *** P < 0.001).
Extended Data Fig. 2
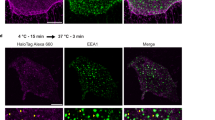
a, confocal image of confluent HUVEC cells in basal conditions showing human DEP1 carrying N-terminal HA-tag (DEP1-HA, red) expression at cell–cell junctions labeled with VE-cadherin (cyan), white arrows, lower panels. Scale bar: 25 μm. b, confocal image of HUVEC cells in basal conditions expressing both human DEP1 carrying N-terminal HA-tag (DEP1-HA, red) and human Sdc2 carrying N-terminal SNAP-tag (SDC2-SNAP, green) proteins. Lower panels show magnification of box in upper panels showing localization of both proteins at the plasma membrane and at the membrane protrusions. Scale bar: 25 μm. c, f, SIM imaging of SDC2/DEP1/Rab5 and SDC2/DEP1/VEGFR2 complexes in HUVECs expressing DEP1-HA and SDC2-SNAP proteins and treated for 20 min with 50 ng/ml VEGFA165. Scale bar: 0.5 μm. d,f, line profile analysis illustrating the distance between DEP1, Sdc2 and Rab5 or DEP1, Sdc2 and VEGFR2 in the same compartment. Plots represent the fluorescent signal in SIM images as a function of position along the line profile in left panel (red dotted line). g, h confocal images of constitutive internalization of DEP1 after incubation for 0 min, 30 min and 60 min in absence of VEGFA in HUVECs control and HUVECs transfected with Sdc2 siRNA. Scale bar: 15 μm. i, confocal image of internalized SDC2–DEP1 complexes in Rab5+ endosomes (white arrows) following constitutive endocytosis in VEGFA-free media. Scale bar: 5 μm. All images are representative images of >3 independent experiments (n). Data are presented as mean values + /- SEM (standard error of the mean). Statistical analysis was performed by two-way ANOVA with Sidak’s multiple comparison test (panels h), (N.S. not significant, * P < 0.05, ** P < 0.01, *** P < 0.001).
Extended Data Fig. 3
a, position and sequence of DEP1-binding motif in human Sdc2. b, direct ELISA comparing Sdc2 pAb (5 µg/ml) binding to mouse Sdc2 vs. mouse Sdc4. c, bEnd.3 cell proliferation in presence of rabbit IgG (5 µg/ml) or Sdc2 pAb (5 µg/ml) measured with xCELLigence system. d-e, ECs migration measured by in vitro wound-healing assay in bEnd.3 cells in presence of rabbit IgG (5 µg/ml) or Sdc2 pAb (5 µg/ml) (n = 3–4) f-g, TTC staining (at 24 h post stroke) and quantification of stroke infarct in WT vs Sdc2iECKO (n = 5–8). h-i, TTC staining (at 24 h post stroke) in Sdc2-/- mice with or without Sdc2 pAb treatment (administered 1 hour before stroke) (n = 4–9). Data are presented as mean values + /- SEM (standard error of the mean). In all figure panels, each dot represents a biological independent experiment (n). Statistical analysis was performed by one-way ANOVA with Sidak’s multiple comparison test (panels e, i), and by unpaired t-student test (panel g), (N.S. not significant, * P < 0.05, ** P < 0.01, *** P < 0.001).
Extended Data Fig. 4
MR imaging of IgG and Sdc2 pAb-treated mice: Representative T2 maps from mouse treated with IgG and Sdc2 pAb showing relatively well defined heterogeneous, hyperintense area within right MCA territory with measured T2 values in a range of 0.05–0.09 ms which were higher compared with contralateral normal tissue average 0.03 ms. On the ADC map, in the same region there is hypointense area with low ADC values, range from 0.4–0.8×10–3mm2/s, due to restricted diffusion. In normal appearing contralateral tissue measured ADC values were 0.9–1.1×10-3mm2/s. Compartmentalization analysis (see Material and Methods for details) shows different compartments of ischemic stroke; core (white), penumbra (light green) and edema (dark green).
Supplementary information
Source data
Source Data Fig. 1
Source data Fig. 1.
Source Data Fig. 1
Western blots Fig. 1.
Source Data Fig. 2
Source data Fig. 2.
Source Data Fig. 2
Western blots Fig. 2.
Source Data Fig. 3
Source data Fig. 3.
Source Data Fig. 3
Western blots Fig. 3.
Source Data Extended Data Fig. 1
Source data Extended Data Fig. 1.
Source Data Extended Data Fig. 1
Western blots Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Source data Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Source data Extended Data Fig. 3.
Rights and permissions
About this article
Cite this article
Corti, F., Ristori, E., Rivera-Molina, F. et al. Syndecan-2 selectively regulates VEGF-induced vascular permeability. Nat Cardiovasc Res 1, 518–528 (2022). https://doi.org/10.1038/s44161-022-00064-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-022-00064-2
This article is cited by
-
Report from the 2023 workshop on endothelial permeability, edema and inflammation
Nature Cardiovascular Research (2023)
-
Biology and therapeutic targeting of vascular endothelial growth factor A
Nature Reviews Molecular Cell Biology (2023)
-
KIF13B mediates VEGFR2 recycling to modulate vascular permeability
Cellular and Molecular Life Sciences (2023)
-
YES to junctions, no to Src
Nature Cardiovascular Research (2022)
-
Targeting VEGF-induced vascular permeability
Nature Cardiovascular Research (2022)