Abstract
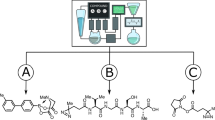
The amount of chemical synthesis literature is growing quickly; however, it takes a long time to share and evaluate new processes among laboratories. Here we present an approach that uses a universal chemical programming language (χDL) to encode and execute synthesis procedures for a variety of chemical reactions, including reductive amination, ring formation, esterification, carbon–carbon bond formation and amide coupling on four different hardware systems in two laboratories. With around 50 lines of code per reaction, our approach uses abstraction to efficiently compress chemical protocols. Our different robotic platforms consistently produce the expected synthesis with yields up to 90% per step, allowing faster and more secure research workflows that can increase the throughput of a process by number-up instead of scale-up. Chemputer-type platforms at the University of Glasgow and the University of British Columbia Vancouver were used, as well as Opentrons robots and multi-axis cobotic robots to distribute and repeat experimental results. Protocols for three case studies involving seven reaction steps and three final compounds were validated and disseminated to be repeated in two international laboratories and on three independent robots.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Code availability
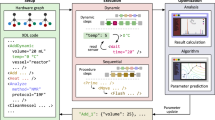
χDL files (.xdl) and Chemputer graph files (.json) can be opened and edited with the ChemIDE app on https://croningroup.gitlab.io/chemputer/xdlapp/. The χDL software standard is linked here: https://croningroup.gitlab.io/chemputer/xdl/standard/index.html. A complete docker image of the synthetic platforms and hardware can be made available by request.
References
Popper, K. R. Logik der Forschung: Zur Erkenntnistheorie der modernen Naturwissenschaft (Springer, 1934).
Gushee, D. E. Factors affecting dissemination of chemical information. J. Chem. Doc. 11, 201–204 (1971).
Arveson, M. H. Economic aspects in the dissemination of chemical knowledge. J. Chem. Doc. 1, 1–3 (1961).
Boga, S. B. et al. Selective functionalization of complex heterocycles via an automated strong base screening platform. React. Chem. Eng. 2, 446–450 (2017).
Burger, B. et al. A mobile robotic chemist. Nature 583, 237–241 (2020).
Chatterjee, S., Guidi, M., Seeberger, P. H. & Gilmore, K. Automated radial synthesis of organic molecules. Nature 579, 379–384 (2020).
Godfrey, A. G., Masquelin, T. & Hemmerle, H. A remote-controlled adaptive medchem lab: an innovative approach to enable drug discovery in the 21st Century. Drug Discov. Today 18, 795–802 (2013).
Legrand, M. & Bolla, P. A fully automatic apparatus for chemical reactions on the laboratory scale. J. Autom. Chem. 7, 513591 (1985).
MacLeod, B. P. et al. Self-driving laboratory for accelerated discovery of thin-film materials. Sci. Adv. 6, eaaz8867 (2020).
Okamoto, H. & Deuchi, K. Design of a robotic workstation for automated organic synthesis. Lab. Robotics Automat. 12, 2–11 (2000).
Orita, A., Yasui, Y. & Otera, J. Automated synthesis: development of a new apparatus friendly to synthetic chemists (MEDLEY). Org. Process Res. Dev. 4, 333–336 (2000).
Tanaka, Y., Fuse, S., Tanaka, H., Doi, T. & Takahashi, T. An efficient synthesis of a cyclic ether key intermediate for 9-membered masked enediyne using an automated synthesizer. Org. Process Res. Dev. 13, 1111–1121 (2009).
Baker, M. 1,500 scientists lift the lid on reproducibility. Nature 533, 452–454 (2016).
Steiner, S. et al. Organic synthesis in a modular robotic system driven by a chemical programming language. Science 363, eaav2211 (2019).
Hammer, A. J. S., Leonov, A. I., Bell, N. L. & Cronin, L. Chemputation and the standardization of chemical informatics. JACS Au 1, 1572–1587 (2021).
Mehr, S. H. M., Craven, M., Leonov, A. I., Keenan, G. & Cronin, L. A universal system for digitization and automatic execution of the chemical synthesis literature. Science 370, 101–108 (2020).
Rohrbach, S. et al. Digitization and validation of a chemical synthesis literature database in the ChemPU. Science 377, 172–180 (2022).
Gromski, P. S., Granda, J. M. & Cronin, L. Universal chemical synthesis and discovery with ‘The Chemputer. Trends Chem. 2, 4–12 (2020).
Wilbraham, L., Mehr, S. H. M. & Cronin, L. Digitizing chemistry using the chemical processing unit: from synthesis to discovery. Accounts Chem. Res. 54, 253–262 (2021).
Angelone, D. et al. Convergence of multiple synthetic paradigms in a universally programmable chemical synthesis machine. Nat. Chem. 13, 63–69 (2021).
XDL documentation. Cronin Group, UoG https://croningroup.gitlab.io/chemputer/xdl/standard/index.html (2022).
Arduengo, A. J. et al. Imidazolylidenes, imidazolinylidenes and imidazolidines. Tetrahedron 55, 14523–14534 (1999).
Buckley, B. R. & Neary, S. P. Thiadiazolidine 1-oxide systems for phosphine-free palladium-mediated catalysis. Tetrahedron 66, 7988–7994 (2010).
Higgins, E. M. et al. pKas of the conjugate acids of N-heterocyclic carbenes in water. Chem. Commun. 47, 1559–1561 (2011).
Blair, D. J. et al. Automated iterative Csp3–C bond formation. Nature 604, 92–97 (2022).
Liu, J., Sato, Y., Yang, F., Kukor, A. J. & Hein, J. E. An adaptive auto-synthesizer using online PAT feedback to flexibly perform a multistep reaction. Chem. Methods 2, e202200009 (2022).
Cronin Group, UofG. χDL code. Zenodo https://zenodo.org/record/6534009 (2022).
Acknowledgements
Financial support for this work was provided by the EPSRC (grants EP/L023652/1, EP/R01308X/1, EP/S019472/1 and EP/P00153X/1), Defence Advanced Research Projects Agency (DARPA) under the Accelerated Molecular Discovery Program (Cooperative Agreement HR00111920027, dated 1 August 2019). Additional support was provided by the Canada Foundation for Innovation (CFI) (CFI-35883) and the Natural Sciences and Engineering Research Council of Canada (NSERC) (RGPIN-2021-03168, Discovery Accelerator Supplement RGPAS-2021-00016). Student support was provided by the German Academic Scholarship Foundation (to R.R.) and an NSERC CGS-D scholarship (to M.G.). We thank D. Thomas, M. Siauciulis and E. Trushina from the Cronin Laboratory at the University of Glasgow and S. Rohrbach from Chemify Ltd for proofreading the manuscript and for support in the laboratory.
Author information
Authors and Affiliations
Contributions
L.C. and J.H. conceived the idea of utilizing χDL protocols between different laboratories and named the concept of this paper ‘ChemTorrent’. L.C. and J.H. together coordinated the research project and mentored R.R. and M.G. All experimental work was completed by R.R. and M.G. in equal contribution with help from the respective research groups in Glasgow and Vancouver. The body of this manuscript and the Supplementary Information were written by R.R. and M.G. with input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Linjiang Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Figs. 1–5 and Tables 1–3.
Supplementary Data 1
HPLC data for the traces of compounds 7, 8, 14 and 16 found in Supplementary Figs. 2 and 3.
Supplementary Code 1
χDL protocols and graph files for experiments detailed in the Supplementary Information.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rauschen, R., Guy, M., Hein, J.E. et al. Universal chemical programming language for robotic synthesis repeatability. Nat. Synth 3, 488–496 (2024). https://doi.org/10.1038/s44160-023-00473-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00473-6
This article is cited by
-
Sharing reproducible synthesis recipes
Nature Synthesis (2024)