Abstract
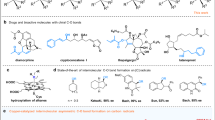
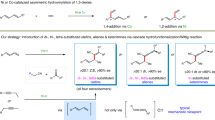
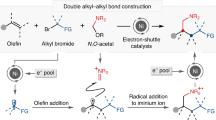
The merging of transition-metal catalysis with radical chemistry offers a versatile platform for three-component difunctionalization of alkenes, allowing the construction of molecular complexity from readily available feedstocks. However, the direct use of hydrocarbon feedstocks as sp3-hybridized carbon radical precursors to participate in catalytic enantioselective functionalization of alkenes remains elusive. Here we describe an asymmetric 1,2-oxidative alkylation of conjugated dienes based on direct functionalization of strong and neutral C(sp3)–H bonds enabled by the combination of hydrogen atom transfer and copper catalysis. This approach allows carbon-centred radicals to be generated by selective activation of aliphatic C–H bonds, which are ubiquitous in hydrocarbons and other radical precursors. These radicals then react with 1,3-dienes to give allylic radicals which participate in copper-mediated asymmetric C–O coupling. This protocol provides a single-step access to various synthetically useful allylic esters directly from widely available chemical feedstocks.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information). Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC 2167286 (compound 73). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
Change history
06 January 2023
A Correction to this paper has been published: https://doi.org/10.1038/s44160-022-00238-7
References
Zheng, C. & You, S.-L. Recent development of direct asymmetric functionalization of inert C–H bonds. RSC Adv. 4, 6173–6214 (2014).
Newton, C. G., Wang, S.-G., Oliveira, C. C. & Cramer, N. Catalytic enantioselective transformations involving C–H bond cleavage by transition-metal complexes. Chem. Rev. 117, 8908–8976 (2017).
He, J., Wasa, M., Chan, K. S. L., Shao, Q. & Yu, J.-Q. Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev. 117, 8754–8786 (2017).
Rouquet, G. & Chatani, N. Catalytic functionalization of C(sp2)–H and C(sp3)–H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 52, 11726–11743 (2013).
He, J. et al. Ligand-controlled C(sp3)–H arylation and olefination in synthesis of unnatural chiral α-amino acids. Science 343, 1216–1220 (2014).
Zhang, F.-L., Hong, K., Li, T.-J., Park, H. & Yu, J.-Q. Functionalization of C(sp3)–H bonds using a transient directing group. Science 351, 252–256 (2016).
Saint-Denis, T. G., Zhu, R.-Y., Chen, G., Wu, Q.-F. & Yu, J.-Q. Enantioselective C(sp3)–H bond activation by chiral transition metal catalysts. Science 359, eaao4798 (2018).
Liao, K., Negretti, S., Musaev, D. G., Bacsa, J. & Davies, H. M. L. Site-selective and stereoselective functionalization of unactivated C–H bonds. Nature 533, 230–234 (2016).
Liao, K. et al. Site-selective and stereoselective functionalization of non-activated tertiary C–H bonds. Nature 551, 609–613 (2017).
Fu, J., Ren, Z., Bacsa, J., Musaev, D. G. & Davies, H. M. L. Desymmetrization of cyclohexanes by site- and stereoselective C–H functionalization. Nature 564, 395–399 (2018).
Cao, H., Tang, X., Tang, H., Yuan, Y. & Wu, J. Photoinduced intermolecular hydrogen atom transfer reactions in organic synthesis. Chem Catal. 1, 523–598 (2021).
Capaldo, L., Ravelli, D. & Fagnoni, M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C–H bonds elaboration. Chem. Rev. 122, 1875–1924 (2022).
Zhang, W. et al. Enantioselective cyanation of benzylic C–H bonds via copper-catalyzed radical relay. Science 353, 1014–1018 (2016).
Murphy, J. J., Bastida, D., Paria, S., Fagnoni, M. & Melchiorre, P. Asymmetric catalytic formation of quaternary carbons by iminium ion trapping of radicals. Nature 532, 218–222 (2016).
Mazzarella, D., Crisenza, G. E. M. & Melchiorre, P. Asymmetric photocatalytic C–H functionalization of toluene and derivatives. J. Am. Chem. Soc. 140, 8439–8443 (2018).
Li, Y., Lei, M. & Gong, L. Photocatalytic regio- and stereoselective C(sp3)–H functionalization of benzylic and allylic hydrocarbons as well as unactivated alkanes. Nat. Catal. 2, 1016–1026 (2019).
Li, Z. L., Fang, G. C., Gu, Q. S. & Liu, X. Y. Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 49, 32–48 (2020).
Zhu, S., Zhao, X., Li, H. & Chu, L. Catalytic three-component dicarbofunctionalization reactions involving radical capture by nickel. Chem. Soc. Rev. 50, 10836–10856 (2021).
Merino, E. & Nevado, C. Addition of CF3 across unsaturated moieties: a powerful functionalization tool. Chem. Soc. Rev. 43, 6598–6608 (2014).
Yan, M., Lo, J. C., Edwards, J. T. & Baran, P. S. Radicals: reactive Intermediates with translational potential. J. Am. Chem. Soc. 138, 12692–12714 (2016).
Kaga, A. & Chiba, S. Engaging radicals in transition metal-catalyzed cross-coupling with alkyl electrophiles: recent advances. ACS Catal. 7, 4697–4706 (2017).
Wang, P.-Z., Xiao, W.-J. & Chen, J.-R. Recent advances in radical-mediated transformations of 1,3-dienes. Chin. J. Catal. 43, 548–557 (2022).
Huang, H.-M. et al. Catalytic radical generation of π-allylpalladium complexes. Nat. Catal. 3, 393–400 (2020).
Zhu, R. & Buchwald, S. L. Enantioselective functionalization of radical intermediates in redox catalysis: copper-catalyzed asymmetric oxytrifluoromethylation of alkenes. Angew. Chem. Int. Ed. 52, 12655–12658 (2013).
Wang, F. et al. Enantioselective copper-catalyzed intermolecular cyanotrifluoromethylation of alkenes via radical process. J. Am. Chem. Soc. 138, 15547–15550 (2016).
Sha, W. et al. Merging photoredox and copper catalysis: enantioselective radical cyanoalkylation of styrenes. ACS Catal. 8, 7489–7494 (2018).
Chierchia, M., Xu, P., Lovinger, G. J. & Morken, J. P. Enantioselective radical addition/cross-coupling of organozinc reagents, alkyl iodides, and alkenyl boron reagents. Angew. Chem. Int. Ed. 58, 14245–14249 (2019).
Lin, J. S. et al. A dual-catalytic strategy to direct asymmetric radical aminotrifluoromethylation of alkenes. J. Am. Chem. Soc. 138, 9357–9360 (2016).
Wei, X., Shu, W., Garcia-Dominguez, A., Merino, E. & Nevado, C. Asymmetric Ni-catalyzed radical relayed reductive coupling. J. Am. Chem. Soc. 142, 13515–13522 (2020).
Guo, L. et al. General method for enantioselective three-component carboarylation of alkenes enabled by visible-light dual photoredox/nickel catalysis. J. Am. Chem. Soc. 142, 20390–20399 (2020).
Tu, H.-Y. et al. Enantioselective three-component fluoroalkylarylation of unactivated olefins through nickel-catalyzed cross-electrophile coupling. J. Am. Chem. Soc. 142, 9604–9611 (2020).
Lu, F. D., Lu, L. Q., He, G. F., Bai, J. C. & Xiao, W. J. Enantioselective radical carbocyanation of 1,3-dienes via photocatalytic generation of allylcopper complexes. J. Am. Chem. Soc. 143, 4168–4173 (2021).
Chen, J. et al. Photoinduced copper-catalyzed asymmetric C–O cross-coupling. J. Am. Chem. Soc. 143, 13382–13392 (2021).
Wang, P. Z. et al. Photoinduced copper-catalyzed asymmetric three-component coupling of 1,3-dienes: an alternative to Kharasch–Sosnovsky reaction. Angew. Chem. Int. Ed. 60, 22956–22962 (2021).
Zhang, Y. et al. Copper-catalyzed photoinduced enantioselective dual carbofunctionalization of alkenes. Org. Lett. 22, 1490–1494 (2020).
Zhu, X. et al. Asymmetric radical carboesterification of dienes. Nat. Commun. 12, 6670 (2021).
Ge, L. et al. Iron-catalysed asymmetric carboazidation of styrenes. Nat. Catal. 4, 28–35 (2021).
Campbell, M. W., Yuan, M., Polites, V. C., Gutierrez, O. & Molander, G. A. Photochemical C–H activation enables nickel-catalyzed olefin dicarbofunctionalization. J. Am. Chem. Soc. 143, 3901–3910 (2021).
Xu, S., Chen, H., Zhou, Z. & Kong, W. Three-component alkene difunctionalization by direct and selective activation of aliphatic C–H bonds. Angew. Chem. Int. Ed. 60, 7405–7411 (2021).
Ouyang, X.-H. et al. Intermolecular dialkylation of alkenes with two distinct C(sp3)–H bonds enabled by synergistic photoredox catalysis and iron catalysis. Sci. Adv. 5, eaav9839 (2019).
Kharasch, M. S. & Sosnovsky, G. The reactions of t-butyl perbenzoate and olefins—a stereospecific reaction. J. Am. Chem. Soc. 80, 756–756 (1958).
Kharasch, M. S., Sosnovsky, G. & Yang, N. C. Reactions of t-butyl peresters. I. The reaction of peresters with olefins. J. Am. Chem. Soc. 81, 5819–5824 (1959).
Lashley, J. C. Copper catalyzed allylic oxidation with peresters. Tetrahedron 58, 845–866 (2002).
Zhang, B., Zhu, S.-F. & Zhou, Q.-L. Copper-catalyzed enantioselective allylic oxidation of acyclic olefins. Tetrahedron Lett. 54, 2665–2668 (2013).
Lumbroso, A., Cooke, M. L. & Breit, B. Catalytic asymmetric synthesis of allylic alcohols and derivatives and their applications in organic synthesis. Angew. Chem. Int. Ed. 52, 1890–1932 (2013).
Zhu, R. & Buchwald, S. L. Versatile enantioselective synthesis of functionalized lactones via copper-catalyzed radical oxyfunctionalization of alkenes. J. Am. Chem. Soc. 137, 8069–8077 (2015).
Tunpanich, P., Limpongsa, E., Pongjanyakul, T., Sripanidkulchai, B. & Jaipakdee, N. Mucoadhesive sustained-release tablets for vaginal delivery of Curcuma comosa extracts: preparation and characterization. J. Drug Deliv. Sci. Technol. 51, 559–568 (2019).
Watthey, J. W. H., Stanton, J. L., Desai, M., Babiarz, J. E. & Finn, B. M. Synthesis and biological properties of (carboxyalkyl)amino-substituted bicyclic lactam inhibitors of angiotensin converting enzyme. J. Med. Chem. 28, 1511–1516 (1985).
Yanagisawa, H. et al. Angiotensin-converting enzyme inhibitors. 2. Perhydroazepin-2-one derivatives. J. Med. Chem. 31, 422–428 (1988).
Liu, L. et al. Nostosins, trypsin inhibitors isolated from the terrestrial cyanobacterium Nostoc sp. strain FSN. J. Nat. Prod. 77, 1784–1790 (2014).
Wang, X. et al. Total synthesis and stereochemical assignment of nostosin B. Mar Drugs 15, 58 (2017).
Urban, F. J. & Moore, B. S. Synthesis of optically active 2-benzyldihydrobenzopyrans for the hypoglycemic agent englitazone. J. Heterocyclic Chem. 29, 431–438 (1992).
Tian, B., Chen, P., Leng, X. & Liu, G. Palladium-catalysed enantioselective diacetoxylation of terminal alkenes. Nat. Catal. 4, 172–179 (2021).
Martynow, J. G. et al. A new synthetic approach to high-purity (15R)-latanoprost. Eur. J. Org. Chem. 2007, 689–703 (2007).
Andrus, M. B. & Zhou, Z. Highly enantioselective copper−bisoxazoline-catalyzed allylic oxidation of cyclic olefins with tert-butyl p-nitroperbenzoate. J. Am. Chem. Soc. 124, 8806–8807 (2002).
Walling, C. & Zavitsas, A. A. The copper-catalyzed reaction of peresters with hydrocarbons. J. Am. Chem. Soc. 85, 2084–2090 (1963).
Acknowledgements
Financial support from the National Key R&D Program of China (2021YFA1500100) and NSFC (22188101) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
L.-Z.G. and L.-F.F. conceived the project. L.-F.F., R.L. and X.-Y.R. performed the experiments and analysed the data. L.-Z.G. and P-S.W. supervised the research. L.-F.F. and L.-Z.G. co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Wangqing Kong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Figs. 1–4, NMR spectra, HPLC data and Tables 1–8.
Supplementary Data 1
Crystallographic data for compound 73, CCDC 2167286.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, LF., Liu, R., Ruan, XY. et al. Asymmetric 1,2-oxidative alkylation of conjugated dienes via aliphatic C–H bond activation. Nat. Synth 1, 946–955 (2022). https://doi.org/10.1038/s44160-022-00172-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00172-8
This article is cited by
-
Cobalt-catalyzed aminoalkylative carbonylation of alkenes toward direct synthesis of γ-amino acid derivatives and peptides
Nature Communications (2023)
-
Radical catalysis breaks and makes bonds
Nature Synthesis (2022)