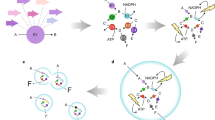
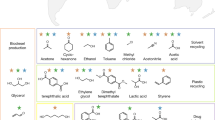
Abstract
Degradation of larger and undesired or harmful molecules into smaller and, ideally, value-added products is one of the most important facets of circular chemistry. However, this task may be cumbersome for chemists who are accustomed to planning syntheses using bond-forming, rather than bond-breaking, methodologies. This work describes a forward-synthesis algorithm that can guide such degradation-oriented analyses. This algorithm uses a broad knowledge-base of degradative and related reactions and applies them to arbitrary small-molecule feeds to generate large synthetic networks within which it then traces degradative pathways that are chemically sound and lead to value-added products. Predictions of the algorithm are validated by proof-of-concept experiments entailing degradation and revalorization of two biomass feeds, d-glucose and quinine.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The User Manual and login details are available in Supplementary Information, section 1. The use of Allchemy’s degradation module is further illustrated in Supplementary Video 1. All degradation pathways described in this work and experimental validation data supporting this study are available in Supplementary Information, sections 2 and 3.
Code availability
The interactive Allchemy web application is freely available for academic users at https://degradation.allchemy.net (due to server capacity, access is limited to five concurrent academic users on a rolling basis and to two-week slots). To obtain access, please send an email (from your academic address) to admin@allchemy.net. Reaction rules are proprietary but their coding is explained in Supplementary Information, section 1.4. The code for the forward-expand reaction networks is provided at https://zenodo.org/records/10034684 or via a DOI search engine under https://doi.org/10.5281/zenodo.10034684.
References
Szymkuć, S. et al. Computer‐assisted synthetic planning: the end of the beginning. Angew. Chem. Int. Ed. 55, 5904–5937 (2016).
Mikulak-Klucznik, B. et al. Computational planning of the synthesis of complex natural products. Nature 588, 83–88 (2020).
Klucznik, T. et al. Efficient syntheses of diverse, medicinally relevant targets planned by computer and executed in the laboratory. Chem 4, 522–532 (2018).
Lin, Y., Zhang, R., Wang, D. & Cernak, T. Computer-aided key step generation in alkaloid total synthesis. Science 379, 453–457 (2023).
Segler, M. H. S., Preuss, M. & Waller, M. P. Planning chemical syntheses with deep neural networks and symbolic AI. Nature 555, 604–610 (2018).
Liu, B. et al. Retrosynthetic reaction prediction using neural sequence-to-sequence models. ACS Cent. Sci. 3, 1103–1113 (2017).
Coley, C. W. et al. A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 365, eaax1566 (2019).
Schwaller, P. et al. Predicting retrosynthetic pathways using transformer-based models and a hyper-graph exploration strategy. Chem. Sci. 11, 3316–3325 (2020).
Rother, D. & Malzacher, S. Computer-aided enzymatic retrosynthesis. Nat. Catal. 4, 92–93 (2021).
Coley, C. W., Green, W. H. & Jensen, K. F. Machine learning in computer-aided synthesis planning. Acc. Chem. Res. 51, 1281–1289 (2018).
de Almeida, A. F., Moreira, R. & Rodrigues, T. Synthetic organic chemistry driven by artificial intelligence. Nat. Rev. Chem. 3, 589–604 (2019).
Schwaller, P. et al. Machine intelligence for chemical reaction space. Wiley Interdiscip. Rev. Comput. Mol. Sci. 12, e1604 (2022).
Strieth-Kalthoff, F., Sandfort, F., Segler, M. H. S. & Glorius, F. Machine learning the ropes: principles, applications and directions in synthetic chemistry. Chem. Soc. Rev. 49, 6154–6168 (2020).
Molga, K., Gajewska, E. P., Szymkuć, S. & Grzybowski, B. A. The logic of translating chemical knowledge into machine-processable forms: a modern playground for physical-organic chemistry. React. Chem. Eng. 4, 1506–1521 (2019).
Molga, K. et al. A computer algorithm to discover iterative sequences of organic reactions. Nat. Synth. 1, 49–58 (2022).
Roszak, R., Beker, W., Molga, K. & Grzybowski, B. A. Rapid and accurate prediction of pKa values of C–H acids using graph convolutional neural networks. J. Am. Chem. Soc. 141, 17142–17149 (2019).
Beker, W., Gajewska, E. P., Badowski, T. & Grzybowski, B. A. Prediction of major regio‐, site‐, and diastereoisomers in Diels–Alder reactions by using machine‐learning: the importance of physically meaningful descriptors. Angew. Chem. Int. Ed. 58, 4515–4519 (2019).
Guan, Y. et al. Regio-selectivity prediction with a machine-learned reaction representation and on-the-fly quantum mechanical descriptors. Chem. Sci. 12, 2198–2208 (2021).
Wołos, A. et al. Synthetic connectivity, emergence, and self-regeneration in the network of prebiotic chemistry. Science 369, eaaw1955 (2020).
Wołos, A. et al. Computer-designed repurposing of chemical wastes into drugs. Nature 604, 668–676 (2022).
Skoraczyński, G. et al. Predicting the outcomes of organic reactions via machine learning: are current descriptors sufficient? Sci. Rep. 7, 3582 (2017).
Gao, W., Mercado, R. & Coley, C. W. Amortized tree generation for bottom-up synthesis planning and synthesizable molecular design. Preprint at https://arxiv.org/abs/2110.06389 (2021).
Granda, J. M., Donina, L., Dragone, V., Long, D. L. & Cronin, L. Controlling an organic synthesis robot with machine learning to search for new reactivity. Nature 559, 377–381 (2018).
Robinson, W. E. et al. Environmental conditions drive self-organization of reaction pathways in a prebiotic reaction network. Nat. Chem. 14, 623–631 (2022).
Coley, C. W., Barzilay, R., Jaakkola, T. S., Green, W. H. & Jensen, K. F. Prediction of organic reaction outcomes using machine learning. ACS Cent. Sci. 3, 434–443 (2017).
Keijer, T., Bakker, V. & Slootweg, J. C. Circular chemistry to enable a circular economy. Nat. Chem. 11, 190–195 (2019).
Kümmerer, K., Clark, J. H. & Zuin, V. G. Rethinking chemistry for a circular economy. Science 367, 369–370 (2020).
Zhang, Y., Qi, M. Y., Tang, Z. R. & Xu, Y. J. Photoredox-catalyzed plastic waste conversion: nonselective degradation versus selective synthesis. ACS Catal. 13, 3575–3590 (2023).
Sobol, Ł., Dyjakon, A., & Soukup, K. Dioxins and furans in biochars, hydrochars and torreficates produced by thermochemical conversion of biomass: a review. Environ. Chem. Lett. 21, 2225–2249 (2023).
Jehanno, C. et al. Critical advances and future opportunities in upcycling commodity polymers. Nature 603, 803–814 (2022).
Coates, G. W. & Getzler, Y. D. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Zhang, F. et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 370, 437–441 (2020).
Trang, B. et al. Low-temperature mineralization of perfluorocarboxylic acids. Science 377, 839–845 (2022).
He, J., Ritalahti, K. M., Yang, K. L., Koenigsberg, S. S. & Löffler, F. E. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424, 62–65 (2003).
Liang, X. et al. Highly efficient NaNO2‐catalyzed destruction of trichlorophenol using molecular oxygen. Angew. Chem. Int. Ed. 44, 5520–5523 (2005).
Kumamaru, T. et al. Enhanced degradation of polychlorinated biphenyls by directed evolution of biphenyl dioxygenase. Nat. Biotechnol. 16, 663–666 (1998).
Meunier, B. Catalytic degradation of chlorinated phenols. Science 296, 270–271 (2002).
Smith, B. M. Catalytic methods for the destruction of chemical warfare agents under ambient conditions. Chem. Soc. Rev. 37, 470–478 (2008).
Rathi, B. S. & Kumar, P. S. Sustainable approach on the biodegradation of azo dyes: a short review. Curr. Opin. Green Sustain. Chem. 33, 100578 (2022).
Antonetti, C., Licursi, D., Fulignati, S., Valentini, G. & Raspolli Galletti, A. M. New frontiers in the catalytic synthesis of levulinic acid: from sugars to raw and waste biomass as starting feedstock. Catalysts 6, 196 (2016).
Liu, F. et al. Continuously processing waste lignin into high-value carbon nanotube fibers. Nat. Commun. 13, 5755 (2022).
Sun, Z., Balint, F., de Santi, A., Saravanakumar, E. & Barta, K. Bright side of lignin depolymerization: toward new platform chemicals. Chem. Rev. 118, 614–678 (2018).
Lee, K., Jing, Y., Wang, Y. & Yan, N. A unified view on catalytic conversion of biomass and waste plastics. Nat. Rev. Chem. 6, 635–652 (2022).
Adams, J. P. et al. Development of GSK’s reagent guides—embedding sustainability into reagent selection. Green Chem. 15, 1542 (2013).
Henderson, R. K., Hill, A. P., Redman, A. M. & Sneddon, H. F. Development of GSK’s acid and base selection guides. Green Chem. 17, 945–949 (2015).
Alder, C. et al. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 18, 3879–3890 (2016).
Zhu, Y., Romain, C. & Williams, C. K. Sustainable polymers from renewable resources. Nature 540, 354–362 (2016).
Zebec, Ž., Poberžnik, M. & Lobnik, A. Enzymatic hydrolysis of textile and cardboard waste as a glucose source for the production of limonene in Escherichia coli. Life 12, 1423 (2022).
Pleissner, D. & Lin, C. S. K. Valorisation of food waste in biotechnological processes. Sustain. Chem. Process. 1, 1–6 (2013).
Woodland, J. G. & Chibale, K. Quinine fever. Nat. Chem. 14, 112–112 (2022).
GlaxoSmithKline Intellectual Property Development Ltd. Chemical compounds. WO patent 2020/202091 (2020).
Trillium Therapeutics Inc. Heteroaromatic-fused imidazolyl amides, compositions and uses thereof as sting agonists. WO patent 2020/10451 (2020).
Hunter Fleming Ltd. Steroid-hormone conjugates with polyamides and their therapeutic use as anti-cancer and angiostatic agents. WO patent 2005/116050 (2005).
Pfizer INC. Adenosine A2A receptor antagonists. US patent 2008/242672 (2008).
Cespi, D. et al. Life cycle assessment comparison of two ways for acrylonitrile production: the SOHIO process and an alternative route using propane. J. Clean. Prod. 69, 17–25 (2014).
Diaminobutane (DAB), unique large scale production. AnQuore https://www.anqore.com/en/products/Diaminobutane (accessed 7 July 2023).
Qi, L. et al. Catalytic conversion of fructose, glucose, and sucrose to 5-(hydroxymethyl) furfural and levulinic and formic acids in γ-valerolactone as a green solvent. ACS Catal. 4, 1470–1477 (2014).
Dutta, S., Wu, L. & Mascal, M. Efficient, metal-free production of succinic acid by oxidation of biomass-derived levulinic acid with hydrogen peroxide. Green Chem. 17, 2335–2338 (2015).
Battelle Memorial Institute. Process for producing N-methyl succinimide. WO patent 2004/58708 (2004).
Ruan, S., Ruan, J., Chen, X. & Zhou, S. Facile dehydration of primary amides to nitriles catalyzed by lead salts: the anionic ligand matters. Mol. Catal. 499, 111313 (2021).
Chemistry and Chemical Engineering Guangdong Laboratory; Beijing Yuji Science & Technology Co Ltd. Method for preparing nitrile through gas phase dehydration. Chinese patent 114057605 (2022).
Zhou, X. et al. Discovery of novel inhibitors of human phosphoglycerate dehydrogenase by activity-directed combinatorial chemical synthesis strategy. Bioorg. Chem. 115, 105159 (2021).
Surivet, J. P. et al. Design, synthesis, and characterization of novel tetrahydropyran-based bacterial topoisomerase inhibitors with potent anti-gram-positive activity. J. Med. Chem. 56, 7396–7415 (2013).
Agouridas, C. et al. Synthesis and antibacterial activity of ketolides (6-O-methyl-3-oxoerythromycin derivatives): a new class of antibacterials highly potent against macrolide-resistant and -susceptible respiratory pathogens. J. Med. Chem. 41, 4080–4100 (1998).
GlaxoSmithKline Beecham PLC. Nitrogen-containing bicyclic heterocycles for use as antibacterials. WO patent 2003/87098 (2003).
Gemma, S. et al. Optimization of 4-aminoquinoline/clotrimazole-based hybrid antimalarials: further structure–activity relationships, in vivo studies, and preliminary toxicity profiling. J. Med. Chem. 55, 6948–6967 (2012).
Beuchel, A. et al. Structure–activity relationship of anti-Mycobacterium abscessus piperidine-4-carboxamides, a new class of NBTI DNA gyrase inhibitors. ACS Med. Chem. Lett. 13, 417–427 (2022).
Hoffmann La Roche. Process for piperidine intermediates for quinine, quinidine and analogs thereof. US patent 3931192 (1976).
Igarashi, J., Katsukawa, M., Wang, Y. G., Acharya, H. P. & Kobayashi, Y. Stereocontrolled synthesis of quinine and quinidine. Tetrahedron Lett. 45, 3783–3786 (2004).
Breman, A. C., Ruiz‐Olalla, A., van Maarseveen, J. H., Ingemann, S. & Hiemstra, H. Synthesis of quinuclidines by intramolecular silver‐catalysed amine additions to alkynes. Eur. J. Org. Chem. 2014, 7413–7425 (2014).
Illa, O., Arshad, M., Ros, A., McGarrigle, E. M. & Aggarwal, V. K. Practical and highly selective sulfur ylide mediated asymmetric epoxidations and aziridinations using an inexpensive, readily available chiral sulfide. Applications to the synthesis of quinine and quinidine. J. Am. Chem. Soc. 132, 1828–1830 (2010).
Kapoor, V. K. & Karwayun, R. 4-Cyano-6-methoxyquinoline from quinine. Indian J. Pharm. Sci. 57, 237 (1995).
Elderfield, R. C. et al. Synthesis of certain simple 4-aminoquinoline derivatives. J. Am. Chem. Soc. 68, 1250–1251 (1946).
von Riesen, C., Jones, P. G. & Hoffmann, H. M. R. From quinidine to new enantiopure materials—tricyclic allylic N,O‐acetals and a stereospecific, onepot conversion of 1,2‐secondary, tertiary diols into spiroepoxides. Eur. J. Chem. 2, 673–679 (1996).
von Riesen, C. & Hoffmann, H. M. R. A tricyclic dehydrorubanone and new isomers of the major quinidine metabolite. Eur. J. Chem. 2, 680–684 (1996).
Friestad, G. K., Ji, A., Baltrusaitis, J., Korapala, C. S. & Qin, J. Scope of stereoselective Mn-mediated radical addition to chiral hydrazones and application in a formal synthesis of quinine. J. Org. Chem. 77, 3159–3180 (2012).
SmithKline Beecham Corporation. Quinoline derivatives as antibacterials. US patent 2004053928A1 (2004).
Clark, J. S., Townsend, R. J., Blake, A. J., Teat, S. J. & Johns, A. A concise enantioselective synthesis of the AB ring system of the manzamine alkaloids by ring-closing enyne metathesis. Tetrahedron Lett. 42, 3235–3238 (2001).
Woo, S. & Shenvi, R. A. Natural product synthesis through the lens of informatics. Acc. Chem. Res. 54, 1157–1167 (2021).
Wan Station Ltd; Tokyo Institute of Technology; Fukuoka University; Kinki University. Rotaxane compound and antitumor agent. EP patent 2348021 (2011).
Peters, J. U. et al. Discovery of potent, balanced and orally active dual NK1/NK3 receptor ligands. Bioorg. Med. Chem. Lett. 20, 3405–3408 (2010).
Levi, N., Khenkin, A. M., Hailegnaw, B. & Neumann, R. Depolymerization of cellulose in water catalyzed by phenylboronic acid derivatives. ACS Sustain. Chem. Eng. 4, 5799–5803 (2016).
Zhang, Z. et al. Conversion of carbohydrates into 5-hydroxymethylfurfural using polymer bound sulfonic acids as efficient and recyclable catalysts. RSC Adv. 3, 9201–9205 (2013).
Li, C., Zhang, Z. & Zhao, Z. K. Direct conversion of glucose and cellulose to 5-hydroxymethylfurfural in ionic liquid under microwave irradiation. Tetrahedron Lett. 50, 5403–5405 (2009).
Li, X., Ho, B., Lim, D. S. & Zhang, Y. Highly efficient formic acid-mediated oxidation of renewable furfural to maleic acid with H2O2. Green Chem. 19, 914–918 (2017).
Tirsoaga, A., El Fergani, M., Parvulescu, V. I. & Coman, S. M. Upgrade of 5-hydroxymethylfurfural to dicarboxylic acids onto multifunctional-based Fe3O4@SiO2 magnetic catalysts. ACS Sustain. Chem. Eng. 6, 14292–14301 (2018).
Kantam, L., Parsharamulu, T. & Manorama, S. V. Layered double hydroxides supported nano palladium: an efficient catalyst for the chemoselective hydrogenation of olefinic bonds. J. Mol. Catal. A 365, 115–119 (2012).
McMaster, L. & Langreck, F. B. On the preparation of fumaric nitrile. The action of hydroxylamine on fumaric nitrile. J. Am. Chem. Soc. 40, 970–973 (1918).
Blomquist, A. T. & Winslow, E. C. Unsaturated nitriles as dienophiles in the diene synthesis. J. Org. Chem. 10, 149–158 (1945).
Chang, F., Kim, H., Lee, B., Park, S. & Park, J. Highly efficient solvent-free catalytic hydrogenation of solid alkenes and nitro-aromatics using Pd nanoparticles entrapped in aluminum oxy-hydroxide. Tetrahedron Lett. 51, 4250–4252 (2010).
Khan, H. A. & Robins, D. J. Pyrrolizidine alkaloid biosynthesis. Synthesis of 13C-labelled putrescines and their incorporation into retronecine. J. Chem. Soc. Perkin Trans. 1, 101–105 (1985).
Chatupheeraphat, A., Liao, H. H., Lee, S. C. & Rueping, M. Nickel-catalyzed C–CN bond formation via decarbonylative cyanation of esters, amides, and intramolecular recombination fragment coupling of acyl cyanides. Org. Lett. 19, 4255–4258 (2017).
Acknowledgements
Development of the Degradation module within the Allchemy platform (by K.M., S.S., M.M., R.R. and B.A.G.) has been supported by internal funds of Allchemy. Calculations, analysis of pathways, and laboratory experiments (by A.Ż.-D. and O.O.K.) were supported by the National Science Centre, Poland (award 2020/39/D/ST4/01890 to A.Ż.-D.). Analysis of results and writing of the paper by B.A.G. was supported by the Institute for Basic Science, Korea (project code IBS-R020-D1 to B.A.G.). We thank the Mcule team for providing access to their catalogue and standardizing the price information therein to per-gramme.
Author information
Authors and Affiliations
Contributions
K.M., S.S., M.M., R.R. and B.A.G. designed and developed the Allchemy platform. A.Ż.-D. performed the analyses and computational calculations described in the paper, most syntheses described in Fig. 4 (d-glucose–1–2–3–4) and all the syntheses described in Fig. 5. O.O.K performed syntheses 3–5 described in Fig. 4. B.A.G. conceived and supervised the research and wrote the paper with help from A.Ż.-D.
Corresponding authors
Ethics declarations
Competing interests
K.M., S.S., M.M. and B.A.G. are consultants and/or stakeholders of Allchemy. Allchemy software is the property of Allchemy. The other authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Calvin Chen, Fabrice Gallou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Supplementary Sections 1–4 (basic information, analysis of results, experimental validation, supplementary references), Figs. 1–81 and Tables 1–7.
Supplementary Video 1
Illustration of the use of Allchemy’s degradation module.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Żądło-Dobrowolska, A., Molga, K., Kolodiazhna, O.O. et al. Computational synthesis design for controlled degradation and revalorization. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00497-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00497-6