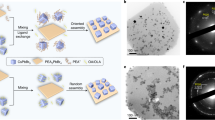
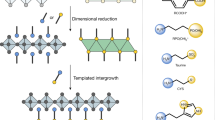
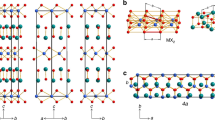
Abstract
Heterolayered structures consist of two or more different types of layer and can exhibit exceptional physical properties. Rational routes to synthesize new members of such compounds are required because most of these compounds have been discovered unintentionally. So far there is no generic method to vertically stack chemically different layers to form two-dimensional compounds owing to a lack of understanding of the synthesis of these materials. Here we report the use of molten hydroxides as unconventional solutions for the rapid stacking of oxide and chalcogenide layers with precise composition control. In addition, the crystal growth of heterolayered phases can be achieved by the reaction of different components at their diffusion front in molten hydroxides. This approach creates conditions in which the building blocks for each heterolayer can coexist, enabling heterolayered structures and bypassing the challenges of traditional solid-state chemistry methods where short reactant diffusion lengths predominate. This crystal growth methodology for heterolayers is also applicable to systems that do not form congruent melts at high temperatures.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available in the Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, with deposition numbers 2109506–2109520, corresponding to the compounds shown in the Supplementary Tables: 1 (Sr2Mn0.6Cu1.6Li0.4S2, CCDC 2109509), 2 (Sr2Mn0.5O2Cu1.31Li0.69Se2, CCDC 2109506), 3 (Sr2CoO2Cu1.81S2, CCDC 2109519), 4 (Sr2CoO2Cu2Se2, CCDC 2109507), 5 (Sr2NiO2Cu2Se2, CCDC 2109510), 6 (Ba2Co0.54Cu2O2Se2, CCDC 2109508), 7 (Sr2Mn0.56O2Ag1.84Li0.16Se2, CCDC 2109514), 8 (Ba3Ag2Fe1.82O3.73Se2, CCDC 2109513), 9 (Ba2Co0.68O2Ag2Se2, CCDC 2109515), 10 (Ba2Cu0.79O1.33Ag1.24Li0.76Se2, CCDC 2109511), 11 (Ba2Zn0.66O1.42Ag2Se2, CCDC 2109512), 12 (CoCu2Li5.51O2Se3, CCDC 2109517), 13 (SrMn0.5OCu3.63S2.5, CCDC 2109518), 14 (Sr2Ni0.7O2Cu2Se2, CCDC 2109516) and 15 (Sr2Cu0.68O2Cu2Se2, CCDC 2109520). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Kageyama, H. et al. Expanding frontiers in materials chemistry and physics with multiple anions. Nat. Commun. 9, 772 (2018).
Ueda, K., Hirose, S., Kawazoe, H. & Hosono, H. Electrical and optical properties of layered oxysulfides with CuS layers: Sr−Cu−M−O−S system (M = Zn, Ga, In). Chem. Mater. 13, 1880–1883 (2001).
Hiramatsu, H. et al. Crystal structures, optoelectronic properties, and electronic structures of layered oxychalcogenides MCuOCh (M = Bi, La; Ch = S, Se, Te): effects of electronic configurations of M3+ ions. Chem. Mater. 20, 326–334 (2008).
Sheng, Z. Z. & Hermann, A. M. Bulk superconductivity at 120 K in the Tl–Ca/Ba–Cu–O system. Nature 332, 138–139 (1988).
Kamihara, Y., Watanabe, T., Hirano, M. & Hosono, H. Iron-based layered superconductor La[O1-xFx]FeAs (x = 0.05−0.12) with Tc = 26 K. J. Am. Chem. Soc. 130, 3296–3297 (2008).
Wu, G. et al. Superconductivity at 56 K in samarium-doped SrFeAsF. J. Phys. Condens. Matter 21, 142203 (2009).
Hsu, F.-C. et al. Superconductivity in the PbO-type structure α-FeSe. Proc. Natl Acad. Sci. USA 105, 14262–14264 (2008).
Lu, X. F. et al. Superconductivity in LiFeO2Fe2Se2 with anti-PbO-type spacer layers. Phys. Rev. B 89, 020507 (2014).
Sun, H. et al. Soft chemical control of superconductivity in lithium iron selenide hydroxides Li1–xFex(OH)Fe1–ySe. Inorg. Chem. 54, 1958–1964 (2015).
Lu, X. F. et al. Coexistence of superconductivity and antiferromagnetism in (Li0.8Fe0.2)OHFeSe. Nat. Mater. 14, 325–329 (2015).
Pachmayr, U. et al. Coexistence of 3d-ferromagnetism and superconductivity in [(Li1−xFex)OH](Fe1−yLiy)Se. Angew. Chem. Int. Ed. 54, 293–297 (2015).
Hamann, D. M. et al. Structural changes as a function of thickness in [(SnSe)1+δ]mTiSe2 heterostructures. ACS Nano 12, 1285–1295 (2018).
Cordova, D. L. M. et al. Designed synthesis and structure–property relationships of kinetically stable [(PbSe)1+δ]m(VSe2)1 (m = 1, 2, 3, 4) heterostructures. Chem. Mater. 31, 8473–8483 (2019).
Mesoza Cordova, D. L., Kam, T. M., Gannon, R. N., Lu, P. & Johnson, D. C. Controlling the self-assembly of new metastable tin vanadium selenides using composition and nanoarchitecture of precursors. J. Am. Chem. Soc. 142, 13145–13154 (2020).
Mugavero, S. J. III, Gemmill, W. R., Roof, I. P. & zur Loye, H.-C. Materials discovery by crystal growth: lanthanide metal containing oxides of the platinum group metals (Ru, Os, Ir, Rh, Pd, Pt) from molten alkali metal hydroxides. J. Solid State Chem. 182, 1950–1963 (2009).
Bugaris, D. E. & zur Loye, H. C. Materials discovery by flux crystal growth: quaternary and higher order oxides. Angew. Chem. Int. Ed. 51, 3780–3811 (2012).
Bugaris, D. E., Smith, M. D. & zur Loye, H.-C. Hydroflux crystal growth of platinum group metal hydroxides: Sr6NaPd2(OH)17, Li2Pt(OH)6, Na2Pt(OH)6, Sr2Pt(OH)8, and Ba2Pt(OH)8. Inorg. Chem. 52, 3836–3844 (2013).
Chance, W. M., Bugaris, D. E., Sefat, A. S. & zur Loye, H.-C. Crystal growth of new hexahydroxometallates using a hydroflux. Inorg. Chem. 52, 11723–11733 (2013).
Albrecht, R., Graßme, F., Doert, T. & Ruck, M. Hydroflux syntheses and crystal structures of hydrogarnets Ba3[RE(OH)6]2 (RE = Sc, Y, Ho–Lu). Z. Naturforsch. B 1, 951–957 (2020).
Lux, H., Kuhn, R. & Niedermaier, T. Reaktionen und Gleichgewichte in Alkalihydroxydschmelzen. III. Peroxydgleichgewichte. Z. Anorg. Allg. Chem. 298, 285–301 (1959).
Flood, H. & Förland, T. The acidic and basic properties of oxides. Acta Chem. Scand. 1, 592–606 (1947).
Elwell, D. & Schell, H. Crystal Growth from High Temperature Solutions (Academic Press, 1975).
Chiotti, P. & Markuszewski, R. Binary systems sodium sulfide-sodium hydroxide and sodium carbonate-sodium hydroxide. J. Chem. Eng. Data 30, 197–201 (1985).
Seefuth, R. N. & Sharma, R. A. Solubility of Li2S in LiCl‐KCl melts. J. Electrochem. Soc. 135, 796 (1988).
Rickard, D. & Luther, G. W. Chemistry of iron sulfides. Chem. Rev. 107, 514–562 (2007).
Byrappa, K. & Yoshimura, M. Handbook of Hydrothermal Technology (William Andrew, 2012).
Albrecht, R. & Ruck, M. Chalcogenides by reduction of their dioxides in ultra-alkaline media. Angew. Chem. Int. Ed. 60, 22570–22577 (2021).
Zhu, W. J. & Hor, P. H. Unusual layered transition-metal oxysulfides: Sr2Cu2MO2S2 (M = Mn, Zn). J. Solid State Chem 130, 319–321 (1997).
Gál, Z. A. et al. Structural chemistry and metamagnetism of an homologous series of layered manganese oxysulfides. J. Am. Chem. Soc. 128, 8530–8540 (2006).
Adamson, P. et al. Competing magnetic structures and the evolution of copper ion/vacancy ordering with composition in the manganite oxide chalcogenides Sr2MnO2Cu1.5(S1–xSex)2. Chem. Mater. 24, 2802–2816 (2012).
Jin, S. et al. Sr2Mn3Sb2O2 type oxyselenides: structures, magnetism, and electronic properties of Sr2AO2M2Se2 (A = Co, Mn; M = Cu, Ag). Inorg. Chem. 51, 10185–10192 (2012).
Clarke, S. J. et al. Structures, physical properties, and chemistry of layered oxychalcogenides and oxypnictides. Inorg. Chem. 47, 8473–8486 (2008).
Otzschi, K., Ogino, H., Shimoyama, J.-I. & Kishio, K. New candidates for superconductors; a series of layered oxysulfides (Cu2S2)(Srn+1MnO3n−1). J. Low Temp. Phys. 117, 729–733 (1999).
Matsumoto, Y. et al. High-pressure synthesis of A2NiO2Ag2Se2 (A = Sr, Ba) with a high-spin Ni2+ in square-planar coordination. Angew. Chem. Int. Ed. 58, 756–759 (2019).
Cario, L. et al. Design and magnetic properties of new compounds containing iron 2D building blocks of the perovskite type. Solid State Sci. 7, 936–944 (2005).
Salter, E. J. T., Blandy, J. N. & Clarke, S. J. Crystal and magnetic structures of the oxide sulfides CaCoSO and BaCoSO. Inorg. Chem. 55, 1697–1701 (2016).
Cassidy, S. J. et al. Complex microstructure and magnetism in polymorphic CaFeSeO. Inorg. Chem. 55, 10714–10726 (2016).
Dawson, J. A., Famprikis, T. & Johnston, K. E. Anti-perovskites for solid-state batteries: recent developments, current challenges and future prospects. J. Mater. Chem. A 9, 18746–18772 (2021).
Rutt, O. J., Williams, G. R. & Clarke, S. J. Reversible lithium insertion and copper extrusion in layered oxysulfides. Chem. Commun. 2869–2871 (2006).
Blandy, J. N. et al. Synthesis, structure, and properties of the layered oxide chalcogenides Sr2CuO2Cu2S2 and Sr2CuO2Cu2Se2. Inorg. Chem. 57, 15379–15388 (2018).
Weiland, A., Felder, J. B., McCandless, G. T. & Chan, J. Y. One Ce, two Ce, three Ce, four? An intermetallic homologous series to explore: An+1BnX3n+1. Chem. Mater. 32, 1575–1580 (2020).
Bennema, P. Crystal growth from solution—theory and experiment. J. Cryst. Growth 24–25, 76–83 (1974).
Pritula, I. & Sangwal, K. in Handbook of Crystal Growth 2nd edn (ed. Rudolph, P) 1185–1227 (Elsevier, 2015).
Hektisch, H. K., Dennis, J. & Hanoka, J. I. Crystal growth in gels. J. Phys. Chem. Solids 26, 493–496 (1965).
Voorhees, P. W. The theory of Ostwald ripening. J. Stat. Phys. 38, 231–252 (1985).
Baldan, A. Review progress in Ostwald ripening theories and their applications to nickel-base superalloys part I: Ostwald ripening theories. J. Mater. Sci. 37, 2171–2202 (2002).
Smura, C. F. et al. High-spin cobalt(II) ions in square planar coordination: structures and magnetism of the oxysulfides Sr2CoO2Cu2S2 and Ba2CoO2Cu2S2 and their solid solution. J. Am. Chem. Soc. 133, 2691–2705 (2011).
Ueda, K. & Hosono, H. Crystal structure of LaCuOS1−xSex oxychalcogenides. Thin Solid Films 411, 115–118 (2002).
Ohki, Y., Takase, K., Takahashi, Y., Takano, Y. & Sekizawa, K. Preparation and crystal structure analysis and physical properties of (LaO)CuTe. J. Alloys Compd 408–412, 98–100 (2006).
Zhou, T. et al. Structures and physical properties of layered oxyselenides Ba2MO2Ag2Se2 (M = Co, Mn). Inorg. Chem. 53, 4154–4160 (2014).
Herkelrath, S. J. C., Saratovsky, I., Hadermann, J. & Clarke, S. J. Fragmentation of an infinite ZnO2 square plane into discrete [ZnO2]2− linear units in the oxyselenide Ba2ZnO2Ag2Se2. J. Am. Chem. Soc. 130, 14426–14427 (2008).
Acknowledgements
This work was supported by the US Department of Energy, Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division. Use of the Center for Nanoscale Materials, including SEM and the ACAT, an Office of Science user facility, was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357. Work at the beamlines 17-BM-B, 15-ID and 20-BM-B at the Advanced Photon Source at Argonne National Laboratory was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-06CH11357. NSF’s ChemMatCARS Sector 15 is supported by the Divisions of Chemistry (CHE) and Materials Research (DMR), National Science Foundation, under grant number NSF/CHE-1834750.
Author information
Authors and Affiliations
Contributions
The work was conceived by X.Z., D.Y.C. and M.G.K. with input from all authors. X.Z. carried out the synthesis, laboratory X-ray diffraction and elemental analysis. C.D.M. and X.Z. analysed the single-crystal diffraction data. X.Z., A.Y. and B.W. collected and analysed the in situ diffraction data. S.G.W. and Y.-S.C. collected the synchrotron single-crystal diffraction data. J.W. performed the TEM analysis and J.W. and L.Y. analysed the EELS spectra. M.B. carried out the XAS experiments. H.-H.W. collected the Raman spectra. D.-Y.C. and M.G.K. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Xiaolong Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–15, Figs. 1–16 and experimental details.
Supplementary Data 1
Crystallographic data for compound Sr2Mn0.6Cu1.6Li0.4S2, CSD 2109509.
Supplementary Data 2
Crystallographic data for compound Sr2Mn0.5O2Cu1.31Li0.69Se2, CSD 2109506.
Supplementary Data 3
Crystallographic data for compound Sr2CoO2Cu1.81S2, CSD 2109519.
Supplementary Data 4
Crystallographic data for compound Sr2CoO2Cu2Se2, CSD 2109507.
Supplementary Data 5
Crystallographic data for compound Sr2NiO2Cu2Se2, CSD 2109510.
Supplementary Data 6
Crystallographic data for compound Ba2Co0.54Cu2O2Se2, CSD 2109508.
Supplementary Data 7
Crystallographic data for compound Sr2Mn0.56O2Ag1.84Li0.16Se2, CSD 2109514.
Supplementary Data 8
Crystallographic data for compound Ba3Ag2Fe1.82O3.73Se2, CSD 2109513.
Supplementary Data 9
Crystallographic data for compound Ba2Co0.68O2Ag2Se2, CSD 2109515.
Supplementary Data 10
Crystallographic data for compound Ba2Cu0.79O1.33Ag1.24Li0.76Se2, CSD 2109511.
Supplementary Data 11
Crystallographic data for compound Ba2Zn0.66O1.42Ag2Se2, CSD 2109512.
Supplementary Data 12
Crystallographic data for compound CoCu2Li5.51O2Se3, CSD 2109517.
Supplementary Data 13
Crystallographic data for compound SrMn0.5OCu3.63S2.5, CSD 2109518.
Supplementary Data 14
Crystallographic data for compound Sr2Ni0.7O2Cu2Se2, CSD 2109516.
Supplementary Data 15
Crystallographic data for compound Sr2Cu0.68O2Cu2Se2, CSD 2109520.
Source data
Source Data Fig. 1
Tab ‘Fig.1c’ shows Li occupancy versus lattice constant c. Tab ‘Fig.1d’ shows lattice constant c and temperature as a function of normalized [LiOH].
Source Data Fig. 2
Tabs ‘Fig2a_400C’, ‘Fig2a_500C’ and ‘Fig2a_600C’ are raw data for 1D diffraction patterns (two-theta versus intensity) for in situ reactions at 400, 500 and 600 °C, respectively. They form the 2D pattern shown in Fig. 2a and the reduced data shown in Fig. 2b. The tab ‘Fig. 2b’ shows temperature (T) versus normalized molar ratio at 400, 500 and 600 °C, respectively.
Source Data Fig. 3
Tab ‘Fig.3b’ contains the calculated concentration profile using 1D Fick’s law of diffusion with concentration as a function of x (in cm). Tab ‘Fig.3c’ contains the calculated concentration profile for x versus C at different reaction times (t) with t = 1, 5, 10 and 24 h.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, X., Malliakas, C.D., Yakovenko, A.A. et al. Coherent approach to two-dimensional heterolayered oxychalcogenides using molten hydroxides. Nat. Synth 1, 729–737 (2022). https://doi.org/10.1038/s44160-022-00130-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00130-4