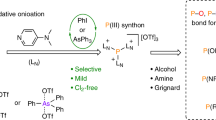
Abstract
Phosphorus and its compounds are a cornerstone of biological life, as well as finding applications in material science, for example, as flame retardants, battery electrolytes or catalysts. In most applications, phosphorus-containing fine chemicals are in the naturally occurring and most stable oxidation state +V of the element; however, syntheses to these compounds from primary PV sources rely on an energy-consuming and wasteful redox detour via elemental white phosphorus (P4) and its subsequent (oxy)chlorination to PCl3, PCl5 and POCl3. Here we report an approach using trifluoromethanesulfonic anhydride (Tf2O) and pyridine to directly cleave P–O bonds in ubiquitous PV sources to form the versatile PO2+ phosphorylation agent (pyridine)2PO2[OTf] (1[OTf]), whose preparation and mechanism of formation is discussed. Harnessing its reactivity towards various nucleophiles such as amines, alcohols and pseudohalogenides, 1[OTf] then provides redox-neutral access to a range of value-added PV chemicals downstream of low-cost phosphoric acid or other phosphate sources.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions of this work are present in the manuscript and/or in Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2232178 (1[OTf]), 2232179 (4), 2232180 (5a[OTf]), 2232181 (5b[OTf]), 2232182 (6[OTf]), 2232183 ([Na]8a), 2232184 ([Na]8b), 2232185 ([Na]8c), 2232186 (9), 2232187 (15), 2232188 (17a) and 2250333 (17b). Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.
Code availability
The code is not available.
References
Jupp, A. R., Beijer, S., Narain, G. C., Schipper, W. & Slootweg, J. C. Phosphorus recovery and recycling—closing the loop. Chem. Soc. Rev. 50, 87–101 (2021).
Beijer, S. & Slootweg, J. C. Encyclopedia of Inorganic and Bioinorganic Chemistry (Wiley, 2021).
Gilmour, R. Phosphoric Acid—Purification, Uses, Technology and Economics (Taylor & Francis Group, LLC, 2014).
Schipper, W. Phosphorus: too big to fail. Eur. J. Inorg. Chem. https://doi.org/10.1002/ejic.201400115 (2014).
Withers, P. J. A. et al. Greening the global phosphorus cycle: how green chemistry can help achieve planetary P sustainability. Green Chem. 17, 2087–2099 (2015).
Scholz, R. W., Roy, A. H., Brand, F. S., Hellums, D. T. & Ulrich, A. E. Sustainable Phosphorus Management (Springer, 2014).
Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability (European Commission, 2020).
Diskowski, H. & Hofmann, T. Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, 2000).
Toxicological Profile for White Phosphorous (Agency for Toxic Substances and Disease Registry, 1997).
Emsley, J. Shocking History of Phosphorus: A Biography of the Devil’s Element (MacMillan, 2001).
Bettermann, G., Krause, W., Riess, G. & Hofmann, T. Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, 2000).
Svara, J., Weferling, N. & Hofmann, T. Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, 2006).
Scheer, M., Balázs, G. & Seitz, A. P4 activation by main group elements and compounds. Chem. Rev. 110, 4236–4256 (2010).
Cossairt, B. M., Piro, N. A. & Cummins, C. C. Early-transition-metal-mediated activation and transformation of white phosphorus. Chem. Rev. 110, 4164–4177 (2010).
Caporali, M., Gonsalvi, L., Rossin, A. & Peruzzini, M. P4 activation by late-transition metal complexes. Chem. Rev. 110, 4178–4235 (2010).
Hoidn, C. M., Scott, D. J. & Wolf, R. Transition-metal-mediated functionalization of white phosphorus. Chem. Eur. J. 27, 1886–1902 (2021).
Giusti, L. et al. Coordination chemistry of elemental phosphorus. Coord. Chem. Rev. 441, 213927 (2021).
Grützmacher, H. White phosphorus first. Nat. Chem. 14, 362–364 (2022).
Scott, D. J. Recent breakthroughs in P4 chemistry: towards practical direct transformations into P1 compounds. Angew. Chem. Int. Ed. 61, e202205019 (2022).
Geeson, M. B. & Cummins, C. C. Let’s make white phosphorus obsolete. ACS Cent. Sci. 6, 848–860 (2020).
Geeson, M. B. & Cummins, C. C. Phosphoric acid as a precursor to chemicals traditionally synthesized from white phosphorus. Science 359, 1383–1385 (2018).
Geeson, M. B., Ríos, P., Transue, W. J. & Cummins, C. C. Orthophosphate and sulfate utilization for C-E (E = P, S) bond formation via trichlorosilyl phosphide and sulfide anions. J. Am. Chem. Soc. 141, 6375–6384 (2019).
Geeson, M. B., Tanaka, K., Taakili, R., Benhida, R. & Cummins, C. C. Photochemical alkene hydrophosphination with bis(trichlorosilyl)phosphine. J. Am. Chem. Soc. 144, 14452–14457 (2022).
Zhai, F., Xin, T., Geeson, M. B. & Cummins, C. C. Sustainable production of reduced phosphorus compounds: mechanochemical hydride phosphorylation using condensed phosphates as a route to phosphite. ACS Cent. Sci. 8, 332–339 (2022).
Dueymes, C., Pirat, C. & Pascal, R. Facile synthesis of simple mono-alkyl phosphates from phosphoric acid and alcohols. Tetrahedron Lett. 49, 5300–5301 (2008).
Lira, L. M., Vasilev, D., Pilli, R. A. & Wessjohann, L. A. One-pot synthesis of organophosphate monoesters from alcohols. Tetrahedron Lett. 54, 1690–1692 (2013).
Sakakura, A., Katsukawa, M. & Ishihara, K. Selective synthesis of phosphate monoesters by dehydrative condensation of phosphoric acid and alcohols promoted by nucleophilic bases. Org. Lett. 7, 1999–2002 (2005).
Sakakura, A., Katsukawa, M. & Ishihara, K. The oxorhenium(VII)-catalyzed direct condensation of phosphoric acid with an alcohol. Angew. Chem. Int. Ed. 46, 1423–1426 (2007).
Sakakura, A., Katsukawa, M., Hayashi, T. & Ishihara, K. Bulky phosphazenium cation catalysis for dehydrative condensation of phosphoric acid with alcohols. Green Chem. 9, 1166 (2007).
Knouse, K. W. et al. Unlocking PV: reagents for chiral phosphorothioate synthesis. Science 361, 1234–1238 (2018).
Huang, Y. et al. A PV platform for oligonucleotide synthesis. Science 373, 1265–1270 (2021).
Donath, M. et al. Direct conversion of white phosphorus to versatile phosphorus transfer reagents via oxidative onioation. Nat. Chem. 14, 384–391 (2022).
Smyth, T. P. & Corby, B. W. Toward a clean alternative to Friedel–Crafts acylation: in situ formation, observation, and reaction of an acyl bis(trifluoroacetyl)phosphate and related structures. J. Org. Chem. 63, 8946–8951 (1998).
Qian, K., Shepard, S. M., Xin, T., Park, G. & Cummins, C. C. Stabilized molecular diphosphorus pentoxide, P2O5L2 (L = N-donor base), in the synthesis of condensed phosphate–organic molecule conjugates. J. Am. Chem. Soc. 145, 6045–6050 (2023).
Rovnaník, P., Kapička, L., Taraba, J. & Černík, M. Base-induced dismutation of POCl3 and POBr3: synthesis and structure of ligand-stabilized dioxophosphonium cations. Inorg. Chem. 43, 2435–2442 (2004).
Weiss, R. & Engel, S. Electrostatic activation of nucleofuges: cationic leaving groups. Angew. Chem. Int. Ed. 31, 216–217 (1992).
Jessen, H. J., Dürr-Mayer, T., Haas, T. M., Ripp, A. & Cummins, C. C. Lost in condensation: poly-, cyclo-, and ultraphosphates. Acc. Chem. Res. 54, 4036–4050 (2021).
Wolf, G.-U. & Meisel, M. Zur Existenz eines Diphosphorsäure-bis(pyrididiumbetains), Py2 · P2O5. Z. Chem. 22, 54 (1982).
Meisel, M., Bock, H., Solouki, B. & Kremer, M. Erzeugung und Ionisationsmuster der iso(valenz)elektronischen Verbindungen ClP(=O)2 und ClP(=S)2. Angew. Chem. 101, 1378–1381 (1989).
Pham Minh, D., Ramaroson, J., Nzihou, A. & Sharrock, P. One-step synthesis of sodium trimetaphosphate (Na3P3O9) from sodium chloride and orthophosphoric acid. Ind. Eng. Chem. Res. 51, 3851–3854 (2012).
Gibard, C., Bhowmik, S., Karki, M., Kim, E. K. & Krishnamurthy, R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 10, 212–217 (2018).
Yadav, M. & Krishnamurthy, R. Bis(dimethylamino)phosphorodiamidate: a reagent for the regioselective cyclophosphorylation of cis-diols enabling one-step access to high-value target cyclophosphates. Org. Lett. 21, 7400–7404 (2019).
Malone, J. A., Toussel, C. E., Fronczek, F. R. & Kartika, R. Brønsted acid-catalyzed formal [2 + 2 + 1] annulation for the modular synthesis of tetrahydroindoles and tetrahydrocyclopenta[b]pyrroles. Org. Lett. 21, 3610–3614 (2019).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Zheng, B., Fox, R. J., Sugiyama, M., Fritz, A. & Eastgate, M. D. Development of efficient processes for the preparation of di-tert-butyl potassium phosphate and di-tert-butyl (chloromethyl) phosphate. Org. Process Res. Dev. 18, 636–642 (2014).
Wilson, A. M. et al. Solvent extraction: the coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 43, 123–134 (2014).
Milien, M. S. et al. Lithium bis(2,2,2-trifluoroethyl)phosphate Li[O2P(OCH2CF3)2]: a high voltage additive for LNMO/graphite cells. J. Electrochem. Soc. 165, A2569–A2576 (2018).
Zeisig, R., Ress, A., Fichtner, I. & Walther, W. Lipoplexes with alkylphospholipid as new helper lipid for efficient in vitro and in vivo gene transfer in tumor therapy. Cancer Gene Ther. 10, 302–311 (2003).
Koehler, J. et al. Lysophospholipid micelles sustain the stability and catalytic activity of diacylglycerol kinase in the absence of lipids. Biochemistry 49, 7089–7099 (2010).
Ichihara, K. et al. Synthesis of phosphatidylcholine: an improved method without using the cadmium chloride complex of sn-glycero-3-phosphocholine. Chem. Phys. Lipids 137, 94–99 (2005).
Huang, H., Denne, J., Yang, C., Wang, H. & Kang, J. Y. Direct aryloxylation/alkyloxylation of dialkyl phosphonates for the synthesis of mixed phosphonates. Angew. Chem. Int. Ed. 57, 6624–6628 (2018).
Huang, H., Ash, J. & Kang, J. Y. Tf2O-promoted activating strategy of phosphate analogues: synthesis of mixed phosphates and phosphinate. Org. Lett. 20, 4938–4941 (2018).
Ash, J., Huang, H. & Kang, J. Y. Metal- and chloride reagent-free synthesis of mixed thiophosphates. Org. Biomol. Chem. 17, 3812–3818 (2019).
Hallett, J. P. & Welton, T. Room-temperature ionic liquids: solvents for synthesis and catalysis. 2. Chem. Rev. 111, 3508–3576 (2011).
Toscano, R. A. et al. Nucleophilic reactions on 1-trifluoromethanesulfonylpyridinium trifluoromethanesulfonate (triflypyridinium triflate, TPT). Ring-opening and ‘unexpected’ 1,4-dihydropyridine reaction products. Chem. Pharm. Bull. 45, 957–961 (1997).
Yogendra, S. et al. Carbodiphosphorane mediated synthesis of a triflyloxyphosphonium dication and its reactivity towards nucleophiles. Chem. Commun. 53, 2954–2957 (2017).
Basnar, B. et al. Layer-by-layer assembly of titania nanoparticles based ionic networks. Chem. Commun. 47, 361–363 (2011).
Acknowledgements
We thank the European Research Council (ERC starting grant, SynPhos-307616), the German Science foundation (WE 4621/6-1) and TU Dresden for financial support. T.S. thanks the Studienstiftung des Deutschen Volkes for a doctoral fellowship. R. Schoemaker and S. Schulz are gratefully acknowledged for their help and discussion regarding this project. We thank P. Lange for experimental assistance and elemental analysis (EA) measurements and U. Schwarzenbolz for high resolution mass spectrometry (HRMS) measurements. Solvay Chemicals is gratefully acknowledged for their donation of the chemicals Me3SiOTf and Tf2O.
Author information
Authors and Affiliations
Contributions
T.S., K.S. and J.J.W. conceptualized the deoxygenation procedure, optimized the reaction conditions and performed mechanistic studies. T.S. and K.S. optimized the synthesis, isolation and purification of products from the phosphorylation reagent. J.F. and J.J.W. were responsible for collecting X-ray data and refinement. K.S. and J.J.W. conceived, oversaw and directed the project. J.J.W. was responsible for funding acquisition. T.S., K.S. and J.J.W. prepared the initial draft of the paper. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
Two patent applications covering the content of this work have been submitted by the TU Dresden (EP 21209296.9 and DE 102022120599.1). The authors declare that they have no other competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Molecular structures of (a) 1+ in 1[OTf] ⋅ 0.5 Py and (b) 4.
Thermal ellipsoids are displayed at 50 % probability level. Hydrogen atoms, solvent molecules and triflate anions are omitted for clarity.
Extended Data Fig. 2 31P NMR spectra (pyridine-d5, 320 K) of the reaction solution of H3PO4 with 2.2 eq. of TfPy[OTf] after varying reaction times.
The ongoing deoxygenation of intermediate 4 (δ(31P) = –17.5 ppm) to phosphinate 1+ (δ(31P) = –15.2 ppm) is observed.
Extended Data Fig. 3 31P{1H} NMR spectra (CH3NO2, C6D6-capillary, 300 K) of the reaction of H3PO4 with different amounts of TfPy[OTf] (1.00 eq., 1.25 eq., 1.50 eq., 1.75 eq.).
Several intermediates were identified by their characteristic signal patterns known to the literature37. PO43−: δ(31P) = 2.4 ppm (s); P2O74−: δ(31P) = – 8.4 ppm (s); P3O93−: δ(31P) = – 20.0 ppm (s); P4O124−: δ(31P) = –21.8 ppm (s); P4O112−: A2X2-spinsystem, δ(A) = – 36.9 ppm (t), δ(X) = – 26.3 ppm (t), 2JPP = 25.5 Hz; P4O136−: AX3-spinsystem, δ(A) = – 33.3 ppm (q), δ(X) = – 23.0 ppm (d), 2JPP = 21.9 Hz; 32−: AX-spinsystem, δ(A) = – 19.3 ppm (d), δ(X) = – 16.8 ppm (d), 2JPP = 18.2 Hz; 4: δ(31P) = – 17.3 ppm (s). Signals of higher, asymmetrically condensed phosphates which could not be assigned a structure are marked with asterisks (*).
Extended Data Fig. 4 Molecular structures of (a) 5a+ in 5a[OTf] ⋅ CH2Cl2; (b) 5b+ in 5b[OTf] and (c) 6+ in 6[OTf].
Thermal ellipsoids are displayed at 50 % probability level. Hydrogen atoms, solvent molecules and triflate anions are omitted for clarity.
Extended Data Fig. 5 (a) Section of the network structure of 17a ⋅ NaOTf ⋅ CH3CN and (b) molecular structure of 17b in 17b ⋅ CH3CN.
Thermal ellipsoids are displayed at 50 % probability level. Hydrogen atoms and non-coordinating solvent molecules are omitted for clarity.
Supplementary information
Supplementary Information
Supplementary materials and methods, text, Figs. 1–146, Tables 1–4 and References 1–19.
Supplementary Data 1
Crystal data of 1, CCDC 2232178.
Supplementary Data 2
Crystal data of 4, CCDC 2232179.
Supplementary Data 3
Crystal data of 5a, CCDC 2232180.
Supplementary Data 4
Crystal data of 5b, CCDC 2232181.
Supplementary Data 5
Crystal data of 6, CCDC 2232182.
Supplementary Data 6
Crystal data of 8a, CCDC 2232183.
Supplementary Data 7
Crystal data of 8b, CCDC 2232184.
Supplementary Data 8
Crystal data of 8c, CCDC 2232185.
Supplementary Data 9
Crystal data of 9, CCDC 2232186.
Supplementary Data 10
Crystal data of 15, CCDC 2232187.
Supplementary Data 11
Crystal data of 17a, CCDC 2232188.
Supplementary Data 12
Crystal data of 17b, CCDC 2250333.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schneider, T., Schwedtmann, K., Fidelius, J. et al. Redox-neutral conversion of ubiquitous PV sources to a versatile PO2+ phosphorylation reagent. Nat. Synth 2, 972–979 (2023). https://doi.org/10.1038/s44160-023-00344-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00344-0
This article is cited by
-
A white phosphorus workaround
Nature Synthesis (2023)