Abstract
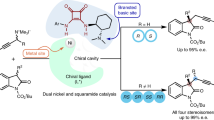
The configuration of biologically active molecules typically alters their physiological properties, which highlights the importance of preparing and fully characterizing all possible stereoisomers of a lead candidate or a given natural product. However, despite many advances in asymmetric synthesis, it remains challenging to completely control both the absolute and relative configuration in catalyst-mediated asymmetric reactions in which contiguous stereogenic centres are created in a single chemical transformation. Here we report a target-oriented stereodivergent propargylic substitution reaction to access four stereoisomers of amathaspiramide D and its analogues. By combining nickel and copper-catalysed stereodivergent propargylation, the key substituted 2-pyrrolidone intermediate was synthesized with excellent selectivity. The scope of the stereoselective propargylation process was demonstrated across a range of propargylic carbonate and aldimine ester substrates. The synthetic utility of the chiral propargylated α-amino ester products was shown through reductive and cyclization transformations.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The experimental data as well as the characterization data for all the compounds prepared during these studies are provided in the Supplementary Information. NMR data in a mnova file format and HPLC traces are available at https://zenodo.org/record/6326770#.YiG1suhBwuU, under the Creative Commons Attribution 4.0 international license. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2112078 ((S,R)-6), 2112074 (anti-8), 2112077 (syn-10), 2112076 (syn-11), 2112075 ((S,S)-11) and 1973954 ((S,R)-29). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. Comprehensive Asymmetric Catalysis (Springer, 1999).
Farina, V., Reeves, J. T., Senanayake, C. H. & Song, J. J. Asymmetric synthesis of active pharmaceutical ingredients. Chem. Rev. 106, 2734–2793 (2006).
Taylor, M. & Jacobsen, E. N. Asymmetric catalysis in complex target synthesis. Proc. Natl Acad. Sci. USA 101, 5368–5373 (2004).
Beletskaya, I. P., Nájera, C. & Yus, M. Stereodivergent catalysis. Chem. Rev. 118, 5080–5200 (2018).
Morris, B. D. & Prinsep, M. R. Amathaspiramides A−F, novel brominated alkaloids from the marine bryozoan Amathia wilsoni. J. Nat. Prod. 62, 688–693 (1999).
Hughes, C. & Trauner, D. The total synthesis of (−)-amathaspiramide F. Angew. Chem. Int. Ed. 41, 4556–4559 (2002).
Sakaguchi, K. et al. Total synthesis of (−)-amathaspiramide F. Org. Lett. 10, 5449–5452 (2008).
Chiyoda, K., Shimokawa, J. & Fukuyama, T. Total syntheses of all the amathaspiramides. Angew. Chem. Int. Ed. 51, 2505–2508 (2012).
O’Connor, M., Sun, C. & Lee, D. Synthesis of amathaspiramides by aminocyanation of enolates. Angew. Chem. Int. Ed. 54, 9963–9966 (2015).
Cai, S.-L., Song, R., Dong, H.-Q., Lin, G.-Q. & Sun, X.-W. Practical asymmetric synthesis of amathaspiramides B, D, and F. Org. Lett. 18, 1996–1999 (2016).
Soheili, A. & Tambar, U. K. Synthesis of (±)-amathaspiramide F and discovery of an unusual stereocontrolling element for the [2,3]-Stevens rearrangement. Org. Lett. 15, 5138–5141 (2013).
Cho, H. et al. Asymmetric Cα-alkylation of proline via chirality transfers of conformationally restricted proline derivative: application to the total synthesis of (−)-amathaspiramide F. Org. Lett. 20, 6121–6125 (2018).
Krautwald, S., Sarlah, D., Schafroth, M. A. & Carreira, E. M. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science 340, 1065–1068 (2013).
Du, Z. T. & Shao, Z.-H. Combining transition metal catalysis and organocatalysis—an update. Chem. Soc. Rev. 42, 1337–1378 (2012).
Chen, D.-F., Han, Z.-Y., Zhou, X.-L. & Gong, L.-Z. Asymmetric organocatalysis combined with metal catalysis: concept, proof of concept, and beyond. Acc. Chem. Res. 47, 2365–2377 (2014).
Romiti, F. et al. Different strategies for designing dual-catalytic enantioselective processes: from fully cooperative to non-cooperative systems. J. Am. Chem. Soc. 141, 17952–17961 (2019).
Schafroth, M. A., Zuccarello, G., Krautwald, S., Sarlah, D. & Carreira, E. M. Stereodivergent total synthesis of Δ9-tetrahydrocannabinols. Angew. Chem. Int. Ed. 53, 13898–13901 (2014).
Ding, C.-H. & Hou, X.-L. Catalytic asymmetric propargylation. Chem. Rev. 111, 1914–1937 (2011).
Sakata, K. & Nishibayashi, Y. Mechanism and reactivity of catalytic propargylic substitution reactions via metal–allenylidene intermediates: a theoretical perspective. Catal. Sci. Technol. 8, 12–25 (2018).
Zhang, D.-Y. & Hu, X.-P. Recent advances in copper-catalyzed propargylic substitution. Tetrahedron Lett. 56, 283–295 (2015).
Smith, S. W. & Fu, G. C. Nickel-catalyzed asymmetric cross-couplings of racemic propargylic halides with arylzinc reagents. J. Am. Chem. Soc. 130, 12645–12647 (2008).
Wendlandt, A. E., Vangal, P. & Jacobsen, E. N. Quaternary stereocentres via an enantioconvergent catalytic SN1 reaction. Nature 556, 447–451 (2018).
Watanabe, K. et al. Nickel-catalyzed asymmetric propargylic amination of propargylic carbonates bearing an internal alkyne group. Org. Lett. 20, 5448–5451 (2018).
Lu, F.-D. et al. Asymmetric propargylic radical cyanation enabled by dual organophotoredox and copper catalysis. J. Am. Chem. Soc. 141, 6167–6172 (2019).
Huo, H., Gorsline, B. J. & Fu, G. C. Catalyst-controlled doubly enantioconvergent coupling of racemic alkyl nucleophiles and electrophiles. Science 367, 559–564 (2020).
Miyazaki, Y., Zhou, B., Tsuji, H. & Kawatsura, M. Nickel-catalyzed asymmetric Friedel–Crafts propargylation of 3-substituted indoles with propargylic carbonates bearing an internal alkyne group. Org. Lett. 22, 2049–2053 (2020).
Chang, X., Zhang, J., Peng, L. & Guo, C. Collective synthesis of acetylenic pharmaceuticals via enantioselective nickel/Lewis acid-catalyzed propargylic alkylation. Nat. Commun. 12, 299 (2021).
Wu, Y., Huo, X. & Zhang, W. Synergistic Pd/Cu catalysis in organic synthesis. Chem. Eur. J. 26, 4895–4916 (2020).
Huo, X., He, R., Zhang, X. & Zhang, W. An Ir/Zn dual catalysis for enantio- and diastereodivergent α-allylation of α-hydroxyketones. J. Am. Chem. Soc. 138, 11093–11096 (2016).
Huo, X. et al. Stereoselective and site-specific allylic alkylation of amino acids and small peptides via a Pd/Cu dual catalysis. J. Am. Chem. Soc. 139, 9819–9822 (2017).
Liang, W., Xu, S.-M., Zhu, Q., Che, C. & Wang, C.-J. Synergistic Cu/Pd catalysis for enantioselective allylic alkylation of aldimine esters: access to α,α-disubstituted α-amino acids. Angew. Chem. Int. Ed. 56, 12312–12316 (2017).
Huo, X., Zhang, J., Fu, J., He, R. & Zhang, W. Ir/Cu dual catalysis: enantio- and diastereodivergent access to α,α-disubstituted α-amino acids bearing vicinal stereocenters. J. Am. Chem. Soc. 140, 2080–2084 (2018).
Wei, L., Zhu, Q., Xu, S.-M., Chang, X. & Wang, C.-J. Stereodivergent synthesis of α,α-disubstituted α-amino acids via synergistic Cu/Ir catalysis. J. Am. Chem. Soc. 140, 1508–1513 (2018).
Jiang, X., Boehm, P. & Hartwig, J. F. Stereodivergent allylation of azaaryl acetamides and acetates by synergistic iridium and copper catalysis. J. Am. Chem. Soc. 140, 1239–1242 (2018).
Zhang, Q. et al. Stereodivergent coupling of 1,3-dienes with aldimine esters enabled by synergistic Pd and Cu catalysis. J. Am. Chem. Soc. 141, 14554–14559 (2019).
He, Z.-T., Jiang, X. & Hartwig, J. F. Stereodivergent construction of tertiary fluorides in vicinal stereogenic pairs by allylic substitution with iridium and copper catalysts. J. Am. Chem. Soc. 141, 13066–13073 (2019).
Xu, S.-M. et al. Stereodivergent assembly of tetrahydro-γ-carbolines via synergistic catalytic asymmetric cascade reaction. Nat. Commun. 10, 5553 (2019).
He, R. et al. Stereodivergent Pd/Cu catalysis for the dynamic kinetic asymmetric transformation of racemic unsymmetrical 1,3-disubstituted allyl acetates. J. Am. Chem. Soc. 142, 8097–8103 (2020).
Zhu, M., Zhang, Q. & Zi, W. Diastereodivergent synthesis of β-amino alcohols by dual-metal-catalyzed coupling of alkoxyallenes with aldimine esters. Angew. Chem. Int. Ed. 60, 6545–6552 (2021).
Zhang, J. et al. Enantio- and diastereodivergent construction of 1,3-nonadjacent stereocenters bearing axial and central chirality through synergistic Pd/Cu catalysis. J. Am. Chem. Soc. 143, 12622–12632 (2021).
Peng, L., He, Z., Xu, X. & Guo, C. Cooperative Ni/Cu-catalyzed asymmetric propargylic alkylation of aldimine esters. Angew. Chem. Int. Ed. 59, 14270–14274 (2020).
Tasker, S. Z., Standley, E. A. & Jamison, T. F. Recent advances in homogeneous nickel catalysis. Nature 509, 299–309 (2014).
Coldham, I. & Hufton, R. Intramolecular dipolar cycloaddition reactions of azomethine ylides. Chem. Rev. 105, 2765–2810 (2005).
Wei, L., Chang, X. & Wang, C.-J. Catalytic asymmetric reactions with N-metallated azomethine ylides. Acc. Chem. Res. 53, 1084–1100 (2020).
Peng, L., Wang, H. & Cuo, C. Copper-catalyzed enantioselective difluoromethylation of amino acids via difluorocarbene. J. Am. Chem. Soc. 143, 6376–6381 (2021).
Jang, H., Romiti, F., Torker, S. & Hoveyda, A. H. Catalytic diastereo- and enantioselective additions of versatile allyl groups to N–H ketimines. Nat. Chem. 9, 1269–1275 (2017).
Zhang, S. et al. Delayed catalyst function enables direct enantioselective conversion of nitriles to NH2-amines. Science 364, 45–51 (2019).
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (grant no. 21971227, C.G.) and the Fundamental Research Funds for the Central Universities (WK2340000090, C.G.).
Author information
Authors and Affiliations
Contributions
C.G. conceived and designed the study and wrote the paper. Z.H. and L.P. performed the experiments and analysed the data. Z.H. performed the stereodivergent total synthesis of amathaspiramide D. L.P. performed the stereodivergent propargylic substitution reactions. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Dirk Trauner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, text and discussion.
Supplementary Data 1
Crystallographic data for compound (S,R)-6 CCDC 2112078.
Supplementary Data 2
Crystallographic data for compound anti-8 CCDC 2112074.
Supplementary Data 3
Crystallographic data for compound syn-10 CCDC 2112077.
Supplementary Data 4
Crystallographic data for compound syn-11 CCDC 2112076.
Supplementary Data 5
Crystallographic data for compound (S,S)-11 CCDC 2112075.
Supplementary Data 6
Crystallographic data for compound (S,R)-29 CCDC 1973954.
Rights and permissions
About this article
Cite this article
He, Z., Peng, L. & Guo, C. Catalytic stereodivergent total synthesis of amathaspiramide D. Nat. Synth 1, 393–400 (2022). https://doi.org/10.1038/s44160-022-00063-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00063-y
This article is cited by
-
Modular access to chiral bridged piperidine-γ-butyrolactones via catalytic asymmetric allylation/aza-Prins cyclization/lactonization sequences
Nature Communications (2024)
-
Chiral aldehyde-nickel dual catalysis enables asymmetric α−propargylation of amino acids and stereodivergent synthesis of NP25302
Nature Communications (2022)