Abstract
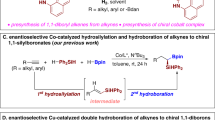
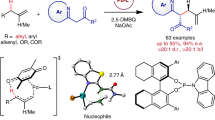
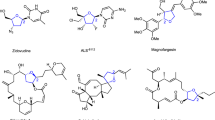
The incorporation of alkenylboronates into axially chiral compounds increases their structural diversity and provides a synthetic handle for late-stage functionalizations. Despite advances made in the synthesis of axially chiral acyclic alkenes, catalytic enantioselective synthesis of tetrasubstituted axially chiral acyclic alkenylboronates still remains a challenge. Here, we report a combined copper- and palladium-catalysed atroposelective arylboration of alkynes, providing access to tetrasubstituted axially chiral alkenylboronates. The process can tolerate a broad range of functional groups and substrates, including unsymmetrical alkynes, providing products with excellent Z-/E-selectivity, regiocontrol and enantiocontrol. The synthetic use of the products has been demonstrated through onwards synthetic transformations to generate various new axially chiral olefins including axially chiral 1,3-enynes. Mechanistic experiments reveal a potential combined copper and palladium catalytic cycle, with the observed stereocontrol originating from a higher-order active palladium catalyst.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the article and its Supplementary Information files. Crystallographic data for the structures reported in this Article have been deposited at the CCDC, under deposition numbers CCDC 2171042 (3), 2171044 (12), 2171040 (17), 2171047 (36), 2171041 (51), 2171043 (70), 2171046 (126) and 2171045 (ent-3). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures.
References
Kumarasamy, E., Raghunathan, R., Sibi, M. P. & Sivaguru, J. Nonbiaryl and heterobiaryl atropisomers: molecular templates with promise for atropselective chemical transformations. Chem. Rev. 115, 11239–11300 (2015).
Smyth, J. E., Butler, N. M. & Keller, P. A. A twist of nature-the significance of atropisomers in biological systems. Nat. Prod. Rep. 32, 1562–1583 (2015).
Bringmann, G., Gulder, T., Gulder, T. A. M. & Breuning, M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 111, 563–639 (2011).
Li, Y., Kwong, F., Yu, W. & Chan, A. Recent advances in developing new axially chiral phosphine ligands for asymmetric catalysis. Coord. Chem. Rev. 251, 2119–2144 (2007).
Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T. & Nussbaumer, A. L. Artificial molecular machines. Chem. Rev. 115, 10081–10206 (2015).
Toenjes, S. T. & Gustafson, J. L. Atropisomerism in medicinal chemistry: challenges and opportunities. Future Med. Chem. 10, 409–422 (2018).
Lassaletta, J. M. (ed.). Atropisomerism and Axial Chirality 1st edn (World Scientific, 2019).
Bao, X., Rodriguez, J. & Bonne, D. Enantioselective synthesis of atropisomers with multiple stereogenic axes. Angew. Chem. Int. Ed. 59, 12623–12634 (2020).
Wencel-Delord, J., Panossian, A., Leroux, F. R. & Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 44, 3418–3430 (2015).
Liao, G., Zhou, T., Yao, Q.-J. & Shi, B.-F. Recent advances in the synthesis of axially chiral biaryls via transition metal-catalysed asymmetric C–H functionalization. Chem. Commun. 55, 8514–8523 (2019).
Liu, C. X., Zhang, W. W., Yin, S. Y., Gu, Q. & You, S. L. Synthesis of atropisomers by transition-metal-catalyzed asymmetric C-H functionalization reactions. J. Am. Chem. Soc. 143, 14025–14040 (2021).
Cheng, J. K., Xiang, S. H., Li, S., Ye, L. & Tan, B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 121, 4805–4902 (2021).
Wu, Y.-J., Liao, G. & Shi, B.-F. Stereoselective construction of atropisomers featuring a C–N chiral axis. Green Synth. Catal. 3, 117–136 (2022).
Mori, K., Ohmori, K. & Suzuki, K. Hydrogen-bond control in axially chiral styrenes: selective synthesis of enantiomerically pure C2-symmetric paracyclophanes. Angew. Chem. Int. Ed. 48, 5638–5641 (2009).
Gu, Z. & Feng, J. Atropisomerism in styrene: synthesis, stability, and spplications. SynOpen 5, 68–85 (2021).
Wu, S., Xiang, S.-H., Cheng, J. K. & Tan, B. Axially chiral alkenes: atroposelective synthesis and applications. Tetrahedron Chem. 1, 100009 (2022).
Defieber, C., Grutzmacher, H. & Carreira, E. M. Chiral olefins as steering ligands in asymmetric catalysis. Angew. Chem. Int. Ed. 47, 4482–4502 (2008).
Feng, X. & Du, H. Synthesis of chiral olefin ligands and their application in asymmetric catalysis. Asian J. Org. Chem. 1, 204–213 (2012).
Li, Y. & Xu, M. H. Simple sulfur-olefins as new promising chiral ligands for asymmetric catalysis. Chem. Commun. 50, 3771–3782 (2014).
Zhang, Z.-X., Zhai, T.-Y. & Ye, L.-W. Synthesis of axially chiral compounds through catalytic asymmetric reactions of alkynes. Chem Catalysis 1, 1378–1412 (2021).
Zheng, S. C. et al. Organocatalytic atroposelective synthesis of axially chiral styrenes. Nat. Commun. 8, 15238 (2017).
Jia, S. et al. Organocatalytic enantioselective construction of axially chiral sulfone-containing styrenes. J. Am. Chem. Soc. 140, 7056–7060 (2018).
Tan, Y. et al. Enantioselective construction of vicinal diaxial styrenes and multiaxis system via organocatalysis. J. Am. Chem. Soc. 140, 16893–16898 (2018).
Wang, Y.-B. et al. Rational design, enantioselective synthesis and catalytic applications of axially chiral EBINOLs. Nat. Catal. 2, 504–513 (2019).
Huang, A. et al. Asymmetric one-pot construction of three stereogenic elements: chiral carbon center, stereoisomeric alkenes, and chirality of axial styrenes. Org. Lett. 21, 95–99 (2019).
Wang, C. S. et al. Axially chiral aryl‐alkene‐indole framework: a nascent member of the atropisomeric family and its catalytic asymmetric construction. Chin. J. Chem. 38, 543–552 (2020).
Li, Q. Z. et al. Organocatalytic enantioselective construction of heterocycle-substituted styrenes with chiral atropisomerism. Org. Lett. 22, 2448–2453 (2020).
Zhang, C. et al. Access to axially chiral styrenes via a photoinduced asymmetric radical reaction involving a sulfur dioxide insertion. Chem. Catalysis 2, 164–177 (2022).
Zhang, W., Song, R., Yang, D. & Lv, J. Construction of axially chiral styrenes linking an indole moiety by chiral phosphoric acid. J. Org. Chem. 87, 2853–2863 (2022).
Yan, J. L. et al. Carbene-catalyzed atroposelective synthesis of axially chiral styrenes. Nat. Commun. 13, 84 (2022).
Wu, S. et al. Urea group-directed organocatalytic asymmetric versatile dihalogenation of alkenes and alkynes. Nat. Catal. 4, 692–702 (2021).
Mi, R. et al. Rhodium-catalyzed atroposelective access to axially chiral olefins via C−H bond activation and directing group migration. Angew. Chem. Int. Ed. 61, e202111860 (2022).
Song, H. et al. Synthesis of axially chiral styrenes through Pd-catalyzed asymmetric C-H olefination enabled by an amino amide transient directing group. Angew. Chem. Int. Ed. 59, 6576–6580 (2020).
Jin, L. et al. Atroposelective synthesis of axially chiral styrenes via an asymmetric C–H functionalization strategy. Chem 6, 497–511 (2020).
Yang, C. et al. Facile synthesis of axially chiral styrene-type carboxylic acids via palladium-catalyzed asymmetric C-H activation. Chem. Sci. 12, 3726–3732 (2021).
Wang, J. et al. Tandem iridium catalysis as a general strategy for atroposelective construction of axially chiral styrenes. J. Am. Chem. Soc. 143, 10686–10694 (2021).
Jin, L., Zhang, P., Li, Y., Yu, X. & Shi, B. F. Atroposelective synthesis of conjugated diene-based axially chiral styrenes via Pd(II)-catalyzed thioether-directed alkenyl C-H olefination. J. Am. Chem. Soc. 143, 12335–12344 (2021).
Dai, D. T., Yang, M. W., Chen, Z. Y., Wang, Z. L. & Xu, Y. H. Chelation-controlled stereospecific cross-coupling reaction between alkenes for atroposelective synthesis of axially chiral conjugated dienes. Org. Lett. 24, 1979–1984 (2022).
Yang, C. et al. Development of axially chiral styrene-type carboxylic acid ligands via palladium-catalyzed asymmetric C-H alkynylation. Org. Lett. 23, 8132–8137 (2021).
Wang, Y. B. et al. Asymmetric construction of axially chiral 2-arylpyrroles by chirality transfer of atropisomeric alkenes. Angew. Chem. Int. Ed. 58, 13443–13447 (2019).
Ma, C. et al. Atroposelective access to oxindole-based axially chiral styrenes via the strategy of catalytic kinetic resolution. J. Am. Chem. Soc. 142, 15686–15696 (2020).
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
Suzuki, A. Cross-coupling reactions of organoboranes: an easy way to construct C-C bonds. Angew. Chem. Int. Ed. 50, 6722–6737 (2011).
Lennox, A. J. & Lloyd-Jones, G. C. Selection of boron reagents for Suzuki-Miyaura coupling. Chem. Soc. Rev. 43, 412–443 (2014).
Armstrong, R. & Aggarwal, V. 50 years of Zweifel olefination: a transition-metal-free coupling. Synthesis 49, 3323–3336 (2017).
Candeias, N. R., Montalbano, F., Cal, P. M. S. D. & Gois, P. M. P. Boronic acids and esters in the petasis-borono mannich multicomponent reaction. Chem. Rev. 110, 6169–6193 (2010).
Shade, R. E., Hyde, A. M., Olsen, J.-C. & Merlic, C. A. Copper-promoted coupling of vinyl boronates and alcohols: a mild synthesis of allyl vinyl ethers. J. Am. Chem. Soc. 132, 1202–1203 (2010).
Carreras, J., Caballero, A. & Perez, P. J. Alkenyl boronates: synthesis and applications. Chem. Asian J. 14, 329–343 (2019).
Zhou, Y., You, W., Smith, K. B. & Brown, M. K. Copper-catalyzed cross-coupling of boronic esters with aryl iodides and application to the carboboration of alkynes and allenes. Angew. Chem. Int. Ed. 53, 3475–3479 (2014).
Yang, K. & Song, Q. Tetracoordinate boron intermediates enable unconventional transformations. Acc. Chem. Res. 54, 2298–2312 (2021).
Yang, K. et al. Construction of axially chiral arylborons via atroposelective miyaura borylation. J. Am. Chem. Soc. 143, 10048–10053 (2021).
Jin, S., Liu, K., Wang, S. & Song, Q. Enantioselective cobalt-catalyzed cascade hydrosilylation and hydroboration of alkynes to access enantioenriched 1,1-silylboryl alkanes. J. Am. Chem. Soc. 143, 13124–13134 (2021).
Lesieur, M., Bidal, Y. D., Lazreg, F., Nahra, F. & Cazin, C. S. J. Versatile relay and cooperative palladium(0) N-heterocyclic carbene/copper (I) N-heterocyclic carbene catalysis for the synthesis of tri- and tetrasubstituted alkenes. Chem. Cat. Chem. 7, 2108–2112 (2015).
Semba, K., Yoshizawa, M., Ohtagaki, Y. & Nakao, Y. Arylboration of internal alkynes by cooperative palladium/copper catalysis. Bull. Chem. Soc. Jpn 90, 1340–1343 (2017).
Huang, Y., Bergmann, A. M. & Brown, M. K. (Hetero)arylboration of alkynes: a strategy for the synthesis of alpha, alpha-bis(hetero)arylketones. Org. Biomol. Chem. 17, 5913–5915 (2019).
Liu, Z.-S. et al. Construction of axial chirality via palladium/chiral norbornene cooperative catalysis. Nat. Cat. 3, 727–733 (2020).
Satyanarayana, T., Abraham, S. & Kagan, H. B. Nonlinear effects in asymmetric catalysis. Angew. Chem. Int. Ed. 48, 456–494 (2009).
Yoshida, H., Takemoto, Y. & Takaki, K. Direct synthesis of boron-protected alkenyl- and alkylborons via copper-catalyzed formal hydroboration of alkynes and alkenes. Asian J. Org. Chem. 3, 1204–1209 (2014).
Acknowledgements
Financial support came from National Natural Science Foundation of China (grant nos. 21931013 to Q.S. and 22001038 to K.Y.), the Natural Science Foundation of Fujian Province (grant no. 2022J02009 to Q.S.) and Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University (to Q.S.) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Q.S. designed and directed the project. W.L. performed the experiments and developed the reactions. S.C., J.X., Z.F. and K.Y. helped collecting some experimental data. Q.S. and W.L. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Tables 1–14, Figs. 1–9 and starting material preparation, experimental procedures, synthetic transformations, mechanistic studies and product characterization.
Supplementary Data 1
Crystallographic data of complex 3 (CCDC 2171042).
Supplementary Data 2
Crystallographic data of complex 12 (CCDC 2171044).
Supplementary Data 3
Crystallographic data of complex 17 (CCDC 2171040).
Supplementary Data 4
Crystallographic data of complex 36 (CCDC 2171047).
Supplementary Data 5
Crystallographic data of complex 51 (CCDC 2171041).
Supplementary Data 6
Crystallographic data of complex 70 (CCDC 2171043).
Supplementary Data 7
Crystallographic data of complex 126 (CCDC 2171046).
Supplementary Data 8
Crystallographic data of complex ent-3 (CCDC 2171045).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, W., Chen, S., Xie, J. et al. Synthesis of axially chiral alkenylboronates through combined copper- and palladium-catalysed atroposelective arylboration of alkynes. Nat. Synth 2, 140–151 (2023). https://doi.org/10.1038/s44160-022-00201-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00201-6
This article is cited by
-
Photocatalytic Z/E isomerization unlocking the stereodivergent construction of axially chiral alkene frameworks
Nature Communications (2024)
-
Ni-catalysed assembly of axially chiral alkenes from alkynyl tetracoordinate borons via 1,3-metallate shift
Nature Chemistry (2024)
-
Atroposelectivity with two catalysts
Nature Synthesis (2023)