Abstract
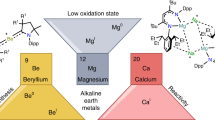
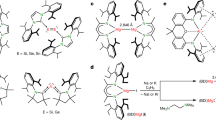
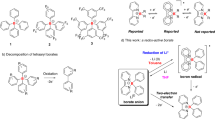
Low-oxidation-state complexes of type (BDI)Mg–Mg(BDI) (BDI, β-diketiminate) show broad reactivity, finding application as soluble, universal reducing agents, enriching the field of early main group metal chemistry. Attempts to isolate similar, but considerably more reactive, Ca–Ca bound complexes have so far failed. As the metal–metal bond strength descending group 2 rapidly decreases, BDI complexes of the heavier AeI (alkaline earth) ions (Ae = Ca, Sr, Ba) probably exist as (BDI)Ae· radicals of untamed reactivity. Here we describe a facile method to prepare stable but highly reactive complexes with heterobimetallic Mg–Ae bonds. Mixing [(BDI)MgˉNa+]2 and [Ae(NR2)2]2 dimers led to anion–cation exchange and exclusive formation of mixed species: (BDI)MgˉNa+/Ae(NR2)2 (Ae = Ca, Sr, Ba; R = SiMe3). Crystal structures feature examples of Mg–Ca and Mg–Sr bonding with formal oxidation states of Mg0–AeII. Best described as polarized Mgδˉ–Aeδ+ bonds, these complexes show potential as alkane-soluble reductants for bond activation, which readily react with benzene by C–H bond cleavage and are oxidized to discrete complexes with N2O.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
X-ray data are available free of charge from the Cambridge Crystallographic Data Centre under reference CCDC 2269821 (1), 2269822 (2), 2269823 (4), 2269824 (5), 2269631 (7), 2269632 (8), 2269633 (9), 2269634 (10) and 2269648 (Na(MgN″3)). Copies of the data can be obtained free of charge at https://www.ccdc.cam.ac.uk/structures/. Spectroscopic data that support the findings of this study as well as complementary crystallographic and computational details are included in Supplementary Information. Raw data are also available from corresponding author on reasonable request.
References
Green, S. P., Jones, C. & Stasch, A. Stable magnesium(I) compounds with Mg–Mg bonds. Science 318, 1754–1757 (2007).
Parkin, G. Valence, oxidation number, and formal charge: three related but fundamentally different concepts. J. Chem. Educ. 83, 791–799 (2006).
Jones, C. Dimeric magnesium(I) β-diketiminates: a new class of quasi-universal reducing agent. Nat. Rev. Chem. 1, 0059 (2017).
Mai, J., Rösch, B., Patel, N., Langer, J. & Harder, S. On the existence of low-valent magnesium–calcium complexes. Chem. Sci. 14, 4724–4734 (2023).
Platts, J. A., Overgaard, J., Jones, C., Iversen, B. B. & Stasch, A. First experimental characterisation of a non-nuclear attractor in a dimeric magnesium(I) compound. J. Phys. Chem. A 115, 194–200 (2011).
Wu, L.-C., Jones, C., Stasch, A., Platts, J. A. & Overgaard, J. Non-nuclear attractor in a molecular compound under external pressure. Eur. J. Inorg. Chem. 2014, 5536–5540 (2014).
Krieck, S., Görls, H., Yu, L., Reiher, M. & Westerhausen, M. Stable ‘inverse’ sandwich complex with unprecedented organocalcium(I): crystal structures of [(thf)2 Mg(Br)-C6H2-2,4,6-Ph3] and [(thf)3Ca{μ-C6H3-1,3,5-Ph3}Ca(thf)3]. J. Am. Chem. Soc. 131, 2977–2958 (2009).
Arrowsmith, M. et al. Neutral zero- valent s-block complexes with strong multiple bonding. Nat. Chem. 8, 890–594 (2016).
Wang, G. et al. A stable, crystalline beryllium radical cation. J. Am. Chem. Soc. 142, 4560–4564 (2020).
Huang, W. & Diaconescu, P. L. Rare-earth metal π-complexes of reduced arenes, alkenes, and alkynes: bonding, electronic structure, and comparison with actinides and other electropositive metals. Dalton Trans. 44, 15360–15371 (2015).
Jędrzkiewicz, D. et al. Access to a labile monomeric magnesium radical by ball-milling. Angew. Chem. Int. Ed. 61, e202200511 (2022).
Breitwieser, K., Bahmann, H., Weiss, R. & Munz, D. Gauging radical stabilization with carbenes. Angew. Chem. Int. Ed. 61, e20220639 (2022).
Gimferrer, M. et al. The oxidation state in low-valent beryllium and magnesium compounds. Chem. Sci. 13, 6583–6591 (2022).
Pan, S. & Frenking, G. Comment on ‘The oxidation state in low-valent beryllium and magnesium compounds’ by M. Gimferrer, S. Danés, E. Vos, C. B. Yildiz, I. Corral, A. Jana, P. Salvador and D. M. Andrada, Chem. Sci. 2022, 13, 6583. Chem. Sci. 14, 379–383 (2023).
Gimferrer, M. et al. Reply to the ‘Comment on ‘The oxidation state in low-valent beryllium and magnesium compounds’ by S. Pan and G. Frenking, Chem. Sci., 2022, 13. Chem. Sci. 14, 384–392 (2023).
Kan, Y. H. Covalent or not? Energy decomposition analysis of metal–metal bonding in alkaline-earth dimetallocene complexes. J. Mol. Struct. THEOCHEM 894, 88–92 (2009).
Boronski, J. T., Crumpton, A. E., Wales, L. L. & Aldridge, S. Diberyllocene, a stable compound of Be(I) with a Be–Be bond. Science 380, 1147–1149 (2023).
Rösch, B. et al. Dinitrogen complexation and reduction at low-valent calcium. Science 371, 1125–1128 (2021).
Rösch, B. et al. Strong reducing magnesium(0) complexes. Nature 592, 717–721 (2021).
Klett, J. Structural motifs of alkali metal superbases in non-coordinating solvents. Chem. Eur. J. 27, 888–904 (2021).
Kremer, T., Harder, S., Junge, M. & Schleyer, P. V. R. Mixed aggregates of alkali metal compounds: structure and stability of ‘superbase’ models. Organometallics 15, 585–595 (1996).
Mullins, J. C. et al. C–H activation of inert arenes using a photochemically activated guanidinato-magnesium(I) compound. Chem. Eur. J. 28, e202202103 (2022).
Causero, A. et al. β-Diketiminate calcium hydride complexes: the importance of solvent effects. Dalton Trans. 46, 1822–1831 (2017).
Bonyhady, S. J. et al. β-Diketiminate-stabilized magnesium(I) dimers and magnesium(II)hydride complexes: synthesis, characterization, adduct formation, and reactivity studies. Chemistry 16, 938–955 (2010).
Liu, H.-Y. et al. Reductive dimerization of CO by a Na/Mg(I) diamide. J. Am. Soc. 143, 17851–17856 (2021).
Friedrich, A., Eyselein, J., Langer, J., Färber, C. & Harder, S. Cationic heterobimetallic Mg(Zn)/Al(Ga) combinations for cooperative C–F bond cleavage. Angew. Chem. Int. Ed. 60, 16492–16499 (2021).
Knizek, J., Krossing, I., Nöth, H., Schwenk, H. & Seifert, T. Synthesis and structures of sodium phenylhydrazides. Chem. Ber. 130, 1053–1062 (1997).
Camp, C. & Arnold, J. On the non-innocence of ‘Nacnacs’: ligand-based reactivity in β-diketiminate supported coordination compounds. Dalton Trans. 45, 14462–14498 (2016).
Postils, V., Delgado-Alonso, C., Luis, J. M. & Salvador, P. An objective alternative to IUPAC’s approach to assign oxidation states. Angew. Chem. Int. Ed. 57, 10525–10529 (2018).
Mackenzie, F. M. & Mulvey, R. E. The first trimetallic lithium–sodium–potassium complex: synthesis and crystal structure of a twelve-vertex Li2Na2K2N4O2 cage molecule containing an amide-alkoxide combination. J. Am. Chem. Soc. 118, 4721–4722 (1996).
Wei, X. et al. Heterotrimetallic salts: synthesis, structures, and superbase reactivity of crystalline tert-butoxides [Li4Na2K2(OtBu)8(μ-L)]n. Angew. Chem. Int. Ed. 47, 3976–3978 (2008).
Gentner, T. X., Kennedy, A. R., Hevia, E. & Mulvey, R. E. Alkali metal (Li, Na, K, Rb, Cs) mediation in magnesium hexamethyldisilazide [Mg(HMDS)2] catalysed transfer hydrogenation of alkenes. Chem. Cat. Chem. 13, 2371–2378 (2021).
Zhang, X.-Y., Du, H.-Z., Zhai, D.-D. & Guan, B.-T. Combined KH/alkaline-earth metal amide catalysts for hydrogenation of alkenes. Org. Chem. Front. 7, 1991–1996 (2020).
Mulvey, R. E. Modern ate chemistry: applications of synergic mixed alkali-metal–magnesium or –zinc reagents in synthesis and structure building. Organometallics 25, 1060–1075 (2006).
Sarazin, Y., Howard, R. H., Hughes, D. L., Humphrey, S. M. & Bochmann, M. Titanium, zinc and alkaline-earth metal complexes supported by bulky O,N,N,O-multidentate ligands: syntheses, characterisation and activity in cyclic esterpolymerisation. Dalton Trans. 14, 340–350 (2006).
Westerhausen, M. Synthesis and spectroscopic properties of bis(trimethyIsily)amides of the alkaline-earth metals magnesium, calcium, strontium, and barium. Inorg. Chem. 30, 96–101 (1991).
Rigaku Oxford Diffraction, CrysAlisPro Software system, version 1.171.40.84a, Rigaku Corporation, Oxford, UK (compound (BDI)MgNa/CaN″2(1)) (Rigaku, 2020).
Rigaku Oxford Diffraction, CrysAlisPro Software system, version 1.171.41.113a, Rigaku Corporation, Oxford, UK (compound (BDI)MgNa/SrN″2(2); (BDI)MgNa/MgN″2(4); (BDI)MgMgN″(5)) (Rigaku, 2021).
Rigaku Oxford Diffraction CrysAlisPro Software system, version 1.171.42.72a, Rigaku Corporation, Oxford, UK (other compounds) (Rigaku, 2022).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009).
Sheldrick, G. M. SHELXT—integrated space-group and crystal-structure determination. Acta Cryst. A 71, 3–8 (2015).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Cryst. C 71, 3–8 (2015).
Frisch, M. J. et al. Gaussian 16 Rev. A.03 (Gaussian, 2016).
Becke, A. D. Density - functional thermochemistry. III. The role of exact change. J. Chem. Phys. 98, 5648–5652 (1993).
Perdew, J. P. et al. Erratum: atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 48, 4978–4978 (1993).
Weigend, F. Accurate coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 8, 1057–1065 (2006).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Glendening, E. D. et al. NBO 7.0 (University of Wisconsin, 2018).
Bader, R. F. W. A quantum theory of molecular structure and its applications. Chem. Rev. 91, 893–928 (1991).
Keith, T. A. AIMAll (Version 17.01.25) (TK Gristmill Software, 2017).
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (DFG) for funding (HA 3218/11-1).
Author information
Authors and Affiliations
Contributions
J. Mai: conceptualization, investigation, validation, formal analysis, writing—original draft, and visualization. J. Maurer: conceptualization, investigation, validation, formal analysis, writing—original draft, and visualization. J.L.: investigation, formal analysis and validation. S.H.: conceptualization, writing—original draft, review and editing, visualization, validation, supervision and project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details and Supplementary Figs. 1–74 and Tables 1–9.
Supplementary Data 1
Crystallographic data for compound 1, CCDC 2269821.
Supplementary Data 2
Crystallographic data for compound 2, CCDC 2269822.
Supplementary Data 3
Crystallographic data for compound 4, CCDC 2269823.
Supplementary Data 4
Crystallographic data for compound 5, CCDC 2269824.
Supplementary Data 5
Crystallographic data for compound 6.
Supplementary Data 6
Crystallographic data for compound 7, CCDC 2269631.
Supplementary Data 7
Crystallographic data for compound 8, CCDC 2269632.
Supplementary Data 8
Crystallographic data for compound 9, CCDC 2269633.
Supplementary Data 9
Crystallographic data for compound 10, CCDC 2269634.
Supplementary Data 10
Crystallographic data for compound Na(MgN″3), CCDC 2269648.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mai, J., Maurer, J., Langer, J. et al. Heterobimetallic alkaline earth metal–metal bonding. Nat. Synth 3, 368–377 (2024). https://doi.org/10.1038/s44160-023-00451-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00451-y