Abstract
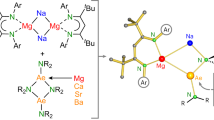
The synthesis of molecules that feature main-group elements in unusual oxidation states and coordination environments is a primary pursuit of main-group chemistry. The p-block elements saw early success towards this goal, and dozens of compounds that contain subvalent p-block metals, semi-metals and non-metals are now known. The development of reliable syntheses for these compounds made it possible to study them in detail, which expanded our understanding of bonding and electronic structure and served as the foundation from which catalysis mediated by main-group elements has emerged. For the group 2 elements, isolating reduced compounds has been a synthetic challenge that has spurred exciting progress in the synthesis of reduced alkaline earth compounds. The past two decades has seen the isolation of stable Be(0), Be(I), Mg(0), Mg(I) and Ca(I) compounds, along with studies of their reactivity profiles. In this Review, we overview the chemistry of isolated low-valent species with a focus on comparing newly discovered chemical trends and features among the different elements in the group. Finally, we discuss future directions and challenges for the field.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Power, P. P. Main-group elements as transition metals. Nature 463, 171–177 (2010).
Anastas, P. T. & Kirchhoff, M. M. Origins, current status, and future challenges of green chemistry. Acc. Chem. Res. 35, 686–694 (2002).
Greenwood, N. N. & Earnshaw, A. Chemistry of the Elements 2nd edn 107–138 (Butterworth-Heinemann, 1997).
Lide, R. D. CRC Handbook of Chemistry and Physics 77th edn (CRC, 1993).
Anker, M. D. & Hill, M. S. in Encyclopedia of Inorganic and Bioinorganic Chemistry (ed. R. A. Scott) 1–23 (John Wiley & Sons, 2017).
Harder, S. Molecular early main group metal hydrides: synthetic challenge, structures and applications. Chem. Commun. 48, 11165–11177 (2012).
Krieck, S. & Westerhausen, M. Kudos and renaissance of s-block metal chemistry. Inorganics 5, 17 (2017).
Hill, M. S., Liptrot, D. J. & Weetman, C. Alkaline earths as main group reagents in molecular catalysis. Chem. Soc. Rev. 45, 972–988 (2016).
Harder, S. Alkaline-Earth Metal Compounds: Oddities and Applications (Springer, 2013).
Jones, C. Open questions in low oxidation state group 2 chemistry. Commun. Chem. 3, 159 (2020).
Rösch, B. & Harder, S. New horizons in low oxidation state group 2 metal chemistry. Chem. Commun. 57, 9354–9365 (2021).
Pritchard, H. & Skinner, H. The concept of electronegativity. Chem. Rev. 55, 745–786 (1955).
Kambe, N., Iwasaki, T. & Terao, J. Pd-catalyzed cross-coupling reactions of alkyl halides. Chem. Soc. Rev. 40, 4937–4947 (2011).
Small, B. L., Brookhart, M. & Bennett, A. M. A. Highly active iron and cobalt catalysts for the polymerization of ethylene. J. Am. Chem. Soc. 120, 4049–4050 (1998).
Nakamura, M., Matsuo, K., Ito, S. & Nakamura, E. Iron-catalyzed cross-coupling of primary and secondary alkyl halides with aryl Grignard reagents. J. Am. Chem. Soc. 126, 3686–3687 (2004).
Bolm, C., Legros, J., Le Paih, J. & Zani, L. Iron-catalyzed reactions in organic synthesis. Chem. Rev. 104, 6217–6254 (2004).
Wenger, O. S. Is iron the new ruthenium? Chem. Eur. J. 25, 6043–6052 (2019).
Amatore, C. & Jutand, A. Anionic Pd(0) and Pd(II) intermediates in palladium-catalyzed Heck and cross-coupling reactions. Acc. Chem. Res. 33, 314–321 (2000).
Krieck, S., Görls, H., Yu, L., Reiher, M. & Westerhausen, M. Stable ‘inverse’ sandwich complex with unprecedented organocalcium(I): crystal structures of [(thf)2Mg(Br)-C6H2-2,4,6-Ph3] and [(thf)3Ca{μ-C6H3-1,3,5-Ph3}Ca(thf)3]. J. Am. Chem. Soc. 1391, 2977–2985 (2009).
Green, S. P., Jones, C. & Stasch, A. Stable magnesium(I) compounds with Mg–Mg bonds. Science 318, 1754–1757 (2007).
Arrowsmith, M. et al. Neutral zero-valent s-block complexes with strong multiple bonding. Nat. Chem. 8, 890–894 (2016).
Green, S. P., Jones, C. & Stasch, A. Stable adducts of a dimeric magnesium(I) compound. Angew. Chem. Int. Ed. 47, 9079–9083 (2008).
Overgaard, J., Jones, C., Stasch, A. & Iversen, B. B. Experimental electron density study of the Mg−Mg bonding character in a magnesium(I) dimer. J. Am. Chem. Soc. 131, 4208–4209 (2009).
Bonyhady, S. J. et al. β-Diketiminate-stabilized magnesium(I) dimers and magnesium(II) hydride complexes: synthesis, characterization, adduct formation, and reactivity studies. Chem. Eur. J. 16, 938–955 (2010).
Stasch, A. & Jones, C. Stable dimeric magnesium(I) compounds: from chemical landmarks to versatile reagents. Dalton Trans. 40, 5659–5672 (2011).
Boutland, A. J., Dange, D., Stasch, A., Maron, L. & Jones, C. Two-coordinate magnesium(I) dimers stabilized by super bulky amido ligands. Angew. Chem. Int. Ed. 55, 9239–9243 (2016).
Jones, D. D. L., Douair, I., Maron, L. & Jones, C. Photochemically activated dimagnesium(I) compounds: reagents for the reduction and selective C−H bond activation of inert arenes. Angew. Chem. Int. Ed. 60, 7087–7092 (2021).
Rösch, B. et al. Strongly reducing magnesium(0) complexes. Nature 592, 717–721 (2021).
Wang, G. et al. A stable, crystalline beryllium radical cation. J. Am. Chem. Soc. 142, 4560–4564 (2020).
Wang, G. et al. Isolation of cyclic(alkyl)(amino) carbene–bismuthinidene mediated by a beryllium(0) complex. Chem. Eur. J. 25, 4335–4339 (2019).
Ma, M. et al. Mg–Mg-bonded compounds with N,N′-dipp-substituted phenanthrene-diamido and o-phenylene-diamino ligands. Dalton Trans. 48, 2295–2299 (2019).
Liu, Y., Li, S., Yang, X.-J., Yang, P. & Wu, B. Magnesium−magnesium bond stabilized by a doubly reduced α-diimine: synthesis and structure of [K(THF)3]2[LMg−MgL] (L = [(2,6-iPr2C6H3)NC(Me)]22−. J. Am. Chem. Soc. 131, 4210–4211 (2009).
Schlenk, W. & Schlenk, W.Jr Über die Konstitution der Grignardschen Magnesiumverbindungen. Ber. Dtsch. Chem. Ges. 62, 920–924 (1929).
Silverman, G. S. & Rakita, P. E. Handbook of Grignard Reagents (CRC, 1996).
Westerhausen, M. 100 years after Grignard: where does the organometallic chemistry of the heavy alkaline earth metals stand today? Angew. Chem. Int. Ed. 40, 2975–2977 (2001).
Westerhausen, M., Koch, A., Görls, H. & Krieck, S. Heavy Grignard reagents: synthesis, physical and structural properties, chemical behavior, and reactivity. Chem. Eur. J. 23, 1456–1483 (2017).
Peltzer, R. M., Eisenstein, O., Nova, A. & Cascella, M. How solvent dynamics controls the Schlenk equilibrium of Grignard reagents: a computational study of CH3MgCl in tetrahydrofuran. J. Phys. Chem. B 121, 4226–4237 (2017).
Sarish, S. P., Nembenna, S., Nagendran, S. & Roesky, H. W. Chemistry of soluble β-diketiminatoalkaline-earth metal complexes with M−X bonds (M = Mg, Ca, Sr; X = OH, halides, H). Acc. Chem. Res. 44, 157–170 (2011).
Edelmann, F. T. N-silylated benzamidines: versatile building blocks in main group and coordination chemistry. Coord. Chem. Rev. 137, 403–481 (1994).
Jones, C. Bulky guanidinates for the stabilization of low oxidation state metallacycles. Coord. Chem. Rev. 254, 1273–1289 (2010).
Ruspic, C. & Harder, S. Big ligands for stabilization of small functionalities in calcium chemistry. Inorg. Chem. 46, 10426–10433 (2007).
Nesterov, V. et al. NHCs in main group chemistry. Chem. Rev. 118, 9678–9842 (2018).
Arduengo, A. J., Davidson, F., Krafczyk, R., Marshall, W. J. & Tamm, M. Adducts of carbenes with group II and XII metallocenes. Organometallics 17, 3375–3382 (1998).
Wolf, R. & Uhl, W. Main-group-metal clusters stabilized by N-heterocyclic carbenes. Angew. Chem. Int. Ed. 48, 6774–6776 (2009).
Hopkinson, M. N., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
Bellemin-Laponnaz, S. & Dagorne, S. Group 1 and 2 and early transition metal complexes bearing N-heterocyclic carbene ligands: coordination chemistry, reactivity, and applications. Chem. Rev. 114, 8747–8774 (2014).
Turner, Z. R. & Buffet, J.-C. Group 1 and 2 cyclic (alkyl)(amino)carbene complexes. Dalton Trans. 44, 12985–12989 (2015).
Wong, Y. O. et al. Two carbenes versus one in magnesium chemistry: synthesis of terminal dihalide, dialkyl, and Grignard reagents. Organometallics 38, 688–696 (2019).
Soleilhavoup, M. & Bertrand, G. Cyclic (alkyl)(amino)carbenes (CAACs): stable carbenes on the rise. Acc. Chem. Res. 48, 256–266 (2015).
Melaimi, M., Jazzar, R., Soleilhavoup, M. & Bertrand, G. Cyclic (alkyl)(amino)carbenes (CAACs): recent developments. Angew. Chem. Int. Ed. 56, 10046–10068 (2017).
Bailey, P. J., Dick, C. M., Fabre, S., Parsons, S. & Yellowlees, L. Complexation of dimethylmagnesium with α-diimines; structural and EPR characterisation of single electron and alkyl transfer products. Dalton Trans 2006, 1602–1610 (2006).
Freeman, L. A. et al. Stepwise reduction at magnesium and beryllium: cooperative effects of carbenes with redox non-innocent α-diimines. Inorg. Chem. 58, 10554–10568 (2019).
Fedushkin, I. L., Khvoinova, N. M., Skatova, A. A. & Fukin, G. K. Oxidative addition of phenylacetylene through C–H bond cleavage to form the MgII–dpp-bian complex: molecular structure of [Mg{dpp-bian(H)}(C≡CPh)(thf)2] and its diphenylketone insertion product [Mg(dpp-bian)·−{OC(Ph2)C≡CPh}(thf)]. Angew. Chem. Int. Ed. 42, 5223–5226 (2003).
Fedushkin, I. L. et al. Monomeric magnesium and calcium complexes containing the rigid, dianionic 1, 2-bis[(2,5-di-tert-butylphenyl)imino]acenaphthene (dtb-BIAN) and 1,2-bis[(2-biphenyl)imino]acenaphthene (bph-BIAN) ligands. Z. Anorg. Allg. Chem. 630, 501–507 (2004).
Fedushkin, I. L., Morozov, A. G., Rassadin, O. V. & Fukin, G. K. Addition of nitriles to alkaline earth metal complexes of 1,2-bis[(phenyl)imino]acenaphthenes. Chem. Eur. J. 11, 5749–5757 (2005).
Fedushkin, I. L., Chudakova, V. A., Skatova, A. A. & Fukin, G. K. Solvent-free alkali and alkaline earth metal complexes of di-imine ligands. Heteroat. Chem. 16, 663–670 (2005).
Gao, J., Liu, Y., Zhao, Y., Yang, X.-J. & Sui, Y. Syntheses and structures of magnesium complexes with reduced α-diimine ligands. Organometallics 30, 6071–6077 (2011).
Ren, W., Fang, X., Sun, W., Gu, D. & Yu, Y. A magnesium complex containing a reduced 2,2′-bipyridyl ligand: synthesis, structure, reactivity, and computational studies. J. Organomet. Chem. 842, 47–53 (2017).
Ren, W. & Gu, D. An azobenzenyl anion radical complex of magnesium: synthesis, structure, and reactivity studies. Inorg. Chem. 55, 11962–11970 (2016).
Couchman, S. A., Holzmann, N., Frenking, G., Wilson, D. J. D. & Dutton, J. L. Beryllium chemistry the safe way: a theoretical evaluation of low oxidation state beryllium compounds. Dalton Trans. 42, 11375–11384 (2013).
Merritt, J. M., Bondybey, V. E. & Heaven, M. C. Beryllium dimer—caught in the act of bonding. Science 324, 1548–1551 (2009).
De, S. & Parameswaran, P. Neutral tricoordinated beryllium(0) compounds—isostructural to BH3 but isoelectronic to NH3. Dalton Trans. 42, 4650–4656 (2013).
Yuan, C., Zhao, X.-F., Wu, Y.-B. & Wang, X. Ultrashort beryllium−beryllium distances rivalling those of metal−metal quintuple bonds between transition metals. Angew. Chem. Int. Ed. 55, 15651–15655 (2016).
Bondybey, V. E. & English, J. H. Laser vaporization of beryllium: gas phase spectrum and molecular potential of Be2. J. Chem. Phys. 80, 568–570 (1984).
Patkowski, K., Špirko, V. & Szalewicz, K. On the elusive twelfth vibrational state of beryllium dimer. Science 326, 1382–1384 (2009).
Sharma, S., Yanai, T., Booth, G. H., Umrigar, C. J. & Chan, G. K.-L. Spectroscopic accuracy directly from quantum chemistry: application to ground and excited states of beryllium dimer. J. Chem. Phys. 140, 104112 (2014).
Deible, M. J., Kessler, M., Gasperich, K. E. & Jordan, K. D. Quantum Monte Carlo calculation of the binding energy of the beryllium dimer. J. Chem. Phys. 143, 084116 (2015).
Liu, X. et al. Beryllium–beryllium double-π bonds in the octahedral cluster of Be2(μ2-X)4 (X = Li, Cu, BeF). Chem. Phys. Lett. 20, 23898–23902 (2018).
Naglav, D., Buchner, M. R., Bendt, G., Kraus, F. & Schulz, S. Off the beaten track—a hitchhiker’s guide to beryllium chemistry. Angew. Chem. Int. Ed. 55, 10562–10576 (2016).
Buchner, M. R. Recent contributions to the coordination chemistry of beryllium. Chem. Eur. J. 25, 12018–12036 (2019).
Herrmann, W. A., Runte, O. & Artus, G. Synthesis and structure of an ionic beryllium–‘carbene’ complex. J. Organomet. Chem. 501, C1–C4 (1995).
Schuster, J. K., Roy, D. K., Lenczyk, C., Mies, J. & Braunschweig, H. New outcomes of beryllium chemistry: Lewis base adducts for salt elimination reactions. Inorg. Chem. 58, 2652–2658 (2019).
Walley, J., Wong, Y.-O., Freeman, L., Dickie, D. & Gilliard, R. N-Heterocyclic carbene-supported aryl- and alkoxides of beryllium and magnesium. Catalysts 9, 934 (2019).
Soleilhavoup, M. & Bertrand, G. Borylenes: an emerging class of compounds. Angew. Chem. Int. Ed. 56, 10282–10292 (2017).
Singh, A. P. et al. A singlet biradicaloid zinc compound and its nonradical counterpart. J. Am. Chem. Soc. 135, 7324–7329 (2013).
Gilliard, R. J. et al. s‐Block multiple bonds: isolation of a beryllium imido complex. Angew. Chem. Int. Ed. 60, 9407–9411 (2021).
Bondybey, V. E. Electronic structure and bonding of Be2. Chem. Phys. Lett. 109, 436–441 (1984).
Brea, O., Mó, O., Yáñez, M., Alkorta, I. & Elguero, J. On the existence of intramolecular one-electron Be–Be bonds. Chem. Commun. 52, 9656–9659 (2016).
Saha, R., Pan, S., Merino, G. & Chattaraj, P. K. Unprecedented bonding situation in viable E2(NHBMe)2 (E = Be, Mg; NHBMe = (HCNMe)2B) complexes: neutral E2 forms a single E−E covalent bond. Angew. Chem. Int. Ed. 58, 8372–8377 (2019).
Walley, J. E. et al. s-Block carbodicarbene chemistry: C(sp3)–H activation and cyclization mediated by a beryllium center. Chem. Commun. 55, 1967–1970 (2019).
Czernetzki, C. et al. A neutral beryllium(I) radical. Angew. Chem. Int. Ed. 60, 20776–20780 (2021).
Paparo, A. et al. N-Heterocyclic carbene, carbodiphosphorane and diphosphine adducts of beryllium dihalides: synthesis, characterisation and reduction studies. Dalton Trans. 50, 7604–7609 (2021).
Arrowsmith, M. et al. Three-coordinate beryllium β-diketiminates: synthesis and reduction chemistry. Inorg. Chem. 51, 13408–13418 (2012).
Paparo, A. & Jones, C. Beryllium halide complexes incorporating neutral or anionic ligands: potential precursors for beryllium chemistry. Chem. Asian J. 14, 486–490 (2019).
Walley, J. E. et al. Cyclic(alkyl)(amino) carbene-promoted ring expansion of a carbodicarbene beryllacycle. Inorg. Chem. 58, 11118–11126 (2019).
Paparo, A., Smith, C. D. & Jones, C. Diagonally related s- and p-block metals join forces: synthesis and characterization of complexes with covalent beryllium–aluminum bonds. Angew. Chem. Int. Ed. 58, 11459–11463 (2019).
Roy, D. K. et al. Isolation and reactivity of an s-block metal antiaromatic. Angew. Chem. Int. Ed. 60, 3812–3818 (2020).
Tjurina, L. A. et al. Synthesis of cluster alkyl and aryl Grignard reagents in solution. Organometallics 23, 1349–1351 (2004).
Velazquez, A., Fernández, I., Frenking, G. & Merino, G. Multimetallocenes. A theoretical study. Organometallics 26, 4731–4736 (2007).
Xie, Y., Schaefer, H. F. & Jemmis, E. D. Characteristics of novel sandwiched beryllium, magnesium, and calcium dimers: C5H5BeBeC5H5, C5H5MgMgC5H5, and C5H5CaCaC5H5. Chem. Phys. Lett. 402, 414–421 (2005).
Petrie, S. Deep space organometallic chemistry. Aust. J. Chem. 56, 259–262 (2003).
Kruczyński, T. et al. From MgBr via single-electron transfer (SET) to a paramagnetic Mg(II) compound and back to Mg(I): [MgBr(L1)·]2 and [K(thf)3]2[Mg2(L1)2], L1 = RN=C(Me)C(Me)=NR, R = 2,6-diisopropylphenyl. Chem. Commun. 50, 15677–15680 (2014).
Arras, J., Kruczyński, T., Bresien, J., Schulz, A. & Schnöckel, H. Magnesium(I) halide versus magnesium metal: differences in reaction energy and reactivity monitored in reduction processes of P−Cl bonds. Angew. Chem. Int. Ed. 131, 726–731 (2019).
Wang, X. & Andrews, L. Infrared spectra of magnesium hydride molecules, complexes, and solid magnesium dihydride. J. Phys. Chem. A 108, 11511–11520 (2004).
Jasien, P. G. & Dykstra, C. E. Simplest magnesium cluster Grignard. Theoretical evidence for strong metal–metal stabilization of RMg2X species. J. Am. Chem. Soc. 105, 2089–2090 (1983).
Jones, C. Dimeric magnesium(I) β-diketiminates: a new class of quasi-universal reducing agent. Nat. Rev. Chem. 1, 0059 (2017).
Huber, R. & Weber, H. G. The collision complex in the exchange reaction Na + Na2. I. An ortho–para pumping experiment on Na2. Chem. Phys. 37, 173–180 (1979).
Kadlecek, S., Anderson, L. W., Erickson, C. J. & Walker, T. G. Spin relaxation in alkali–metal \({{\,}^1}{\Sigma}_{g}^{+}\) dimers. Phys. Rev. A 64, 052717 (2001).
Terrabuio, L. A., Teodoro, T. Q., Matta, C. F. & Haiduke, R. L. A. Nonnuclear attractors in heteronuclear diatomic systems. J. Phys. Chem. A 120, 1168–1174 (2016).
Timerghazin, Q. K. & Peslherbe, G. H. Non-nuclear attractor of electron density as a manifestation of the solvated electron. J. Chem. Phys. 127, 064108 (2007).
Platts, J. A., Overgaard, J., Jones, C., Iversen, B. B. & Stasch, A. First experimental characterization of a non-nuclear attractor in a dimeric magnesium(I) compound. J. Phys. Chem. A 115, 194–200 (2011).
Yuvaraj, K., Douair, I., Paparo, A., Maron, L. & Jones, C. Reductive trimerization of CO to the deltate dianion using activated magnesium (I) compounds. J. Am. Chem. Soc. 141, 8764–8768 (2019).
Boutland, A. J. et al. Reversible insertion of a C═C bond into magnesium(I) dimers: generation of highly active 1,2-dimagnesioethane compounds. J. Am. Chem. Soc. 139, 18190–18193 (2017).
Lalrempuia, R. et al. Activation of CO by hydrogenated magnesium(I) dimers: sterically controlled formation of ethenediolate and cyclopropanetriolate complexes. J. Am. Chem. Soc. 137, 8944–8947 (2015).
Bakewell, C., White, A. J. P. & Crimmin, M. R. Addition of carbon–fluorine bonds to a Mg(I)–Mg(I) bond: an equivalent of Grignard formation in solution. J. Am. Chem. Soc. 138, 12763–12766 (2016).
Paparo, A. et al. Reductive hexamerization of CO involving cooperativity between magnesium(I) reductants and [Mo(CO)6]: synthesis of well-defined magnesium benzenehexolate complexes. Angew. Chem. 133, 640–644 (2021).
Rösch, B. et al. Mg–Mg bond polarization induced by a superbulky β-diketiminate ligand. Chem. Commun. 56, 11402–11405 (2020).
Turner, Z. R. Chemically non-innocent cyclic (alkyl)(amino)carbenes: ligand rearrangement, C−H and C−F bond activation. Chem. Eur. J. 22, 11461–11468 (2016).
Hicks, J., Juckel, M., Paparo, A., Dange, D. & Jones, C. Multigram syntheses of magnesium(I) compounds using alkali metal halide supported alkali metals as dispersible reducing agents. Organometallics 37, 4810–4813 (2018).
Krieck, S., Görls, H. & Westerhausen, M. Mechanistic elucidation of the formation of the inverse Ca(I) sandwich complex[(thf)3Ca(μ-C6H3-1,3,5-Ph3)Ca(thf)3] and stability of aryl-substituted phenylcalcium complexes. J. Am. Chem. Soc. 132, 12492–12501 (2010).
Rösch, B. et al. Dinitrogen complexation and reduction at low-valent calcium. Science 371, 1125–1128 (2021).
Wu, X. et al. Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals. Science 361, 912–916 (2018).
Koch, D., Chen, Y., Golub, P. & Manzhos, S. Revisiting π backbonding: the influence of d orbitals on metal–CO bonds and ligand red shifts. Chem. Phys. Lett. 21, 20814–20821 (2019).
Koch, D., Chen, Y., Golub, P. & Manzhos, S. Reply to the ‘Comment on “Revisiting π backbonding: the influence of d orbitals on metal–CO bonds and ligand red shifts”’ by G. Frenking and S. Pan, Phys. Chem. Chem. Phys., 2019, 22. Phys. Chem. Chem. Phys. 22, 5380–5382 (2020).
Pan, S. & Frenking, G. Comment on “Revisiting π backbonding: the influence of d orbitals on metal–CO bonds and ligand red shifts” by D. Koch, Y. Chen, P. Golub and S. Manzhos, Phys. Chem. Chem. Phys., 2019, 21, 20814. Chem. Phys. Lett. 22, 5377–5379 (2020).
Zhao, L., Pan, S., Zhou, M. & Frenking, G. Response to Comment on “Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals”. Science 365, eaay5021 (2019).
Zhou, M. & Frenking, G. Transition-metal chemistry of the heavier alkaline earth atoms Ca, Sr, and Ba. Acc. Chem. Res. 54, 3071–3082 (2021).
Gilliard, R. J. et al. Isolation of cyclic(alkyl)(amino) carbene–bismuthinidene mediated by a beryllium(0) complex. Chem. Eur. J. 25, 4335–4339 (2019).
Acknowledgements
We are grateful to the University of Virginia for support of the Gilliard Research Group’s projects on alkaline earth metal chemistry. A special thanks is also extended to Gilliard Group members past and present, collaborators and other main-group teams that continue to advance alkaline earth metal chemistry.
Author information
Authors and Affiliations
Contributions
L.A.F. and J.E.W. wrote the review; R.J.G. directed the preparation and revision of the review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Conor Pranckevicius and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Freeman, L.A., Walley, J.E. & Gilliard, R.J. Synthesis and reactivity of low-oxidation-state alkaline earth metal complexes. Nat. Synth 1, 439–448 (2022). https://doi.org/10.1038/s44160-022-00077-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00077-6
This article is cited by
-
Reactivity of the magnesium bisamide complex towards C=C=O-, N=C=O-, and N—N=O-containing substrates
Russian Chemical Bulletin (2024)
-
Alkali metal reduction of alkali metal cations
Nature Communications (2023)