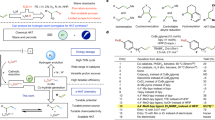
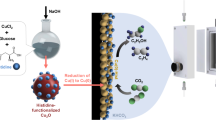
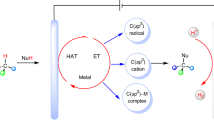
Abstract
The formation of C–S bonds is an important step in the synthesis of pharmaceutical, biological and chemical products. Electrocatalysis using abundant precursors is an attractive and green route to C–S bond-containing species, but examples within this context are largely absent from the literature. To this end, this work demonstrates the use of CO2 and SO32− as cheap building blocks that couple on the surface Cu-based heterogeneous catalysts. Hydroxymethanesulfonate, sulfoacetate and methanesulfonate are formed, with Faradaic efficiencies of up to 6.8%. A combination of operando measurements and computational modelling reveal that the *CHOH intermediate formed on metallic Cu is a key electrophilic species that is nucleophilically attacked by SO32− in the principal C–S bond-forming step. The proof-of-concept for electrocatalytic C–S bond formation and mechanistic insights gained will broaden the scope of the emerging field of electrosynthesis.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data will be deposited in ChemRxiv, a publicly accessible repository (https://chemrxiv.org/engage/chemrxiv/article-details/6397d9a0cfb5ff102468e064).
References
Lagadec, M. F. & Grimaud, A. Water electrolysers with closed and open electrochemical systems. Nat. Mater. 19, 1140–1150 (2020).
Ross, M. B. et al. Designing materials for electrochemical carbon dioxide recycling. Nat. Catal. 2, 648–658 (2019).
Masel, R. I. et al. An industrial perspective on catalysts for low-temperature CO2 electrolysis. Nat. Nanotechnol. 16, 118–128 (2021).
Zhang, Y., Li, J. & Kornienko, N. Strategies for heterogeneous small-molecule electrosynthesis. Cell Rep. Phys. Sci. 2, 100682 (2021).
Perry, S. C. et al. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 3, 442–458 (2019).
Suryanto, B. H. R. et al. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2, 290–296 (2019).
Li, J., Zhang, Y., Kuruvinashetti, K. & Kornienko, N. Construction of C–N bonds from small-molecule precursors through heterogeneous electrocatalysis. Nat. Rev. Chem. 6, 303–319 (2022).
Fontecave, M., Ollagnier-de-Choudens, S. & Mulliez, E. Biological radical sulfur insertion reactions. Chem. Rev. 103, 2149–2166 (2003).
Mansy, S. S. & Cowan, J. A. Iron–sulfur cluster biosynthesis: toward an understanding of cellular machinery and molecular mechanism. Acc. Chem. Res. 37, 719–725 (2004).
Zhao, C., Rakesh, K. P., Ravidar, L., Fang, W.-Y. & Qin, H.-L. Pharmaceutical and medicinal significance of sulfur (SVI)-containing motifs for drug discovery: a critical review. Eur. J. Med. Chem. 162, 679–734 (2019).
Devendar, P. & Yang, G.-F. in Sulfur Chemistry (ed. Jiang, X.) 35–78 (Springer International Publishing, 2019).
Rosenman, A. et al. Review on Li-sulfur battery systems: an integral perspective. Adv. Energy Mater. 5, 1500212 (2015).
Barbarella, G., Melucci, M. & Sotgiu, G. The versatile thiophene: an overview of recent research on thiophene-based materials. Adv. Mater. 17, 1581–1593 (2005).
Sakakura, A., Yamada, H. & Ishihara, K. Enantioselective Diels–Alder reaction of α-(acylthio)acroleins: a new entry to sulfur-containing chiral quaternary carbons. Org. Lett. 14, 2972–2975 (2012).
Wu, X.-S., Chen, Y., Li, M.-B., Zhou, M.-G. & Tian, S.-K. Direct substitution of primary allylic amines with sulfinate salts. J. Am. Chem. Soc. 134, 14694–14697 (2012).
Mukhopadhyay, S. & Bell, A. T. Catalyzed sulfonation of methane to methanesulfonic acid. J. Mol. Catal. A: Chem. 211, 59–65 (2004).
Uraguchi, D., Kinoshita, N., Nakashima, D. & Ooi, T. Chiral ionic Brønsted acid–achiral Brønsted base synergistic catalysis for asymmetric sulfa-Michael addition to nitroolefins. Chem. Sci. 3, 3161–3164 (2012).
Savateev, A., Kurpil, B., Mishchenko, A., Zhang, G. & Antonietti, M. A “waiting” carbon nitride radical anion: a charge storage material and key intermediate in direct C–H thiolation of methylarenes using elemental sulfur as the “S”-source. Chem. Sci. 9, 3584–3591 (2018).
Su, F. et al. Aerobic oxidative coupling of amines by carbon nitride photocatalysis with visible light. Angew. Chem. Int. Ed. 50, 657–660 (2011).
Britschgi, J., Kersten, W., Waldvogel, S. R. & Schüth, F. Electrochemically initiated synthesis of methanesulfonic acid. Angew. Chem. Int. Ed. 61, e202209591 (2022).
Li, J. & Kornienko, N. Electrochemically driven C–N bond formation from CO2 and ammonia at the triple-phase boundary. Chem. Sci. 13, 3957–3964 (2022).
Chen, C. et al. Coupling N2 and CO2 in H2O to synthesize urea under ambient conditions. Nat. Chem. 12, 717–724 (2020).
Wu, Y., Jiang, Z., Lin, Z., Liang, Y. & Wang, H. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nat. Sustain. 4, 725–730 (2021).
Meng, N., Huang, Y., Liu, Y., Yu, Y. & Zhang, B. Electrosynthesis of urea from nitrite and CO2 over oxygen vacancy-rich ZnO porous nanosheets. Cell Rep. Phys. Sci. 2, 100378 (2021).
Rayner-Canham, G. Isodiagonality in the periodic table. Found. Chem. 13, 121–129 (2011).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Barberá, J. J., Metzger, A. & Wolf, M. in Ullmann’s Encyclopedia of Industrial Chemistry (2000). https://onlinelibrary.wiley.com/doi/10.1002/14356007.a25_477
Fatehi, P. & Ni, Y. in Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass ACS Symposium Series (eds Zhu, J. Y., Zhang, X., & Pan, X.) 1067, 409–441 (American Chemical Society, 2011).
Taylor, S. L., Higley, N. A. & Bush, R. K. in Advances in Food Research Vol. 30 (eds Chichester, C. O., Mrak, E. M. & Schweigert, B. S.) 1–76 (Academic Press, 1986).
Nascimento, B. et al. Application of cellulose sulfoacetate obtained from sugarcane bagasse as an additive in mortars. J. App. Polym. Sci. 124, 510–517 (2012).
Seeponkai, N. & Wootthikanokkhan, J. Proton conductivity and methanol permeability of sulfonated poly(vinyl alcohol) membranes modified by using sulfoacetic acid and poly(acrylic acid). J. App. Polym. Sci. 105, 838–845 (2007).
D Gernon, M., Wu, M., Buszta, T. & Janney, P. Environmental benefits of methanesulfonic acid. Comparative properties and advantages. Green Chem. 1, 127–140 (1999).
Balaji, R. & Pushpavanam, M. Methanesulphonic acid in electroplating related metal finishing industries. Trans. IMF 81, 154–158 (2003).
Nacsa, E. D. & Lambert, T. H. Cyclopropenone catalyzed substitution of alcohols with mesylate ion. Org. Lett. 15, 38–41 (2013).
V Makarov, S. Recent trends in the chemistry of sulfur-containing reducing agents. Russ. Chem. Rev. 70, 885–895 (2001).
Sulphonate Additives Market (Fact.MR, 2022).
Methane Sulfonic Acid Market (Market Research Future, 2023).
Ho, J.-Y. & Huang, M. H. Synthesis of submicrometer-sized Cu2O crystals with morphological evolution from cubic to hexapod structures and their comparative photocatalytic activity. J. Phys. Chem. C 113, 14159–14164 (2009).
Boutin, E., Salamé, A., Merakeb, L., Chatterjee, T. & Robert, M. On the existence and role of formaldehyde during aqueous electrochemical reduction of carbon monoxide to methanol by cobalt phthalocyanine. Chem. Eur. J 28, e202200697 (2022).
Scott, S. B. et al. Absence of oxidized phases in Cu under CO reduction conditions. ACS Energy Letters 4, 803–804 (2019).
Yang, S. et al. Near-unity electrochemical CO2 to CO conversion over Sn-doped copper oxide nanoparticles. ACS Catal 12, 15146–15156 (2022).
Markin, A. V., Markina, N. E., Popp, J. & Cialla-May, D. Copper nanostructures for chemical analysis using surface-enhanced Raman spectroscopy. TrAC Trends in Analytical Chemistry 108, 247–259 (2018).
Chernyshova Irina, V., Somasundaran, P. & Ponnurangam, S. On the origin of the elusive first intermediate of CO2 electroreduction. Proc. Natl Acad. Sci. USA 115, E9261–E9270 (2018).
Firet, N. J. & Smith, W. A. Probing the reaction mechanism of CO2 electroreduction over Ag films via operando infrared spectroscopy. ACS Catal. 7, 606–612 (2017).
Gunathunge, C. M. et al. Spectroscopic observation of reversible surface reconstruction of copper electrodes under CO2 reduction. J. Phys. Chem. C 121, 12337–12344 (2017).
Chen, X. et al. Controlling speciation during CO2 reduction on Cu-Alloy electrodes. ACS Catal. 10, 672–682 (2020).
Li, X. et al. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 4, 690–699 (2019).
Zhao, Y. et al. Speciation of Cu surfaces during the electrochemical CO reduction reaction. J. Am. Chem. Soc. 142, 9735–9743 (2020).
Niu, Z.-Z. et al. Hierarchical copper with inherent hydrophobicity mitigates electrode flooding for high-rate CO2 electroreduction to multicarbon products. J. Am. Chem. Soc. 143, 8011–8021 (2021).
Pan, Z. et al. Intermediate adsorption states switch to selectively catalyze electrochemical CO2 reduction. ACS Catal. 10, 3871–3880 (2020).
Al-Mahayni, H., Wang, X., Harvey, J.-P., Patience, G. S. & Seifitokaldani, A. Experimental methods in chemical engineering: density functional theory. Can. J. Chem. Eng. 99, 1885–1911 (2021).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Jouny, M. et al. Formation of carbon–nitrogen bonds in carbon monoxide electrolysis. Nat. Chem. 11, 846–851 (2019).
Meng, N. et al. Electrosynthesis of formamide from methanol and ammonia under ambient conditions. Nat. Commun. 13, 5452 (2022).
Rooney, C. L., Wu, Y., Tao, Z. & Wang, H. Electrochemical reductive N-methylation with CO2 enabled by a molecular catalyst. J. Am. Chem. Soc. 143, 19983–19991 (2021).
Zhang, Y. et al. Oxy-reductive CN bond formation via pulsed electrolysis. Preprint at ChemRxiv (2022).
Feng, Y. et al. Te-doped Pd nanocrystal for electrochemical urea production by efficiently coupling carbon dioxide reduction with nitrite reduction. Nano Letters 20, 8282–8289 (2020).
Cao, N. et al. Oxygen vacancies enhanced cooperative electrocatalytic reduction of carbon dioxide and nitrite ions to urea. J. Colloid Interface Sci. 577, 109–114 (2020).
Yuan, M. et al. Highly selective electroreduction of N2 and CO2 to urea over artificial frustrated Lewis pairs. Energy Environ. Sci. 14, 6605–6615 (2021).
Muramoto, E. et al. Toward benchmarking theoretical computations of elementary rate constants on catalytic surfaces: formate decomposition on Au and Cu. Chem. Sci. 13, 804–815 (2022).
Ojeda, M. & Iglesia, E. Formic acid dehydrogenation on Au-based catalysts at near-ambient temperatures. Angew. Chem. Int. Ed. 48, 4800–4803 (2009).
Ammon, C. et al. Dissociation and oxidation of methanol on Cu(110). Surface Science 507-510, 845–850 (2002).
Chen, W.-K., Liu, S.-H., Cao, M.-J., Yan, Q.-G. & Lu, C.-H. Adsorption and dissociation of methanol on Au(1 1 1) surface: a first-principles periodic density functional study. J. Mol. Struct. 770, 87–91 (2006).
Ben David, R., Ben Yaacov, A., Head, A. R. & Eren, B. Methanol decomposition on copper surfaces under ambient conditions: mechanism, surface kinetics, and structure sensitivity. ACS Catal. 12, 7709–7718 (2022).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
Gu, J. et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat. Catal. 5, 268–276 (2022).
Li, J. & Kornienko, N. Electrocatalytic carbon dioxide reduction in acid. Chem. Catalysis 2, 29–38 (2022).
Kim, J. Y. T. et al. Recovering carbon losses in CO2 electrolysis using a solid electrolyte reactor. Nat. Catal. 5, 288–299 (2022).
Acknowledgements
N.K. and J. L. acknowledge the NSERC for its Discovery Grant RGPIN-2019-05927. A.S. acknowledges the NSERC for its Discovery Grant RGPIN-2020-04960 and Canada Research Chair (950-23288). The computations in this research were enabled in part by support provided by Calcul Quebec and Compute Canada.
Author information
Authors and Affiliations
Contributions
J.L., D.C. and N.K. carried out electrochemical and operando studies. H.A.-M. and A.S. carried out computational work. All authors contributed to analysis and to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks H. Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Extended methods description and Supplementary figs. 1–41.
Source data
Source Data Fig. 2
Unprocessed data of: NMR spectra for standards and reaction solution (a), linear scan voltammetry traces (b), FE for products (c,d) and partial current density (e).
Source Data Fig. 3
Potential and time-dependent XRD and Raman spectra.
Source Data Fig. 5
Energies of reaction intermediates in the calculated free energy landscape.
Source Data Fig. 6
Raw values of product formation rates.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Al-Mahayni, H., Chartrand, D. et al. Electrochemical formation of C–S bonds from CO2 and small-molecule sulfur species. Nat. Synth 2, 757–765 (2023). https://doi.org/10.1038/s44160-023-00303-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00303-9
This article is cited by
-
Electrochemical hydrogenation and oxidation of organic species involving water
Nature Reviews Chemistry (2024)
-
Sustainable electrosynthesis of sulfur-containing chemical feedstocks
Nature Synthesis (2023)