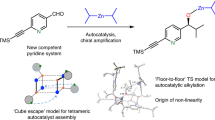
Abstract
Asymmetric catalysis has expanded the range of chiral products readily accessible through increasingly efficient synthetic catalysts. The development of these catalysts often starts with a result obtained by systematic screening of known privileged chiral structures and assumes that the active species would be an isolated monomolecular species. Here we report the study of three proline-derived ligands, diphenyl-N-methyl-prolinol, diphenylprolinol and 5-(hydroxydiphenylmethyl)-2-pyrrolidinone, in the zinc-catalysed alkylation of benzaldehyde. The three ligands exhibit different system-level behaviour, characterized by multiple levels of aggregation that may be catalytically active simultaneously. While diphenyl-N-methyl-prolinol behaves as expected from a mechanistic point of view, diphenylprolinol shows enantiodivergence during the reaction due to an asymmetric autoinduction process. With 5-(hydroxydiphenylmethyl)-2-pyrrolidinone, we were able to establish the possibility of at least trimeric active species in equilibrium with less aggregated active species. Simulations using a mathematical model confirm the possibility of such systems-level behaviour. Parallel study of the three systems reveals three distinct system-level behaviours that are central to the efficiency of the catalytic reaction.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the Supporting Information. Source data are provided with this paper.
References
Yoon, T. P. & Jacobsen, E. N. Privileged chiral catalysts. Science 299, 1691–1693 (2003).
Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (eds) Comprehensive Asymmetric Catalysis Vols 1–3 (Springer, 1999).
Ojima, I. (ed.) Catalytic Asymmetric Synthesis (Wiley, 2010).
Ma, L., Abney, C. & Lin, W. Enantioselective catalysis with homochiral metal–organic frameworks. Chem. Soc. Rev. 38, 1248–1256 (2009).
Huang, S., Yu, H. & Li, Q. Supramolecular chirality transfer toward chiral aggregation: asymmetric hierarchical self-assembly. Adv. Sci. 8, 2002132 (2021).
Puchot, C. et al. Nonlinear effects in asymmetric synthesis. Examples in asymmetric oxidations and aldolization reactions. J. Am. Chem. Soc. 108, 2353–2357 (1986).
Guillaneux, D., Zhao, S.-H., Samuel, O., Rainford, D. & Kagan, H. B. Nonlinear effects in asymmetric catalysis. J. Am. Chem. Soc. 116, 9430–9439 (1994).
Girard, C. & Kagan, H. B. Nonlinear effects in asymmetric synthesis and stereoselective reactions: ten years of investigation. Angew. Chem. Int. Ed. 37, 2922–2959 (1998).
Satyanarayana, T., Abraham, S. & Kagan, H. B. Nonlinear effects in asymmetric catalysis. Angew. Chem. Int. Ed. 48, 456–494 (2009).
Klussmann, M. et al. Thermodynamic control of asymmetric amplification in amino acid catalysis. Nature 441, 621–623 (2006).
Kalek, M. & Fu, G. C. Caution in the use of nonlinear effects as a mechanistic tool for catalytic enantioconvergent reactions: intrinsic negative nonlinear effects in the absence of higher-order species. J. Am. Chem. Soc. 139, 4225–4229 (2017).
Ali, C., Blackmond, D. G. & Burés, J. Kinetic rationalization of nonlinear effects in asymmetric catalytic cascade reactions under Curtin–Hammett conditions. ACS Catal. 441, 5776–5785 (2022).
Geiger, Y., Achard, T., Maisse-François, A. & Bellemin-Laponnaz, S. Hyperpositive nonlinear effects in asymmetric catalysis. Nat. Catal. 3, 422–426 (2020).
Geiger, Y., Achard, T., Maisse‐François, A. & Bellemin‐Laponnaz, S. Observation of hyperpositive non-linear effect in catalytic asymmetric organozinc additions to aldehydes. Chirality 32, 1250–1256 (2020).
Geiger, Y., Achard, T., Maisse-François, A. & Bellemin-Laponnaz, S. Hyperpositive non-linear effects: enantiodivergence and modelling. Chem. Sci. 11, 12453–12463 (2020).
Geiger, Y., Achard, T., Maisse‐François, A. & Bellemin‐Laponnaz, S. Absence of non‐linear effects despite evidence for catalyst aggregation. Eur. J. Org. Chem. 2021, 2916–2922 (2021).
Thierry, T., Geiger, Y. & Bellemin-Laponnaz, S. Observation of hyperpositive non-linear effect in asymmetric organozinc alkylation in presence of N-pyrrolidinyl norephedrine. Molecules 27, 3780 (2022).
Zhou, Q.-L. (ed.) Privileged Chiral Ligands and Catalysts (Wiley, 2011).
Kitamura, M., Okada, S., Suga, S. & Noyori, R. Enantioselective addition of dialkylzincs to aldehydes promoted by chiral amino alcohols. Mechanism and nonlinear effect. J. Am. Chem. Soc. 111, 4028–4036 (1989).
Kitamura, M., Suga, S., Oka, H. & Noyori, R. Quantitative analysis of the chiral amplification in the amino alcohol-promoted asymmetric alkylation of aldehydes with dialkylzincs. J. Am. Chem. Soc. 120, 9800–9809 (1998).
Pu, L. & Yu, H.-B. Catalytic asymmetric organozinc additions to carbonyl compounds. Chem. Rev. 101, 757–824 (2001).
Noyori, R. Asymmetric Catalysis in Organic Synthesis (Wiley, 1994).
Soai, K., Ookawa, A., Ogawa, K. & Kaba, T. Complementary catalytic asymmetric induction in the enantioselective addition of diethylzinc to aldehydes. Chem. Commun. 7, 467–468 (1987).
Soai, K., Ookawa, A., Kaba, T. & Ogawa, K. Catalytic asymmetric induction. Highly enantioselective addition of dialkylzincs to aldehydes using chiral pyrrolidinylmethanols and their metal salts. J. Am. Chem. Soc. 109, 7111–7115 (1987).
Geiger, Y. & Bellemin‐Laponnaz, S. Non‐linear effects in asymmetric catalysis: impact of catalyst precipitation. ChemCatChem 14, e202200165 (2022).
Burés, J. A simple graphical method to determine the order in catalyst. Angew. Chem. Int. Ed. 55, 2028–2031 (2016).
Burés, J. Variable time normalization analysis: general graphical elucidation of reaction orders from concentration profiles. Angew. Chem. Int. Ed. 55, 16084–16087 (2016).
Nielsen, C. D.-T. & Burés, J. Visual kinetic analysis. Chem. Sci. 10, 348–353 (2019).
Gesslbauer, S., Hutchinson, G., White, A. J. P., Burés, J. & Romain, C. Chirality-induced catalyst aggregation: insights into catalyst speciation and activity using chiral aluminum catalysts in cyclic ester ring-opening polymerization. ACS Catal. 11, 4084–4093 (2021).
Alamillo-Ferrer, C., Hutchinson, G. & Burés, J. Mechanistic interpretation of orders in catalyst greater than one. Nat. Rev. Chem. 7, 26–34 (2023).
Nakano, K., Nozaki, K. & Hiyama, T. Asymmetric alternating copolymerization of cyclohexene oxide and CO2 with dimeric zinc complexes. J. Am. Chem. Soc. 125, 5501–5510 (2003).
Nakano, K., Hiyama, T. & Nozaki, K. Asymmetric amplification in asymmetric alternating copolymerization of cyclohexene oxide and carbon dioxide. Chem. Commun. 6, 467–468 (2005).
Dreisbach, C., Wischnewski, G., Kragl, U. & Wandrey, C. Changes of enantioselectivity with the substrate ratio for the addition of diethylzinc to aldehydes using a catalyst coupled to a soluble polymer. J. Chem. Soc. Perkin Trans. 1, 875–878 (1995).
Trost, B. M., Fettes, A. & Shireman, B. T. Direct catalytic asymmetric aldol additions of methyl ynones. Spontaneous reversal in the sense of enantioinduction. J. Am. Chem. Soc. 126, 2660–2661 (2004).
Bryliakov, K. P. Dynamic nonlinear effects in asymmetric catalysis. ACS Catal. 9, 5418–5438 (2019).
Griffiths, G. J. & Warm, A. Proposed mechanism for the enantioselective alkynylation of an aryl trifluoromethyl ketone, the key step in the synthesis of efavirenz. Org. Process Res. Dev. 20, 803–813 (2016).
Mayer, L. C., Heitsch, S. & Trapp, O. Nonlinear effects in asymmetric catalysis by design: concept, synthesis, and applications. Acc. Chem. Res. 55, 3345–3361 (2022).
Trapp, O. in Supramolecular Catalysis Ch. 4 (eds van Leeuwen, P. W. N. M. & Raynal, M.) 55–67 (Wiley, 2022).
Storch, G. & Trapp, O. By-design enantioselective self-amplification based on non-covalent product–catalyst interactions. Nat. Chem. 9, 179–187 (2017).
Scholtes, J. F. & Trapp, O. Design and synthesis of a stereodynamic catalyst with reversal of selectivity by enantioselective self-inhibition. Chirality 31, 1028–1042 (2019).
Menke, J.-M. & Trapp, O. Controlling the enantioselectivity in an adaptable ligand by biomimetic intramolecular interlocking. J. Org. Chem. 87, 11165–11171 (2022).
Torres, M., Maisse-François, A. & Bellemin-Laponnaz, S. Highly recyclable self-supported chiral catalysts for the enantioselective α-hydrazination of β-ketoesters. ChemCatChem 5, 3078–3085 (2013).
Bissessar, D., Achard, T. & Bellemin-Laponnaz, S. Robust and recyclable self-supported chiral nickel catalyst for the enantioselective Michael addition. Adv. Synth. Catal. 358, 1982–1988 (2016).
Enthaler, S. Rise of the zinc age in homogeneous catalysis? ACS Catal. 3, 150–158 (2013).
Athavale, S. V., Simon, A., Houk, K. N. & Denmark, S. E. Demystifying the asymmetry-amplifying, autocatalytic behaviour of the Soai reaction through structural, mechanistic and computational studies. Nat. Chem. 12, 412–423 (2020).
Athavale, S. V., Simon, A., Houk, K. N. & Denmark, S. E. Structural contributions to autocatalysis and asymmetric amplification in the Soai reaction. J. Am. Chem. Soc. 142, 18387–18406 (2020).
Trapp, O. et al. In situ mass spectrometric and kinetic investigations of Soai’s asymmetric autocatalysis. Chemistry 26, 15871–15880 (2020).
Trapp, O. Efficient amplification in Soai’s asymmetric autocatalysis by a transient stereodynamic catalyst. Front. Chem. 8, 615800 (2020).
Soai, K., Shibata, T., Morioka, H. & Choji, K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature 378, 767–768 (1995).
Soai, K., Kawasaki, T. & Matsumoto, A. Asymmetric autocatalysis of pyrimidyl alkanol and related compounds. Self-replication, amplification of chirality and implication for the origin of biological enantioenriched chirality. Tetrahedron 74, 1973–1990 (2018).
Geiger, Y. One Soai reaction, two mechanisms? Chem. Soc. Rev. 51, 1206–1211 (2022).
Xiang, S.-H. & Tan, B. Advances in asymmetric organocatalysis over the last 10 years. Nat. Commun. 11, 3786 (2020).
Han, B. et al. Asymmetric organocatalysis: an enabling technology for medicinal chemistry. Chem. Soc. Rev. 50, 1522–1586 (2021).
Aukland, M. H. & List, B. Organocatalysis emerging as a technology. Pure Appl. Chem. 93, 1371–1381 (2021).
Acknowledgements
This research was supported by the Interdisciplinary Thematic Institute ITI-CSC via the IdEx Unistra (ANR-10-IDEX-0002) within the programme Investissement d’Avenir (T.T.) and the Ministère de l’Enseignement Supérieur et de la Recherche (MESR) for a PhD grant to Y.G. We thank the NMR department of CNRS FR2010 Strasbourg and in particular B. Vincent for his valuable studies. We also thank T. Achard, A. Maisse-François and E. Couzigné from IPCMS Strasbourg.
Author information
Authors and Affiliations
Contributions
T.T. performed the synthetic experiments. Y.G. made the mathematical models and computed simulations. T.T., Y.G. and S.B.-L. performed the data analyses. S.B.-L. conceptualized and supervised the study, and wrote the manuscript with T.T. and Y.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Oliver Trapp and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
All details of syntheses, experimental procedures and mathematical treatments.
Supplementary Data 1
Spreadsheet program for ML models (Supplementary Information, sections 3.3 and 3.4).
Supplementary Data 2
Numerical experimental data for Supplementary Fig. 1.
Supplementary Data 3
Numerical experimental data for Supplementary Fig. 2.
Supplementary Data 4
Numerical experimental data for Supplementary Fig. 3.
Supplementary Data 5
Numerical experimental data for Supplementary Fig. 4.
Supplementary Data 6
Numerical experimental data for Supplementary Fig. 5.
Supplementary Data 7
Numerical experimental data for Supplementary Fig. 6.
Supplementary Data 8
Numerical experimental data for Supplementary Fig. 7.
Supplementary Data 9
Numerical experimental data for Supplementary Fig. 8.
Supplementary Data 10
Numerical experimental data for Supplementary Fig. 9.
Supplementary Data 11
Numerical experimental data for Supplementary Fig. 10.
Supplementary Data 12
Numerical experimental data for Supplementary Fig. 11.
Supplementary Data 13
Numerical simulation data for Supplementary Fig. 12.
Supplementary Data 14
Numerical experimental data for Supplementary Fig. 13.
Supplementary Data 15
Numerical experimental data for Supplementary Fig. 14.
Supplementary Data 16
Numerical experimental data for Supplementary Fig. 15.
Supplementary Data 17
Numerical experimental data for Supplementary Fig. 16.
Supplementary Data 18
Numerical experimental data for Supplementary Fig. 17.
Supplementary Data 19
Numerical simulation data for Supplementary Fig. 19.
Source data
Source Data Fig. 3
Numerical experimental data.
Source Data Fig. 4
Numerical experimental data.
Source Data Fig. 5
Numerical experimental data.
Source Data Fig. 7
Numerical simulation data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thierry, T., Geiger, Y. & Bellemin-Laponnaz, S. Divergence of catalytic systems in the zinc-catalysed alkylation of benzaldehyde mediated by chiral proline-based ligands. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00491-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00491-y