Abstract
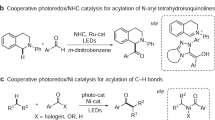
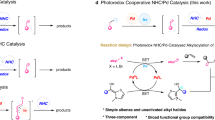
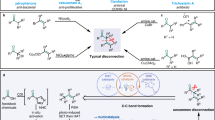
meta-Selective functionalization of electron-rich arenes provides a complementary route to that of traditional organic synthesis. In classical electrophilic aromatic substitution reactions of electron-donating group-pendant arenes, C–H functionalization occurs at the ortho- or para-positions. There have been numerous efforts to overcome this selectivity, and various synthetic methods have been developed, typically using transition metal catalysis. Here we report a combined N-heterocyclic carbene- and organic photoredox-catalysed method for meta-selective acylation of electron-rich arenes, using acyl imidazoles as acylating reagents. This approach proceeds without directing groups or steric factors required in transition metal-catalysed processes, resulting in the opposite regioselectivity to conventional approaches such as Friedel–Crafts acylation. Mechanistic studies reveal the process involves a sequence of single-electron oxidation of an electron-rich arene followed by the radical–radical coupling between a ketyl radical and an arene radical cation.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Details on the procedures, optimization and characterization, including spectra of new compounds and compounds made using the reported method, are available in Supplementary Information.
References
Friedel, C. & Crafts, J.-M. Sur une méthode générale nouvelle de synthèse d’hydrocarbures d’acetones. Compt. Rend. 84, 1450–1454 (1877).
Crafts, J. M. & Ador, E. Ueber die Einwirkung des Chlorkohlenoxyds auf Toluol in Gegenwart von Chloraluminium. Ber. Dtsch. Chem. Ges. 10, 2173–2176 (1877).
Leitch, J. A. & Frost, C. G. Ruthenium-catalysed σ-activation for remote meta-selective C–H functionalisation. Chem. Soc. Rev. 46, 7145–7153 (2017).
Gandeepan, P. & Ackermann, L. Transient directing groups for transformative C–H activation by synergistic metal catalysis. Chem 4, 199–222 (2018).
Mihai, M. T., Genov, G. R. & Phipps, R. J. Access to the meta position of arenes through transition metal catalysed C–H bond functionalisation: a focus on metals other than palladium. Chem. Soc. Rev. 47, 149–171 (2018).
Dey, A., Sinha, S. K., Achar, T. K. & Maiti, D. Accessing remote meta‐ and para‐C(sp2)−H bonds with covalently attached directing groups. Angew. Chem. Int. Ed. 58, 10820–10843 (2019).
Phipps, R. J. & Gaunt, M. J. A meta-selective copper-catalysed C–H bond arylation. Science 323, 1593–1597 (2009).
Leow, D., Li, G., Mei, T.-S. & Yu, J.-Q. Activation of remote meta-C–H bonds assisted by an end-on template. Nature 486, 518–522 (2012).
Wang, X.-C. et al. Ligand-enabled meta-C–H activation using a transient mediator. Nature 519, 334–338 (2015).
Kuninobu, Y., Ida, H., Nishi, M. & Kanai, M. A meta-selective C–H borylation directed by a secondary interaction between ligand and substrate. Nat. Chem. 7, 712–717 (2015).
Shi, H., Herron, A. N., Shao, Y., Shao, Q. & Yu, J.-Q. Enantioselective remote meta-C–H arylation and alkylation via a chiral transient mediator. Nature 558, 581–585 (2018).
Ramadoss, B., Jin, Y., Asako, S. & Ilies, L. Remote steric control for undirected meta-selective C–H activation of arenes. Science 375, 658–663 (2022).
Cao, H., Cheng, Q. & Studer, A. Radical and ionic meta-C–H functionalization of pyridines, quinolines, and isoquinolines. Science 378, 779–785 (2022).
Ishii, T., Nagao, K. & Ohmiya, H. Recent advances in N-heterocyclic carbene-based radical catalysis. Chem. Sci. 11, 5630–5636 (2020).
Ohmiya, H. N-heterocyclic carbene-based catalysis enabling cross-coupling reactions. ACS Catal. 10, 6862–6869 (2020).
Liu, K., Schwenzer, M. & Studer, A. Radical NHC catalysis. ACS Catal. 12, 11984–11999 (2022).
Bay, A. V. & Scheidt, K. A. Single-electron carbene catalysis in redox processes. Trends Chem. 4, 277–290 (2022).
Ishii, T., Kakeno, Y., Nagao, K. & Ohmiya, H. N-heterocyclic carbene-catalysed decarboxylative alkylation of aldehydes. J. Am. Chem. Soc. 141, 3854–3858 (2019).
Ishii, T., Ota, K., Nagao, K. & Ohmiya, H. N-heterocyclic carbene-catalysed radical relay enabling vicinal alkylacylation of alkenes. J. Am. Chem. Soc. 141, 14073–14077 (2019).
Davies, A. V., Fitzpatrick, K. P., Betori, R. C. & Scheidt, K. A. Combined photoredox and carbene catalysis for the synthesis of ketones from carboxylic acids. Angew. Chem. Int. Ed. 59, 9143–9148 (2020).
Kakeno, Y., Kusakabe, M., Nagao, K. & Ohmiya, H. Direct synthesis of dialkyl ketones from aliphatic aldehydes through radical N-heterocyclic carbene catalysis. ACS Catal. 10, 8524–8529 (2020).
Zuo, Z., Daniliuc, C. G. & Studer, A. Cooperative NHC/photoredox catalysed ring‐opening of aryl cyclopropanes to 1‐aroyloxylated‐3‐acylated alkanes. Angew. Chem. Int. Ed. 60, 25252–25257 (2021).
Matsuki, Y. et al. Aryl radical-mediated N-heterocyclic carbene catalysis. Nat. Commun. https://doi.org/10.1038/s41467-021-24144-2 (2021).
Liu, K. & Studer, A. Direct α-acylation of alkenes via N-heterocyclic carbene, sulfinate, and photoredox cooperative triple catalysis. J. Am. Chem. Soc. 143, 4903–4909 (2021).
Meng, Q.-Y., Lezius, L. & Studer, A. Benzylic C−H acylation by cooperative NHC and photoredox catalysis. Nat. Commun. https://doi.org/10.1038/s41467-021-22292-z (2021).
Sato, Y. et al. Light-driven N-heterocyclic carbene catalysis using alkylborates. ACS Catal. https://doi.org/10.1021/acscatal.1c04153 (2021).
Ren, S.-C. et al. Carbene and photocatalyst-catalyzed decarboxylative radical coupling of carboxylic acids and acyl imidazoles to form ketones. Nat. Commun. https://doi.org/10.1038/s41467-022-30583-2 (2022).
Bay, A. V., Farnam, E. J. & Scheidt, K. A. Synthesis of cyclohexanones by a tandem photocatalyzed annulation. J. Am. Chem. Soc. 144, 7030–7037 (2022).
Yu, X., Meng, Q. Y., Daniliuc, C. G. & Studer, A. Aroyl fluorides as bifunctional reagents for dearomatizing fluoroaroylation of benzofurans. J. Am. Chem. Soc. https://doi.org/10.1021/jacs.2c01735 (2022).
Han, Y.-F. et al. Photoredox cooperative N-heterocyclic carbene/palladium-catalysed alkylacylation of alkenes. Nat. Commun. 13, 5754 (2022).
Yang, W., Hu, W., Dong, X., Li, X. & Sun, J. N-heterocyclic carbene catalyzed γ-dihalomethylenation of enals by single-electron transfer. Angew. Chem. Int. Ed. 55, 15783–15786 (2016).
DiRocco, D. A. & Rovis, T. Catalytic asymmetric α-acylation of tertiary amines mediated by a dual catalysis mode: N-heterocyclic carbene and photoredox catalysis. J. Am. Chem. Soc. 134, 8094–8097 (2012).
Leifert, D. & Studer, A. The persistent radical effect in organic synthesis. Angew. Chem. Int. Ed. 59, 74–108 (2020).
Ohkubo, K., Mizushima, K., Iwata, R. & Fukuzumi, S. Selective photocatalytic aerobic bromination with hydrogen bromidevia an electron-transfer state of 9-mesityl-10-methylacridinium ion. Chem. Sci. 2, 715–722 (2011).
Romero, N. A., Margrey, K. A., Tay, N. E. & Nicewicz, D. A. Site-selective arene C–H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Fukuzumi, S. et al. Electron-transfer state of 9-mesityl-10-methylacridinium ion with a much longer lifetime and higher energy than that of the natural photosynthetic reaction center. J. Am. Chem. Soc. 126, 1600–1601 (2004).
Margrey, K. A., McManus, J. B., Bonazzi, S., Zecri, F. & Nicewicz, D. A. Predictive model for site-selective aryl and heteroaryl C–H functionalisation via organic photoredox catalysis. J. Am. Chem. Soc. 139, 11288–11299 (2017).
Yan, H., Song, J., Zhu, S. & Xu, H.-C. Synthesis of acridinium photocatalysts via site-selective C–H alkylation. CCS Chem. 3, 317–325 (2021).
Truong, C. C., Kim, J., Lee, Y. & Kim, Y. J. Well-defined cesium benzotriazolide as an active catalyst for generating disubstituted ureas from carbon dioxide and amines. ChemCatChem 9, 247–252 (2017).
Ji, P. et al. Selective skeletal editing of polycyclic arenes using organophotoredox dearomative functionalization. Nat. Commun. https://doi.org/10.1038/s41467-022-32201-7 (2022).
Acknowledgements
This work was supported by JSPS KAKENHI grant numbers JP21H04681, JP23H04912 and JST, PRESTO grant number JPMJPR19T2 to H.O. We thank K. Nagao (Kyoto University) for helpful discussion about computational study.
Author information
Authors and Affiliations
Contributions
Y.G., M.S., Y.S. and H.O. designed, performed and analysed the experiments. Y.S. and H.O. co-wrote the paper. All authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Wei Wang, Song Ye and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11 and Tables 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goto, Y., Sano, M., Sumida, Y. et al. N-heterocyclic carbene- and organic photoredox-catalysed meta-selective acylation of electron-rich arenes. Nat. Synth 2, 1037–1045 (2023). https://doi.org/10.1038/s44160-023-00378-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00378-4
This article is cited by
-
Carbene- and photocatalysis redefine arene acylation
Nature Synthesis (2023)