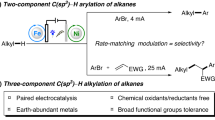
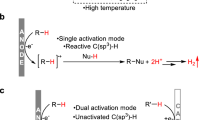
Abstract
Oxidative C(sp3)–H bond functionalization is a powerful tool in organic synthesis, but the stoichiometric oxidants required for bond formation raise environmental concerns. Electrochemical synthesis is typically carried out under environmentally benign conditions and hence is increasingly used in organic reactions. Recent advances revealed that electrochemical oxidative cross-coupling reactions employing either free radicals or carbocations can be conducted in the absence of external oxidants. In this Review, we outline the electrochemical oxidative C(sp3)–H cross-coupling to C(sp3)–C(X) (X = N, O, S, P, F, I and Br) bonds with hydrogen evolution under external oxidant-free conditions. Two commonly used electrochemical methods, namely direct and indirect oxidation electrolysis, are discussed for C(sp3)–H activation. The outline of electrochemical oxidative C(sp3)–H cross-coupling is organized based on the reactive intermediates (C(sp3)–M, a C(sp3) radical or a C(sp3) cation). Electrochemical asymmetric C(sp3)–H cross-coupling and late-stage functionalization of C(sp3)–H bonds in complex molecules are included to demonstrate the utility. Future perspectives for the development of electrochemical C(sp3)–H bond functionalization are proposed.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Girard, S. A., Knauber, T. & Li, C. J. The cross-dehydrogenative coupling of C(sp3)–H bonds: a versatile strategy for C–C bond formations. Angew. Chem. Int. Ed. 53, 74–100 (2014).
Liu, C., Liu, D. & Lei, A. Recent advances of transition-metal catalysed radical oxidative cross-couplings. Acc. Chem. Res. 47, 3459–3470 (2014).
Liu, C. et al. Oxidative coupling between two hydrocarbons: an update of recent C–H functionalizations. Chem. Rev. 115, 12138–12204 (2015).
Li, C. J. et al. Cross-dehydrogenative coupling (CDC): exploring C–C bond formations beyond functional group transformations. Acc. Chem. Res. 42, 335–344 (2009).
Yeung, C. S. & Dong, V. M. Catalytic dehydrogenative cross-coupling: forming carbon–carbon bonds by oxidizing two carbon–hydrogen bonds. Chem. Rev. 111, 1215–1292 (2011).
Le Bras, J. & Muzart, J. Intermolecular dehydrogenative Heck reactions. Chem. Rev. 111, 1170–1214 (2011).
An, X.-D. & Xiao, J. Recent advances in hydride transfer-involved C(sp3)–H activation reactions. Org. Chem. Front. 8, 1364–1383 (2021).
Chen, J. Y., Wu, W., Li, Q. & Wei, W. T. Visible‐light induced C(sp3)−H functionalization for the formation of C−N bonds under metal catalyst‐free conditions. Adv. Synth. Catal. 362, 2770–2777 (2020).
Liu, X.-Y., Li, Z.-L., Wei, H. & Zhang, Z.-H. Recent advances in radical-involved alkynylation of unactivated C(sp3)–H bonds by hydrogen atom abstraction. Synlett. 32, 362–369 (2020).
Mishra, A. A., Subhedar, D. & Bhanage, B. M. Nickel, cobalt and palladium catalysed C–H functionalization of un-activated C(sp3)–H bond. Chem. Rec. 19, 1829–1857 (2019).
Sarkar, S., Cheung, K. P. S. & Gevorgyan, V. C–H functionalization reactions enabled by hydrogen atom transfer to carbon-centered radicals. Chem. Sci. 11, 12974–12993 (2020).
Xing, Y., Wang, N.-X., Zhang, L.-Y. & Wu, Y.-H. C(sp3)–H bond functionalization of alcohols, ketones, nitriles, ethers and amides using tert-butyl hydroperoxide as a radical initiator. Synlett. 32, 23–29 (2020).
Chen, X., Engle, K. M., Wang, D. H. & Yu, J. Q. Palladium(II)-catalyzed C–H activation/C–C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 48, 5094–5115 (2009).
Newhouse, T. & Baran, P. S. If C–H bonds could talk: selective C–H bond oxidation. Angew. Chem. Int. Ed. 50, 3362–3374 (2011).
Yamaguchi, J., Yamaguchi, A. D. & Itami, K. C–H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 51, 8960–9009 (2012).
Jiang, Y., Xu, K. & Zeng, C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 118, 4485–4540 (2018).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Yang, Q.-L., Fang, P. & Mei, T.-S. Recent advances in organic electrochemical C–H functionalization. Chin. J. Chem. 36, 338–352 (2018).
Frontana-Uribe, B. A., Little, R. D., Ibanez, J. G., Palma, A. & Vasquez-Medrano, R. Organic electrosynthesis: a promising green methodology in organic chemistry. Green Chem. 12, 2099–2119 (2010).
Horn, E. J., Rosen, B. R. & Baran, P. S. Synthetic organic electrochemistry: an enabling and innately sustainable method. ACS Cent. Sci. 2, 302–308 (2016).
Francke, R. & Little, R. D. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 43, 2492–2521 (2014).
Jutan, Anny Contribution of electrochemistry to organometallic catalysis. Chem. Rev. 108, 2300–2347 (2008).
Schäfer, H. J. Contributions of organic electrosynthesis to green chemistry. C. R. Chim. 14, 745–765 (2011).
Yoshida, J., Kataoka, K., Horcajada, R. & Nagaki, A. Modern strategies in electroorganic synthesis. Chem. Rev. 108, 2265–2299 (2008).
Tang, S., Zeng, L. & Lei, A. Oxidative R1–H/R2–H cross-coupling with hydrogen evolution. J. Am. Chem. Soc. 140, 13128–13135 (2018).
Moeller, K. D. Using physical organic chemistry to shape the course of electrochemical reactions. Chem. Rev. 118, 4817–4833 (2018).
Tang, S., Liu, Y. & Lei, A. Electrochemical oxidative cross-coupling with hydrogen evolution: a green and sustainable way for bond formation. Chem 4, 27–45 (2018).
Smith, M. B. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure 7th edn (John Wiley & Sons, Inc., 2013).
Huang, P., Wang, P., Wang, S., Tang, S. & Lei, A. Electrochemical oxidative [4 + 2] annulation of tertiary anilines and alkenes for the synthesis of tetrahydroquinolines. Green Chem. 20, 4870–4874 (2018).
Guan, Z. et al. Electrochemical synthesis of α-amino amides via C(sp3)–H bond activation. Green Chem. 24, 3964–3968 (2022).
Wu, Z. J. & Xu, H. C. Synthesis of C3-fluorinated oxindoles through reagent-free cross-dehydrogenative coupling. Angew. Chem. Int. Ed. 56, 4734–4738 (2017).
Wu, Z. J., Li, S. R., Long, H. & Xu, H. C. Electrochemical dehydrogenative cyclization of 1,3-dicarbonyl compounds. Chem. Commun. 54, 4601–4604 (2018).
Wu, Z. J., Li, S. R. & Xu, H. C. Synthesis of N-heterocycles by dehydrogenative annulation of N-allyl amides with 1,3-dicarbonyl compounds. Angew. Chem. Int. Ed. 57, 14070–14074 (2018).
Jie, L. H., Guo, B., Song, J. & Xu, H. C. Organoelectrocatalysis enables direct cyclopropanation of methylene compounds. J. Am. Chem. Soc. 144, 2343–2350 (2022).
Guan, Z. et al. Synthesis of cyclopentene derivatives via electrochemically induced intermolecular selective (3+2) annulation. Angew. Chem. Int. Ed. 61, e202207059 (2022).
Chen, M., Wu, Z. J., Song, J. & Xu, H. C. Electrocatalytic allylic C–H alkylation enabled by a dual-function cobalt catalyst. Angew. Chem. Int. Ed. 61, e202115954 (2022).
Gao, H., Zha, Z., Zhang, Z., Ma, H. & Wang, Z. A simple and efficient approach to realize difunctionalization of arylketones with malonate esters via electrochemical oxidation. Chem. Commun. 50, 5034–5036 (2014).
Qian, P. et al. Electrocatalytic C–H/N–H Coupling of 2′-aminoacetophenones for the synthesis of isatins. J. Org. Chem. 82, 6434–6440 (2017).
He, T. J., Ye, Z., Ke, Z. & Huang, J. M. Stereoselective synthesis of sulfur-containing β-enaminonitrile derivatives through electrochemical C(sp3)–H bond oxidative functionalization of acetonitrile. Nat. Commun. 10, 833 (2019).
Wang, F. & Stahl, S. S. Merging photochemistry with electrochemistry: functional-group tolerant electrochemical amination of C(sp3)–H bonds. Angew. Chem. Int. Ed. 58, 6385–6390 (2019).
Xu, P., Chen, P. Y. & Xu, H. C. Scalable photoelectrochemical dehydrogenative cross-coupling of heteroarenes with aliphatic C–H bonds. Angew. Chem. Int. Ed. 59, 14275–14280 (2020).
Blanksby, S. J. & Ellison, G. B. Bond dissociation energies of organic molecules. Acc. Chem. Res. 36, 255–263 (2003).
Horn, E. J. et al. Scalable and sustainable electrochemical allylic C–H oxidation. Nature 533, 77–81 (2016).
Masui, M. et al. Electrochemical oxidation of olefins using N–hydroxyphthalimide as a mediator. Chem. Pharm. Bull. 33, 4798–4802 (1985).
Masui, M. et al. Anodic oxidation of compounds having benzylic or allylic carbon and α-carbon to heteroatom using N-hydroxyphthalimide as a mediator. Chem. Pharm. Bull. 31, 4209–4211 (1983).
Masui, M. et al. N-hydroxyphthalimide as an effective mediator for the oxidation of alcohols by electrolysis. J. Chem. Soc. Chem. Commun. 1983, 479–480 (1983).
Rafiee, M., Wang, F., Hruszkewycz, D. P. & Stahl, S. S. N-hydroxyphthalimide-mediated electrochemical iodination of methylarenes and comparison to electron-transfer-initiated C–H functionalization. J. Am. Chem. Soc. 140, 22–25 (2018).
Niu, L. et al. Manganese-catalysed oxidative azidation of C(sp3)–H bonds under electrophotocatalytic conditions. J. Am. Chem. Soc. 142, 17693–17702 (2020).
Zhang, S. et al. Scalable electrochemical dehydrogenative lactonization of C(sp2)/(sp3)–H bonds. Org. Lett. 20, 252–255 (2018).
Kawamata, Y. et al. Scalable, electrochemical oxidation of unactivated C–H bonds. J. Am. Chem. Soc. 139, 7448–7451 (2017).
Hu, X., Zhang, G., Bu, F., Nie, L. & Lei, A. Electrochemical-oxidation-induced site-selective intramolecular C(sp3)–H amination. ACS Catal. 8, 9370–9375 (2018).
Huang, H., Strater, Z. M. & Lambert, T. H. Electrophotocatalytic C–H functionalization of ethers with high regioselectivity. J. Am. Chem. Soc. 142, 1698–1703 (2020).
Capaldo, L., Quadri, L. L., Merli, D. & Ravelli, D. Photoelectrochemical cross-dehydrogenative coupling of benzothiazoles with strong aliphatic C–H bonds. Chem. Commun. 57, 4424–4427 (2021).
Meyer, T. H., Samanta, R. C., Del Vecchio, A. & Ackermann, L. Mangana(III/IV)electro-catalysed C(sp3)–H azidation. Chem. Sci. 12, 2890–2897 (2020).
Zhou, Z. et al. Synergy of anodic oxidation and cathodic reduction leads to electrochemical C–H halogenation. Chin. J. Chem. 37, 611–615 (2019).
Zhang, L. et al. Ritter-type amination of C(sp3)–H bonds enabled by electrochemistry with SO42–. Nat. Commun. 13, 4138 (2022).
Luo, Y.-R., Comprehensive Handbook of Chemical Bond Energies 1st edn (CRC, 2007).
Kvasovs, N. & Gevorgyan, V. Contemporary methods for generation of aryl radicals. Chem. Soc. Rev. 50, 2244–2259 (2021).
Kurandina, D. et al. Transition-metal- and light-free directed amination of remote unactivated Csp3–H bonds of alcohols. J. Am. Chem. Soc. 141, 8104–8109 (2019).
Parasram, M., Chuentragool, P., Sarkar, D. & Gevorgyan, V. Photoinduced formation of hybrid aryl Pd-radical species capable of 1,5-HAT: selective catalytic oxidation of silyl ethers into silyl enol ethers. J. Am. Chem. Soc. 138, 6340–6343 (2016).
Ratushnyy, M., Kvasovs, N., Sarkar, S. & Gevorgyan, V. Visible-light-induced palladium-catalyzed generation of aryl radicals from aryl triflates. Angew. Chem. Int. Ed. 59, 10316–10320 (2020).
Ashikari, Y., Nokami, T. & Yoshida, J. Integrated electrochemical-chemical oxidation mediated by alkoxysulfonium ions. J. Am. Chem. Soc. 133, 11840–11843 (2011).
Jun-ichi, Yoshida et al. Oxidative hydroxylation mediated by alkoxysulfonium ions. Org. Lett. 14, 938–941 (2012).
Meng, L. et al. Direct electrosynthesis of ketones from benzylic methylenes by electrooxidative C–H activation. Chemistry 19, 5542–5545 (2013).
Xiong, P. et al. Site-selective electrooxidation of methylarenes to aromatic acetals. Nat. Commun. 11, 2706 (2020).
Baba, D. & Fuchigami, T. Anodic methoxylation and acetoxylation of imines and imidates. Tetrahedron Lett. 44, 3133–3136 (2003).
Okimoto, M. et al. Electrooxidative cyclization of hydroxyamino compounds possessing a benzyl group. Synthesis 44, 1315–1322 (2012).
Akinori, Konno et al. Electrolytic partial fluorination of organic compounds. 23.1 Regioselective anodic difluorination of sulfides using novel fluorine source Et4NF·4HF. J. Org. Chem. 62, 8579–8581 (1997).
Yuan, Y. et al. Exogenous-oxidant-free electrochemical oxidative C–H phosphonylation with hydrogen evolution. Chem. Commun. 55, 4230–4233 (2019).
Wang, H. et al. Electrochemical oxidation-induced etherification via C(sp3)─H/O─H cross-coupling. Sci. Adv. 6, eaaz05 (2020).
Hou, Z. W. et al. Site-selective electrochemical benzylic C–H amination. Angew. Chem. Int. Ed. 60, 2943–2947 (2021).
Yang, Y. Z., Wu, Y. C., Song, R. J. & Li, J. H. Electrochemical dehydrogenative cross-coupling of xanthenes with ketones. Chem. Commun. 56, 7585–7588 (2020).
Shen, T. & Lambert, T. H. C–H amination via electrophotocatalytic Ritter-type reaction. J. Am. Chem. Soc. 143, 8597–8602 (2021).
Shao, X., Tian, L. & Wang, Y. C–N coupling of azoles or imides with carbocations generated by electrochemical oxidation. Eur. J. Org. Chem. 2019, 4089–4094 (2019).
Wang, Y., Lin, Z., Oliveira, J. C. A. & Ackermann, L. Electro-oxidative intermolecular allylic C(sp3)–H aminations. J. Org. Chem. 22, 15935–15945 (2021).
Wu, J., Zhou, Y., Zhou, Y., Chiang, C.-W. & Lei, A. Electro-oxidative C(sp3)–H amination of azoles via intermolecular oxidative C(sp3)–H/N–H cross-coupling. ACS Catal. 7, 8320–8323 (2017).
Wang, P. et al. Electrochemical oxidative C(sp3)–H/N–H cross-coupling for N-Mannich bases with hydrogen evolution. ChemSusChem 12, 3073–3077 (2019).
Wan, Z. et al. Electrochemical oxidative C(sp3)–H azolation of lactams under mild conditions. Green Chem. 22, 3742–3747 (2020).
Wang, H. et al. Electrochemical oxidation enables regioselective and scalable α-C(sp3)–H acyloxylation of sulfides. J. Am. Chem. Soc. 143, 3628–3637 (2021).
Lennox, A. J. J. et al. Electrochemical aminoxyl-mediated α-cyanation of secondary piperidines for pharmaceutical building block diversification. J. Am. Chem. Soc. 140, 11227–11231 (2018).
Colby, D. A., Bergman, R. G. & Ellman, J. A. Rhodium-catalysed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 110, 624–655 (2010).
Rit, R. K., Yadav, M. R. & Sahoo, A. K. Pd(II)-catalysed primary-C(sp3)–H acyloxylation at room temperature. Org. Lett. 14, 3724–3727 (2012).
Thompson, S. J., Thach, D. Q. & Dong, G. Cyclic ether synthesis via palladium-catalysed directed dehydrogenative annulation at unactivated terminal positions. J. Am. Chem. Soc. 137, 11586–11589 (2015).
Wang, M. et al. Pd-catalysed α-selective C(sp3)–H acetoxylation of amides through an unusual cyclopalladation mechanism. Chem. Commun. 51, 3219–3222 (2015).
Ye, X. et al. 1,2,3-Triazoles as versatile directing group for selective sp2 and sp3 C–H activation: cyclization vs substitution. Chem. Sci. 4, 3712–3716 (2013).
Zhang, S. Y. et al. Efficient alkyl ether synthesis via palladium-catalysed, picolinamide-directed alkoxylation of unactivated C(sp3)–H and C(sp2)–H bonds at remote positions. J. Am. Chem. Soc. 134, 7313–7316 (2012).
Bercaw, J. E. et al. Electrocatalytic functionalization of alkanes using aqueous platinum salts. J. Mol. Catal. 87, 11–15 (1994).
Yang, Q. L. et al. Palladium-catalysed C(sp3)–H oxygenation via electrochemical oxidation. J. Am. Chem. Soc. 139, 3293–3298 (2017).
Shrestha, A., Lee, M., Dunn, A. L. & Sanford, M. S. Palladium-catalysed C–H bond acetoxylation via electrochemical oxidation. Org. Lett. 20, 204–207 (2018).
Jutand, A., Amatore, C. & Cammoun, C. Palladium/benzoquinone-catalyzed electrochemical oxidation of alcohols under anaerobic conditions. Synlett. 2007, 2173–2178 (2007).
Tsuchida, K., Kochi, T. & Kakiuchi, F. Copper-catalyzed electrochemical chlorination of 1,3-dicarbonyl compounds using hydrochloric acid. Asian J. Org. Chem. 2, 935–937 (2013).
Jacobsen, E. N., Pfaltz, A & Yamamoto, H., Comprehensive Asymmetric Catalysis Vols I–III, Suppl. I & II (Springer, 1999).
Qin, Y., Zhu, L. & Luo, S. Organocatalysis in inert C–H bond functionalization. Chem. Rev. 117, 9433–9520 (2017).
MacMillan, D. W. The advent and development of organocatalysis. Nature 455, 304–308 (2008).
Bui, N.-N. & Ho, X.-H. Mho, S.-I. & Jang, H.-Y. Organocatalysed α-oxyamination of aldehydes using anodic oxidation. Eur. J. Org. Chem. 31, 5309–5312 (2009).
Ho, X.-H., Mho, S.-i, Kang, H. & Jang, H.-Y. Electro-organocatalysis: enantioselective α-alkylation of aldehydes. Eur. J. Org. Chem. 23, 4436–4441 (2010).
Fu, N., Li, L., Yang, Q. & Luo, S. Catalytic asymmetric electrochemical oxidative coupling of tertiary amines with simple ketones. Org. Lett. 19, 2122–2125 (2017).
Li, L., Li, Y., Fu, N., Zhang, L. & Luo, S. Catalytic asymmetric electrochemical α-arylation of cyclic β-ketocarbonyls with anodic benzyne intermediates. Angew. Chem. Int. Ed. 59, 14347–14351 (2020).
Huang, X., Zhang, Q., Lin, J., Harms, K., & Meggers, E. Electricity-driven asymmetric Lewis acid catalysis. Nat. Catal. 2, 34–40 (2018).
Zhang, Q., Chang, X., Peng, L. & Guo, C. Asymmetric Lewis acid catalysed electrochemical alkylation. Angew. Chem. Int. Ed. 58, 6999–7003 (2019).
Gao, P. S. et al. Cu(II)/TEMPO-catalysed enantioselective C(sp3)–H alkynylation of tertiary cyclic amines through Shono-type oxidation. Angew. Chem. Int. Ed. 59, 15254–15259 (2020).
Jensen, K. L., Franke, P. T., Nielsen, L. T., Daasbjerg, K. & Jorgensen, K. A. Anodic oxidation and organocatalysis: direct regio- and stereoselective access to meta-substituted anilines by α-arylation of aldehydes. Angew. Chem. Int. Ed. 49, 129–133 (2010).
Wang, Z. H. et al. TEMPO-enabled electrochemical enantioselective oxidative coupling of secondary acyclic amines with ketones. J. Am. Chem. Soc. 143, 15599–15605 (2021).
Chang, X., Zhang, J., Zhang, Q. & Guo, C. Merging electrosynthesis and bifunctional squaramide catalysis in the asymmetric detrifluoroacetylative alkylation reactions. Angew. Chem. Int. Ed. 59, 18500–18504 (2020).
Saito, M. et al. N-ammonium ylide mediators for electrochemical C–H oxidation. J. Am. Chem. Soc. 143, 7859–7867 (2021).
Frankowski, K. J., Liu, R., Milligan, G. L., Moeller, K. D. & Aube, J. Practical electrochemical anodic oxidation of polycyclic lactams for late stage functionalization. Angew. Chem. Int. Ed. 54, 10555–10558 (2015).
Wu, T. & Moeller, K. D. Organic electrochemistry: expanding the scope of paired reactions. Angew. Chem. Int. Ed. 60, 12883–12890 (2021).
Ma, Y. et al. Direct arylation of α-amino C(sp3)–H bonds by convergent paired electrolysis. Angew. Chem. Int. Ed. 58, 16548–16552 (2019).
Zhang, L. & Hu, X. Nickel catalysis enables convergent paired electrolysis for direct arylation of benzylic C–H bonds. Chem. Sci. 11, 10786–11079 (2020).
Gunasekera, D. et al. Controlling one- or two-electron oxidation for selective amine functionalization by alternating current frequency. J. Am. Chem. Soc. 144, 9874–9882 (2022).
Acknowledgements
This work was supported by the National Key R&D Program of China (no. 2022YFA1505100 and 2021YFA1500100), National Natural Science Foundation of China (22031008) and Science Foundation of Wuhan (2020010601012192). This research work was funded by the Institution Fund Projects under grant IFPRP 263-135-1442. Also, we gratefully acknowledge technical and financial support from the Ministry of Education and Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia
Author information
Authors and Affiliations
Contributions
A.L. and H.Y. designed and directed the investigations and composed the manuscript with revisions provided by the other authors.. Z.Y., H.A. and W.S. completed the full text of the writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Z., Shi, W., Alhumade, H. et al. Electrochemical oxidative C(sp3)–H cross-coupling with hydrogen evolution. Nat. Synth 2, 217–230 (2023). https://doi.org/10.1038/s44160-022-00221-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00221-2