Abstract
Background
Islet autoantibodies form the foundation for type 1 diabetes (T1D) diagnosis and staging, but heterogeneity exists in T1D development and presentation. We hypothesized that autoantibodies can identify heterogeneity before, at, and after T1D diagnosis, and in response to disease-modifying therapies.
Methods
We systematically reviewed PubMed and EMBASE databases (6/14/2022) assessing 10 years of original research examining relationships between autoantibodies and heterogeneity before, at, after diagnosis, and in response to disease-modifying therapies in individuals at-risk or within 1 year of T1D diagnosis. A critical appraisal checklist tool for cohort studies was modified and used for risk of bias assessment.
Results
Here we show that 152 studies that met extraction criteria most commonly characterized heterogeneity before diagnosis (91/152). Autoantibody type/target was most frequently examined, followed by autoantibody number. Recurring themes included correlations of autoantibody number, type, and titers with progression, differing phenotypes based on order of autoantibody seroconversion, and interactions with age and genetics. Only 44% specifically described autoantibody assay standardization program participation.
Conclusions
Current evidence most strongly supports the application of autoantibody features to more precisely define T1D before diagnosis. Our findings support continued use of pre-clinical staging paradigms based on autoantibody number and suggest that additional autoantibody features, particularly in relation to age and genetic risk, could offer more precise stratification. To improve reproducibility and applicability of autoantibody-based precision medicine in T1D, we propose a methods checklist for islet autoantibody-based manuscripts which includes use of precision medicine MeSH terms and participation in autoantibody standardization workshops.
Plain language summary
Islet autoantibodies are markers found in the blood when insulin-producing cells in the pancreas become damaged and can be used to predict future development of type 1 diabetes. We evaluated published literature to determine whether characteristics of islet antibodies (type, levels, numbers) could improve prediction and help understand differences in how individuals with type 1 diabetes respond to treatments. We found existing evidence shows that islet autoantibody type and number are most useful to predict disease progression before diagnosis. In addition, the age when islet autoantibodies first appear strongly influences rate of progression. These findings provide important information for patients and care providers on how islet autoantibodies can be used to understand future type 1 diabetes development and to identify individuals who have the potential to benefit from intervention or prevention therapy.
Similar content being viewed by others
Introduction
Type 1 diabetes (T1D) results from the immune-mediated destruction of insulin-producing pancreatic beta cells1. Clinical disease is characterized by progressive hyperglycemia that, if left untreated, leads to ketoacidosis and death. T1D can be managed with exogenous insulin, and while technology surrounding glucose monitoring and insulin delivery have revolutionized diabetes care, effective disease management remains difficult, time-consuming, and costly. Islet autoantibodies that recognize insulin (IAA), glutamic acid decarboxylase (GADA), protein phosphatase-like IA-2 (IA-2A), zinc transporter 8 (ZnT8A), and islet cell cytoplasmic antigen (ICA), are well-validated predictors of risk and disease progression and have been proposed as diagnostic markers of presymptomatic stages of T1D. Stage 1 T1D is defined by the presence of multiple islet autoantibodies and normal glucose tolerance. This progresses to stage 2 T1D (multiple islet autoantibodies and dysglycemia) and ultimately stage 3 T1D (meet American Diabetes Association (ADA) criteria for diabetes, usually with onset of clinical symptoms, typically requiring treatment with exogenous insulin)2. Understanding the pathophysiology that drives T1D progression through these stages remains critical to developing interventions to pause or reverse disease progression. However, vast heterogeneity exists in T1D progression, presentation, and responses to interventions. These differences suggest that differences in clinical features or presentation of disease progression and response to treatment could reflect discreet pathophysiological mechanisms. Along these lines, if distinct etiologic mechanisms are responsible for different forms of disease, it may be that specific subsets of individuals with T1D will respond better to specific disease-modifying therapies with improved risk/benefit ratios. Therefore, precision approaches to diagnosis may be necessary to effect disease modification in T1D.
The Precision Medicine in Diabetes Initiative (PMDI) was established in 2018 by the ADA in partnership with the European Association for the Study of Diabetes (EASD). The ADA/EASD PMDI includes global thought leaders in precision diabetes medicine who are working to address the burgeoning need for better diabetes prevention and care through precision medicine3. This T1D Diagnostics-focused Systematic Review is written on behalf of the ADA/EASD PMDI as part of a comprehensive evidence evaluation in support of the 2nd International Consensus Report on Precision Diabetes Medicine4.
Multiple observational studies and clinical trials have investigated the impact of genetics, immune markers, metabolic function, and environmental factors, on the development and progression of T1D5. Some of these works have identified subgroups of individuals who may theoretically derive greater benefit than others from particular therapies. In this systematic review, we sought to identify aspects of precision medicine that have the potential to be adopted into clinical practice over the next 10 years. Given the substantial body of work focused on optimization, reproducibility, and validation of islet autoantibodies as biomarkers of islet autoimmunity6,7,8,9,10, and their increased use in clinical practice since the development of the T1D staging system2, we chose to focus on islet autoantibodies as an individual feature of disease. We explored and summarized evidence that islet autoantibodies can be used to identify unique phenotypes of disease presentation and progression at four clinically-relevant timepoints: prior to clinical (stage 3) T1D diagnosis, at stage 3 T1D onset, after stage 3 T1D diagnosis, and in response to disease-modifying therapy at diagnosis (new onset trials) or before the time of stage 3 T1D diagnosis (prevention trials).
Here we show that autoantibody type/target was most frequently examined, followed by autoantibody number. Recurring themes included correlations of autoantibody number, type, and titers with progression, differing phenotypes based on order of autoantibody seroconversion, and interactions with age and genetics. Our findings suggest that the application of autoantibody features, specifically in relation to age and genetic risk, prior to diagnosis has the most potential to more precisely define and understand differences in T1D progression. To improve reproducibility and applicability of autoantibody-based precision medicine in T1D, we propose a methods checklist for islet autoantibody-based manuscripts which includes use of precision medicine MeSH terms and participation in autoantibody standardization workshops.
Methods
Data Source
We developed a search strategy using an iterative process that involved identification of Medical Subject Headings (MeSH) and text words, followed by refinement based on a sensitivity check for key articles identified by group members. On 10/25/21 “Precision Medicine”[Mesh] AND (Latent Autoimmune Diabetes in Adults [Mesh] OR “Diabetes Mellitus, Type 1 “[Mesh]) was applied as an initial search strategy to PubMed. Based on identification of only 128 papers (mostly narrative reviews), the search strategy was expanded to include additional terms linked to precision medicine (Supplementary Note 1). This strategy was applied to PubMed and EMBASE databases on 6/14/2022.
Study selection
The Covidence platform was used for stages of systematic review. To be included, studies must have involved individuals with high genetic risk (based on family history or genotype), single islet autoantibody positivity, stage 1 T1D (multiple islet autoantibody positivity and normal glucose tolerance), stage 2 T1D (positive islet autoantibodies and abnormal glucose tolerance), or stage 3 T1D (overt hyperglycemia, clinical symptoms of untreated T1D). Individuals with stage 3 T1D must have been within 1 year of diagnosis. Eligible study types included randomized controlled trials; systematic reviews or meta-analyses of randomized controlled trials; cross-sectional studies; open-label extension studies; prospective observational studies; retrospective observational studies; and post hoc analyses. Studies must have had a total sample size ≥10 per experimental or control group studied and have been published as a full paper in English in a peer-reviewed journal within 10 years of the search (2011–2022). Studies of non-T1D populations, unclearly classified diabetes populations, or mixed populations that included T1D among other diabetes types (type 2 diabetes, Latent Autoimmune Diabetes in Adults (LADA), gestational diabetes, or hypothetical cohort) were excluded. Several key articles identified by the group of experts that also met inclusion criteria but were not included in the search results because of search restrictions made to improve search feasibility, were also included in the analysis. In addition, clinical trials testing autoantibody features associated with response to disease-modifying therapies from the last 25 years were also added, given the modest number of clinical trials identified during the specified search period. These papers are denoted in their respective tables and reference lists by an asterisk (*).
Investigators independently screened and reviewed each potentially relevant article according to preliminary eligibility criteria determined by members of the review team. For Level 1 screening two investigators per article screened each title and abstract. Discordant assessments were discussed and resolved by consensus or arbitration after consultation with a member of the review leadership team (JLF, RO, KJG, MJR, or EKS). In January of 2022, to improve review feasibility, the decision was made to limit articles at the Level 2 screening step using additional inclusion criteria. Here articles were further limited to exposures testing detection of abnormal islet autoantibodies (i.e., presence, total number, type, or titer) and addressing outcomes related to progression to multiple antibody positivity or diabetes, heterogenous presentation of disease, progression of C-peptide loss after diabetes develops, or response to treatment. For Level 2 screening of eligible articles, full texts were retrieved and reviewed by two independent reviewers using the inclusion/exclusion criteria. Discordant assessments were similarly discussed and resolved.
Data extraction
Two independent investigators from the writing group extracted data from each article meeting inclusion criteria, with consensus determined by a member of the leadership team. Extracted data included details on participant characteristics, intervention outcomes, methods, and conclusions of precision analyses on disease progression or treatment response. Investigators performed quality assessments using a modified version of the Joanna Briggs Institute’s critical appraisal checklist tool for cohort studies (https://jbi.global/critical-appraisal-tools) in tandem for each eligible study to determine overall risk of bias. Discordant extractions and quality assessments were resolved as above.
Data analysis and synthesis
Because of heterogeneity of included studies (i.e., design, population, exposure, and outcomes tested) we were unable to perform a meta-analysis. Instead, we provide a list of key themes extracted from all studies in Table 1, and completed summaries of relevant studies (Tables 2 and 3 and Supplementary Data 1 and 2).
The protocol of this review was registered at PROSPERO (CRD42022340047) prior to implementation (available at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022340047).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Literature search and screening results
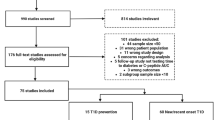
Of the 11,192 papers evaluated, 152 ultimately met inclusion criteria for extraction (Fig. 1). We categorized studies based on clinically relevant timepoints assessed: 91 characterized differences in rates of progression and clinical features prior to stage 3 T1D and were categorized as “prior to diagnosis” (Supplementary Data 1); 44 assessed differences in metabolic or immune features at stage 3 T1D onset and were categorized as “at diagnosis” (Supplementary Data 2; abbreviations and references for supplementary data provided in Supplementary Data 4 and 5, respectively); 11 characterized metabolic decline after diagnosis and were categorized as “after diagnosis” (Table 2); and 13 assessed differences in responses to disease-modifying therapies tested in clinical trials and were categorized as “treatment response” (Table 3). Of note, some papers included multiple studies of several outcomes; therefore, a total of 159 studies were identified from 152 papers. While the size prohibited inclusion of the list of studies from the “prior to diagnosis” and “at diagnosis” periods in the main text, a list of key themes extracted from all studies is included as Table 1.
For study classification, “Prior to diagnosis” refers to studies that assessed differences in rates of progression and clinical features during the period leading up to stage 3 T1D diagnosis. “At diagnosis” refers to studies that assessed heterogeneity in clinical features at the time of stage 3 T1D diagnosis. “After diagnosis” refers to studies that used features before or at the time of stage 3 T1D diagnosis to characterize subsequent metabolic decline (and preservation of endogenous insulin production). “Treatment response” refers to Studies that assessed heterogeneity in responses to disease-modifying therapies tested in clinical trials in subjects at or before stage 3 T1D diagnosis.
All papers in the analysis assessed established islet autoantibodies (IAA, GADA, IA-2A, ZnT8A, ICA) or novel autoantibodies targeting other islet autoantigens. Islet autoantibodies were a primary focus for a majority of the papers identified (101/152, 66%), while others included autoantibody assessments as part of a larger precision analysis or clinical trial follow-up. The most frequent autoantibody feature studied was autoantibody type/target protein (137/152, 90%), followed by autoantibody number (98/152, 64%), autoantibody titer (50/152, 33%), age at seroconversion (40/152, 26%), rate of seroconversion from single to multiple autoantibodies (32/152, 21%), order of autoantibody appearance after seroconversion (28/152, 18%), novel islet autoantibody/epitope identification (13/152, 9%), and autoantibody affinity (6/152, 4%). Four of 152 papers (3%) assessed the use of different autoantibody assays to improve specificity of autoantibody testing.
Only 10/152 (7%) studies focused on a population that did not feature primarily European ancestry (described in Supplementary Table 1). In 110 studies, race and ethnicity were not reported, and those that did report race and ethnicity used inconsistent approaches to reporting (e.g., combined vs. separated race and ethnicity categories). Of studies that reported race and ethnicity, the median percentage of participants identifying as non-Hispanic white was 89% (IQR 84%–97%).
Prior to diagnosis
The majority of the literature using autoantibody features to define heterogeneity in T1D focused on the period leading up to stage 3 T1D diagnosis (91/152, 60%). Studies included in the “prior to diagnosis” group are summarized in Supplementary Data 1, and key themes identified after systematic review of these papers are listed in Table 1. Of these, 85 evaluated longitudinal or cross-sectional cohorts (summarized in Table 4), typically testing differences in rates of diabetes progression. Median sample size was 510 (IQR 134–2239). Pediatric only populations were included in 61% (55/91); one study included only adults. The remainder (35/91, 38%) included pediatric and adult populations combined. Impact of age on findings was tested in 75/91 studies; with a significant impact of age reported in 85% (64/75). Assessment of islet autoantibody features during progression to T1D highlighted phenotypes characterized by age and genetic risk that were more clearly delineated with the addition of autoantibody type. Key recurring themes are summarized below.
Risk for progression to stage 3 T1D increases with autoantibody number
In 2013, a combined analysis of large birth cohorts (DAISY, DIPP, BABYDIAB, BABYDIET) from different countries showed that T1D risk progressively increased with increasing numbers of positive autoantibodies. Of the 585 children who developed at least 2 autoantibodies, 84% developed type 1 diabetes within 15 years of follow-up11. This appreciation that lifetime risk of diabetes progression nears 100% once multiple positive islet autoantibodies have developed informs the current T1D staging system2, and the impact of autoantibody number on risk of progression to stage 3 has been corroborated in numerous studies of additional cohorts12,13,14,15,16,17,18,19,20.
Younger age at seroconversion results in faster progression to stage 3 T1D
Longitudinal assessment of over 2500 children with genetic risk for T1D followed in the DAISY cohort revealed that speed of progression to T1D diagnosis is strongly correlated with age at seroconversion21. These findings have been replicated in many subsequent T1D screening studies including the 2013 combined analysis of over 13,000 children from multiple birth cohorts mentioned above11. The ongoing TrialNet Pathway to Prevention natural history study has followed over 30,000 first and second degree relatives of individuals with T1D and shown that frequency of seroconversion from single to multiple autoantibody positivity decreases with age (cumulative incidence 2% for age 10 and under, 0.7% for those over 10 years)22. The clear relationship between younger age and faster progression was particularly strong prior to puberty23. A recent analysis from TEDDY described an exponential decline in risk and rate of development of single and multiple autoantibodies with increasing age24.
Islet autoantibody type (IAA, GADA, IA-2A, ZnT8A, ICA) influences progression
In addition to autoantibody number, autoantibody type can be used to stratify risk more precisely for T1D progression. Overall, IA-2A and ZnT8A positivity have both been associated with increased T1D pathogenicity. Multiple studies described an increased risk of progression from single to multiple autoantibody positivity or to stage 3 T1D associated with IA-2A positivity15,18,19,25,26,27,28,29,30,31,32,33. This was most clearly seen in pediatric populations, as IA-2A positivity was preceded by or accompanied by development of other islet autoantibodies in 98% of the IA-2A positive children followed in the BABYDIAB cohort29. However, when pediatric and adult populations were evaluated together, ZnT8A positivity was most commonly associated with development of other autoantibodies, and in single autoantibody positive subjects, if the single autoantibody was ZnT8A, risk of progression to T1D was higher compared to single positivity for IAA, GADA, or IA-2A34. Positivity for IAA and GADA was more often associated with decreased risk or slower progression to T1D. In analysis of pediatric and adult subjects, IAA or GADA positive first degree relatives progressed more slowly to T1D than double autoantibody positive subjects positive for IA-2A and ZnT8A15. Reversion from single autoantibody positivity to autoantibody negativity was frequent for GADA and IAA, but not IA-2A and ZnT8A14. Multivariate analysis of subjects <20 years old showed that IA-2A, IAA, ICA, and ZnT8A positivity, but not GADA, could all independently predict diabetes progression34.
Order of autoantibody development varies by age and impacts risk for progression
Multiple longitudinal studies have shown that the first autoantibody to appear differs significantly depending on age of seroconversion. Analysis from the Finnish Type 1 Diabetes Prediction and Prevention (DIPP) study showed that in children 2 years old and younger, abnormal IAA titers most frequently develop first, while children ages 3–5 years more frequently seroconvert to GADA positivity35. A smaller analysis from the Diabetes Auto Immunity Study in the Young (DAISY) cohort found that higher IAA levels were associated with younger age at diagnosis, and that nearly all young children who progressed to T1D were IAA positive36. Analysis of the BABYDIAB and BABYDIET pediatric cohorts also found that earliest autoantibody development (peak incidence 9 months) was most commonly development of a single IAA which progressed to multiple autoantibodies. The Environmental Determinants of Diabetes in the Young (TEDDY) study has also suggested that IAA vs. GADA at seroconversion is linked to differing disease phenotypes, with additional features linked to IAA as the first autoantibody including specific single-nucleotide polymorphisms, male sex, father or siblings as a diabetic proband, introduction of probiotics at less than 1 month of age, and weight at 12 months37. Analysis of both pediatric and adult subjects combined revealed that the risk of progression from single to multiple autoantibodies decreased rapidly with increasing age when IAA was the first to develop. A decrease in risk with increasing age was also observed when GADA was the first to develop, but the risk reduction was less robust than that of IAA first38.
The addition of autoantibodies improves performance of genetic risk stratification to predict progression
Highest genetic risk for T1D is associated with genes that encode MHC class II molecules39. Multiple studies suggest that genetic risk stratification with other identified risk variants has the potential to be improved by the consideration of autoantibody features. While MHC class II-associated genetic risk is well defined, less is known about risk associated with MHC class I genes. In a study of Belgian adult and pediatric first-degree relatives who were carriers of the high-risk MHC class II HLA-DQ2/DQ8, risk was further increased by the presence of MHC class I HLA-A*24 if subjects were also positive for IA-2A, but not if subjects were IA-2A negative. Additional screening for MHC class I HLA-B*18 with HLA-DQ2/DQ8, HLA-A*24, and IA-2A and/or ZnT8A increased the sensitivity of detecting rapid progressors32. For single autoantibody positive relatives, combinations of autoantibody positivity and high-risk alleles improved risk prediction, with younger age, HLA-DQ2/DQ8 genotype, and IAA positivity acting as independent predictors of more rapid seroconversion to multiple autoantibody positivity. The addition of autoantibody features to predict progression in genetically at-risk, multiple autoantibody positive relatives was less useful for this cohort, as most multiple autoantibody positive relatives progress to T1D within 20 years. Progression did occur more rapidly in the presence of IA-2A or ZnT8A, regardless of age, HLA-DQ genotype, and autoantibody number15. Among single and multiple autoantibody subjects, the non-HLA risk variant PTPN22 risk allele (T/T) was associated with faster progression to T1D after appearance of the first and second autoantibodies, indicating a higher risk subgroup, while the INS risk allele had no impact on the risk of progression to T1D40. Performance of genetic risk scores calculated using multiple different genetic factors to predict disease progression were also shown to have the potential to be improved by the addition of autoantibody features. For example, the positive predictive value of a 30 T1D associated single-nucleotide polymorphism genetic risk score to predict T1D development in autoantibody positive individuals could be improved when the number of positive autoantibodies was also included in the model41.
Positive predictive value of autoantibody titer and affinity varies by type
In addition to autoantibody type, autoantibody titers and affinities were also measured in many studies. However, only higher IAA and IA-2A titers and affinities have been shown to be linked to more rapid disease progression42. In pediatric and adult first degree relatives followed in the DPT-1 cohort, IA-2A titers increase and GADA titers decrease in the years prior to T1D diagnosis28. Similar findings were supported in young European children with HLA-DQB1-conferred disease susceptibility and advanced beta-cell autoimmunity where, in addition to young age, higher BMI SDS, and reduced first phase insulin response, higher IAA and IA-2A levels predicted T1D43. In persistently autoantibody positive children in the TEDDY study, higher mean IAA and IA-2A levels, but not GADA levels were associated with increased T1D risk12,17. The addition of islet autoantibody features to existing metabolic measures alone will likely be less impactful in stratifying risk, particularly after development of abnormalities in glucose tolerance. In the DPT-1 study cohort, the addition of autoantibody titers did not improve a prediction model based on oral glucose tolerance testing, and IAA titers did not provide significant prediction value in subgroups with abnormal glucose tolerance44.
Specific autoantibody assay methods impact risk stratification
While autoantibody assay methods described were primarily radiobinding (RBA) or radioimmunoassays (RIA), other methods used included ELISA, other competitive and non-competitive binding assays, and indirect immunofluorescence. Four papers assessed differences between traditional radiobinding (RBA) assays and newer electrochemiluminescent (ECL) assays. Overall, this work suggested that ECL assays had higher positive predictive value, were more sensitive, and defined seroconversion earlier than traditional RBA assays45,46,47. This association was most pronounced in single autoantibody positive populations46. In the DAISY cohort, only 3 of 11 single autoantibody positive children testing positive for ZnT8A by RBA were also positive for ZnT8A by ECL. All 3 progressed to T1D, suggesting that ECL assays may identify a subset of higher risk, single autoantibody positive individuals47.
At diagnosis
We identified 44 relevant studies that assessed the use of antibodies to define heterogeneous phenotypes at stage 3 T1D onset in the “at diagnosis” group (Supplementary Data 2). Median sample size was 561 (IQR 266–1036). Pediatric only populations were included in 28/44 (64%).
Multiple studies demonstrated differences in autoantibody type by age at diagnosis. Compared to children at onset, adults were less likely to be ICA positive or IA-2A positive; however, there were no differences in GADA positivity rates48. In Chinese individuals with T1D, children with acute onset T1D showed higher prevalence of IA-2A, ZnT8A, and multiple autoantibody positivity than adults, and children diagnosed under 10 years had the highest frequency of IA-2A positivity and multiple antibody positivity49. Studies from the DAISY cohort found that age at diagnosis is strongly correlated with age at seroconversion and IAA levels21; however, this does not hold true for adults, where GADA is more commonly positive at diagnosis50. Children who develop autoantibodies and progress to T1D early in life have less functional beta-cell mass and higher rates of diabetic ketoacidosis (DKA) at diagnosis. No specific positive autoantibody type (GADA, IA-2A, IAA, ZnT8A) was consistently associated with DKA severity in children51.
After diagnosis
A total of 11 studies that assessed the use of autoantibodies to characterize progression after diagnosis were identified and summarized in Table 2. In these studies, autoantibodies were identified at diagnosis, prior to initiation of insulin; therefore, IAA positivity reflects loss of self-tolerance, rather than immune response to exogenous insulin. All studies used C-peptide measures to assess endogenous insulin production. Median sample size was smaller for this group (247, IQR 129–367). The majority (9/11, 82%) included only pediatric populations.
Conclusions from this set of studies varied widely; however, a general theme was that less autoimmunity at diagnosis (as reflected by autoantibody titer and number) was more commonly associated with greater residual C-peptide and more pronounced partial remission. Persistent autoantibody negative status was associated with preserved residual C-peptide in multiple studies52. Mean C-peptide 2 years post-diagnosis was correlated with absence of ICA or IAA at diagnosis in European ethnic groups53. Higher antibody number at diagnosis was associated with lower rates of partial remission54. In a study of new onset pediatric patients, when controlling for age, IAA positivity was associated with more rapid C-peptide decline post-diagnosis, while no relation was identified for GADA or IA-2A positivity55.
Treatment response
The “response to treatment” group included 13 primary randomized controlled trials of disease-modifying therapies tested prior to or at stage 3 T1D diagnosis (Table 3). Three of these trials were designed to test agents targeting a specific antigen in individuals who were positive for specific autoantibodies, with overall negative findings. The TrialNet oral insulin study, designed to test a subgroup identified as part of the DPT-1 study, where individuals with high IAA titers exhibited significant delay in time to diabetes compared to placebo, ultimately did not show an impact of oral insulin on time to diabetes in this population overall56. Similarly, studies testing whether a GADA antigen-based immunotherapy was effective in GADA positive individuals did not identify a treatment response in the overall study population57,58. Responses to immunomodulatory therapies were frequently reported to potentially differ by autoantibody type. Cyclosporin immune suppression tended to work more poorly in IA-2A positive individuals but reduced insulin requirements and increased C-peptide secretion in IA-2A negative individuals59. In the teplizumab anti-CD3 prevention trial, treatment response was greater when ZnT8A was negative, while the presence or absence of other autoantibodies was not as strongly associated with clinical response60. The B cell-depleting agent rituximab suppressed IAAs compared with placebo but had a much smaller effect on all other antibodies61,62. However, analysis of whether IAA positivity was associated with treatment response was not done. Importantly, all these trials tested combined pediatric and adult populations without considering age effects in autoantibody subgroup analyses, although none identified a statistically significant impact of age itself on treatment efficacy.
Risk of bias analyses
Reviewers performed assessments of specific metrics related to autoantibody assay quality as well as overall study design (Fig. 2). Metrics to assess performance of autoantibody assays are shown in Fig. 2a. Seventy-four percent (112/152) of studies applied the same assay to all participants tested; this either did not occur or was not clearly described in the remaining 26% (40/152) of studies. Methods used to measure autoantibodies were described in 72% (109/152) of papers. The 43 papers that did not give specific assay information typically either referenced another paper for methods (33/152, 22%) or did not focus on antibodies as a main outcome (8/152, 5%). About half (74/152, 49%) described characteristics of autoantibody assays utilized such as sensitivity, specificity, and assay variation. Papers that did not describe assay characteristics also most commonly referenced another paper for methods (43/74, 58%) or did not primarily focus on autoantibodies (20/74, 27%). Although references for antibody methods and standardization may have been included, only forty-four percent of total papers (67/152) specifically mentioned participation in an autoantibody standardization program in the manuscript text. Over half of the 85 papers that did not mention this type of program primarily focused on autoantibodies.
Quality assessments also touched on other aspects of study design. Reviewers judged that study participant groups were all recruited or identified from similar populations in most (134/152, 88%) papers. Confounding factors were presented in 87% (132/152) of papers, but only addressed in the analysis in 71% (108/152). Multiple analyses or comparisons were tested in the vast majority of papers (143/152, 94%), but only 24 of these (17%) described corrections for multiple comparisons. Statistical analyses were judged as clearly documented and able to be replicated in 85% (129/152) of papers, not clearly documented in 8% (12/152) and documented but with concerns raised for approach in 7% (11/152).
Diagnosis of T1D was considered an applicable outcome 91% (138/152) of papers and of these, 126/138 (91%) included valid and reliable measures of T1D diagnosis. For the 111 papers with dichotomous outcomes over a period of follow-up, 90% (100/111) clearly described methods to ensure that participants were free of the outcome at study start. 111 studies included longitudinal follow-up. Specific descriptions of the follow-up period were typically included; this was most commonly over >5 years (61/111, 55%), with 30% (33/111) followed for 2-5 years, and 11% (12/111) followed for <2 years. Duration of follow-up was clearly described in 106/111 (95%). Loss to follow up was much less commonly described. Specifics were only included in 29% (32/111) of applicable papers, and strategies to address loss to follow-up were only described in 19% (21/111) of applicable papers.
Discussion
This work explored and summarized evidence that islet autoantibodies could be used to identify and define specific phenotypes prior to, at, and after stage 3 T1D diagnosis, and in response to disease-modifying therapy. We systematically reviewed the application of antibody measurements to define heterogeneity at diagnostic timepoints before and after the onset of clinically symptomatic disease in 152 papers published over the past 10 years. The large majority of studies identified assessed antibody features prior to diagnosis, suggesting that overall, the application of antibody features to T1D precision diagnostics will be most impactful on defining differences in T1D phenotypes during this period of disease development.
Although multiple individual features (immune signatures, genetics, metabolic measures) could be applied to differentiate disease phenotypes, in this effort, we chose to focus on autoantibodies because standardized measures are currently available and their implementation as precision diagnostic tools in T1D has the potential to be rapidly implemented. As a well-established marker of islet autoimmunity, autoantibodies benefit from prior harmonization efforts, existing standardization workshops that compare assays using clinical samples, and for the most-established assays, easy accessibility to clinicians6,7,8,9. Indeed, the application of autoantibody number as a precision diagnostic tool that stratifies future disease risk has moved beyond the T1D research field, as the with the T1D staging system2 which is now being applied as part of clinical care guidelines to define stages of T1D63. Our review supports broad application of T1D staging using autoantibody number to guide individuals and clinicians on T1D risk. Furthermore, our findings support conclusions that have been drawn by others64 that additional antibody features, such as antibody titer, type, and order of appearance, could be utilized together and in concert with autoantibody number to more precisely stratify the current staging paradigm.
Our analysis supports existing evidence of the strong impact of age on heterogeneity of T1D development65. Specifically, our analysis confirms that younger age at seroconversion increases risk and rate for progression to stage 3 T1D, and this age-related risk can be further stratified using islet autoantibody type, titer, combined with number at seroconversion. The significant impact of age when considering use of autoantibodies to stratify risk suggests that (1) recommendations for use of autoantibodies in screening and prediction studies will need to consider stratification by age groups, and (2) that the analytic approach to autoantibody studies should include strategies to address impacts of age.
While most studies on autoantibodies in the period before Stage 3 T1D diagnosis focused on autoantibody number, type, or timing of seroconversion, fewer studies that passed our criteria for review assessed the immune responses that drive these changes. Therefore, there is continued need to understand how and when tolerance is broken in the context of clinical studies and how this leads to heterogeneous phenotypes. The few studies that did assess immune signatures66,67,68,69 in multiple autoantibody positive relatives revealed both proinflammatory and partially regulated (protective) phenotypes, which were also associated with autoantibody number. Interestingly, autoantibody negative relatives were characterized by the partially regulated phenotype66, suggesting that progression to T1D may be the result of insufficient suppressive mechanisms, rather than differences in antigen targets. Immunoregulatory signatures were also identified in high HLA-risk siblings of subjects with T1D who were autoantibody negative70, though this study was excluded from our review due to sample size.
Precision diagnostics has particular utility in stratifying risk beyond autoantibody number in single autoantibody positive individuals, a group that is considered lower risk for T1D progression overall, and consequently, often do not meet inclusion criteria for clinical trials that require multiple positive autoantibodies. Studies of ECL vs. RBA assays suggest that ECL assays can identify a subset of higher risk, single autoantibody positive individuals. Autoantibody type was identified in this review as a common approach to stratify risk among single autoantibody positive individuals. For example, given the rarity of IA-2A as the initial autoantibody at seroconversion in birth cohorts, individuals who are cross-sectionally single autoantibody positive for IA-2A may reflect a higher risk group that has reverted to single autoantibody positivity, and are at higher risk of progression to multiple autoantibody positivity and ultimately T1D. The T1DI analysis of over 24,000 children at increased genetic risk for T1D from prospective cohort studies in Finland, Germany, Sweden, and the US, revealed that HLA-DR-DQ genotypes can stratify risk progression among children retaining a single autoantibody71.
Evidence for use of antibodies at, after, and in response to disease-modifying therapies was less robust, and far fewer studies were identified. At diagnosis, the presence or absence of specific islet autoantibodies was also correlated with age, which might be expected given the differences in autoantibody presentation at seroconversion. However, multiple studies suggested that the primary autoantibodies at seroconversion had often disappeared at the time of diagnosis14,72,73,74,75; this is an open question in the field. While some evidence suggested that declining islet autoantibody titers and numbers after diagnosis corresponded to preserved residual C-peptide, we did not find convincing evidence to support the use of islet autoantibodies to define heterogeneity in metabolic outcomes after stage 3 diagnosis. Evidence for use of islet autoantibody features to predict responses to disease-modifying therapies was modest. One potential explanation for this finding could be epitope spreading and neoantigen expansion that accompanies T1D disease progression, making the impact of a specific antigen (and its corresponding autoantibody) less significant by the time an individual has reached more advanced stages of disease.
Of note, our initial search strategy that targeted papers with combined use of MeSH (Medical Subject Headings) terms for “Precision Medicine” and “Type 1 Diabetes” identified only a small number of papers which were predominantly commentaries or reviews with very few original research articles. This likely represents the relatively recent application of precision medicine concepts to the field as well as a broader issue surrounding nebulous definitions of precision medicine76. Moving forward, inclusion of “Precision Medicine” as a MeSH term in manuscripts focused on T1D heterogeneity, stratification, or endotypes will be critical to allow researchers to easily access relevant studies in this area.
Many of the studies we reviewed emanated from prospective longitudinal cohort studies either from prevention trials or natural history cohorts, such as DPT-1, DAISY, BABYDIAB, TEDDY, Fr1da, DIPP, and the TrialNet Pathway to Prevention study. Likely related to this, overall, outcomes were judged to be reliably ascertained, and participants had substantial durations of follow-up (55% documented follow-up beyond 5 years). These qualities highlight the exceptional value that natural history cohorts have brought to the field of T1D precision medicine overall. However, our review has also highlighted quality concerns that will benefit from being addressed moving forward. An area that is particularly high yield is the use of autoantibody standardization workshops aimed at improving the performance and concordance of immunoassays used to measure islet autoantibodies. Despite standardization programs being available throughout the timeframe studied in this review77, participation in theses workshops was not uniformly described, even among papers with autoantibodies as a primary focus. Especially with more novel assays, clear reporting of methods and validation efforts are critical to the reproducibility of findings6. The fact that the framework has already been set to do this through the establishment of existing standardization programs makes more consistent and explicitly identified participation in these workshops “low hanging fruit” for improvement of study quality.
Loss to follow-up in longitudinal studies was not frequently documented. While analysis strategies frequently addressed differences in follow-up duration, systematic differences in loss to follow-up amongst different populations could theoretically still impact findings. Additionally, given the frequent reporting of interactions of autoantibody findings with age, consideration of relationships with age and other confounding factors is critical. This did not appear to be addressed in 30% of papers reviewed. Finally, many of the analyses of autoantibody subgroups in clinical trials were post hoc assessments, such that robust association of features with treatment response will require confirmation in a trial specifically designed for these subgroups.
The vast majority of studies assessed emanated from cohorts that were composed of groups of individuals of primarily European ancestry. Specific reporting on race and ethnicity were uncommon (only present in about ¼ of papers) and were inconsistently applied. Validation of antibodies as a tool for precision diagnosis across diverse populations, such as has been performed with genetic T1D risk scores78 will be important to ensure broader applicability.
There are some limitations to this analysis. While our original goal was a meta-analysis of studies conducted at each time point, given the significant heterogeneity of exposures, outcomes, and study conditions, we were limited to a systematic review of the state of the literature. Mainly for review feasibility, we limited our search to a 10-year period and to outcomes related to islet autoantibodies. Because of this, important papers in the field that did not meet inclusion criteria or published earlier or later than our search were not included as part of this review. Since the first estimation of T1D risk using ICA and HLA in relatives of individuals with T1D in the 1988 analysis of the Barts-Windsor Family study79, leaders in the field of T1D prediction have been using autoantibody features to stratify T1D risk. This review indeed stands upon the shoulders of giants whose work was recently elegantly highlighted by Bonifacio and Achenbach in their 2019 review of islet autoantibody history80. Importantly, the key finding that the use of autoantibodies for application of precision medicine is highest yield in the period prior to T1D diagnosis is supported by papers published prior to 2011.
More recently published papers were not included based on timing of our literature search, but are nonetheless important. For example, the Fr1da-study group recently published data that showed IA-2A positivity and titer, in combination with hemoglobin A1c and OGTT glucose values, could be used to generate a progression likelihood score that effectively identified presymptomatic multiple autoantibody positive children at very high risk of progression to clinical disease81. More recently, multiple studies generated from the harmonized data of five prospective cohorts into the combined T1DI cohort have been published, highlighting important insights gained from analysis of this cohort, including how the stringency of the definition of multiple Aab positivity markedly alters the risk of progression82, suggesting that initial screening for islet autoantibodies at two ages (2 and 6 years) may be a sensitive and efficient approach to T1D population screening83, and identifying trajectories of progression based on autoantibody positivity and titer84,85. We anticipate that data generated from this powerful, harmonized cohort will continue to have important implications on the application of autoantibody status to precision diagnostics.
Our review inevitably excluded populations of adult individuals with T1D who have been historically misclassified as having type 2 diabetes. Along these lines, we decided not to include LADA in this review due to significant inconsistencies in the definition of LADA in many studies, but this group of individuals certainly contribute to T1D heterogeneity. Whether the findings noted here can be replicated in studies of LADA populations deserves future study/review.
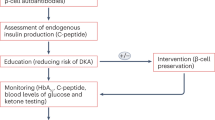
Notwithstanding these limitations, overall, our findings suggest that islet autoantibodies are likely to be most useful to define T1D heterogeneity prior to clinical diagnosis, supporting prior efforts to use autoantibodies as part of precision T1D staging. Further benefit may be gained by their incorporation into risk scores that include features beyond autoantibody number and also consider age and genetics. Moving forward, thoughtfully designed, prospective analyses to test these relationships with disease-modifying therapies will be critical for further application of these observations and development of precision medicine approaches to T1D disease-modifying therapies. To aid in potential precision application and assessment of potential risk of bias, in Fig. 3 we provide a suggested checklist for studies applying islet autoantibodies to phenotypic heterogeneity before and after T1D diagnosis. Additionally, systematic review of other individual features, such as genetics, metabolic function, and other immune findings, will likely provide further insight into the current evidence for strategies to apply these features to precision T1D diagnostics.
Data availability
All studies reviewed were identified and can be accessed via publicly available databases (PubMed and Embase). Source data can be found in Supplementary Data 3. A full list of included studies is available in Supplementary Data 6. Article review data supporting the findings of this study are available upon reasonable request from the corresponding author.
References
DiMeglio, L. A., Evans-Molina, C. & Oram, R. A. Type 1 diabetes. Lancet 391, 2449–2462 (2018).
Insel, R. A. et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 38, 1964–1974 (2015).
Nolan, J. J. et al. ADA/EASD precision medicine in diabetes initiative: an international perspective and future vision for precision medicine in diabetes. Diabetes Care 45, 261–266 (2022).
Tobias, D. K. et al. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat. Med. 29, 2438–2457 (2023).
Battaglia, M. et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 43, 5–12 (2020).
Lampasona, V. et al. Islet Autoantibody Standardization Program 2018 Workshop: interlaboratory comparison of glutamic acid decarboxylase autoantibody assay performance. Clin. Chem. 65, 1141–1152 (2019).
Bonifacio, E. et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J. Clin. Endocrinol. Metab. 95, 3360–3367 (2010).
Mire-Sluis, A. R., Gaines Das, R. & Lernmark, Å. The World Health Organization international collaborative study for islet cell antibodies. Diabetologia 43, 1282–1292 (2000).
Bingley, P. J. & Williams, A. J. K. Validation of autoantibody assays in type 1 diabetes: workshop programme. Autoimmunity 37, 257–260 (2004).
Marzinotto, I. et al. Islet autoantibody standardization program: interlaboratory comparison of insulin autoantibody assay performance in 2018 and 2020 workshops. Diabetologia 66, 897–912 (2023).
Ziegler, A. G. et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. J. Am. Med. Assoc. 309, 2473–2479 (2013).
Steck, A. K. et al. Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 38, 808–813 (2015).
Giannopoulou, E. Z. et al. Islet autoantibody phenotypes and incidence in children at increased risk for type 1 diabetes. Diabetologia 58, 2317–2323 (2015).
Vehik, K. et al. Reversion of β-cell autoimmunity changes risk of type 1 diabetes: TEDDY study. Diabetes Care 39, 1535–1542 (2016).
Gorus, F. K. et al. Twenty-year progression rate to clinical onset according to autoantibody profile, age, and HLA-DQ genotype in a registry-based group of children and adults with a first-degree relative with type 1 diabetes. Diabetes Care 40, 1065–1072 (2017).
Pöllänen, P. M. et al. Characterisation of rapid progressors to type 1 diabetes among children with HLA-conferred disease susceptibility. Diabetologia 60, 1284–1293 (2017).
Steck, A. K. et al. Predicting progression to diabetes in islet autoantibody positive children. J. Autoimmun. 90, 59–63 (2018).
Jacobsen, L. M. et al. Predicting progression to type 1 diabetes from ages 3 to 6 in islet autoantibody positive TEDDY children. Pediatr. Diabetes 20, 263–270 (2019).
Pöllänen, P. M. et al. Characteristics of slow progression to type 1 diabetes in children with increased HLA-conferred disease risk. J. Clin. Endocrinol. Metab. 104, 5585–5594 (2019).
Korneva, K. G. et al. Diagnostic capabilities of islet autoantibodies in children with new-onset type 1 diabetes mellitus and healthy siblings. Sovrem. Tekhnologii Med. 12, 29–35 (2021).
Steck, A. K. et al. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care 34, 1397–1399 (2011).
Vehik, K. et al. Development of autoantibodies in the TrialNet natural history study. Diabetes Care 34, 1897–1901 (2011).
Parikka, V. et al. Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia 55, 1926–1936 (2012).
Bonifacio, E. et al. An age-related exponential decline in the risk of multiple islet autoantibody seroconversion during childhood. Diabetes Care 44, 2260–2268 (2021).
Jacobsen, L. M. et al. The risk of progression to type 1 diabetes is highly variable in individuals with mulitple autoantibodies following screening. Diabetologia 63, 588–596 (2020).
Vehik, K. et al. Hierarchical order of distinct autoantibody spreading and progression to type 1 diabetes in the TEDDY study. Diabetes Care 43, 2066–2073 (2020).
Eising, S. et al. Danish children born with glutamic acid decarboxylase-65 and islet antigen-2 autoantibodies at birth had an increased risk to develop type 1 diabetes. Eur. J. Endocrinol. 164, 247 (2011).
Sosenko, J. M. et al. A longitudinal study of GAD65 and ICA512 autoantibodies during the progression to type 1 diabetes in diabetes prevention trial-type 1 (DPT-1) participants. Diabetes Care 34, 2435–2437 (2011).
Krause, S. et al. IA-2 autoantibody affinity in children at risk for type 1 diabetes. Clin. Immunol. 145, 224–229 (2012).
Achenbach, P. et al. Characteristics of rapid vs slow progression to type 1 diabetes in multiple islet autoantibody-positive children. Diabetologia 56, 1615–1622 (2013).
Gorus, F. K. et al. Screening for insulinoma antigen 2 and zinc transporter 8 autoantibodies: a cost-effective and age-independent strategy to identify rapid progressors to clinical onset among relatives of type 1 diabetic patients. Clin. Exp. Immunol. 171, 82–90 (2013).
Mbunwe, E. et al. In antibody-positive first-degree relatives of patients with type 1 diabetes, HLA-A*24 and HLA-B*18, but not HLA-B*39, are predictors of impending diabetes with distinct HLA-DQ interactions. Diabetologia 56, 1964–1970 (2013).
Xu, P. & Krischer, J. P. Prognostic classification factors associated with development of multiple autoantibodies, dysglycemia, and type 1 diabetes—a recursive partitioning analysis. Diabetes Care 39, 1036–1044 (2016).
Yu, L. et al. Zinc transporter-8 autoantibodies improve prediction of type 1 diabetes in relatives positive for the standard biochemical autoantibodies. Diabetes Care 35, 1213–1218 (2012).
Ilonen, J. et al. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes 62, 3636–3640 (2013).
Yu, L. et al. Proinsulin/insulin autoantibodies measured with electrochemiluminescent assay are the earliest indicator of prediabetic islet autoimmunity. Diabetes Care 36, 2266–2270 (2013).
Krischer, J. P. et al. Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: the TEDDY study. Diabetes Care 40, 1194–1202 (2017).
Bosi, E. et al. Impact of age and antibody type on progression from single to multiple autoantibodies in type 1 diabetes relatives. J. Clin. Endocrinol. Metab. 102, 2881–2886 (2017).
Redondo, M. J., Steck, A. K. & Pugliese, A. Genetics of type 1 diabetes. Pediatr. Diabetes 19, 346–353 (2018).
Lempainen, J. et al. Effect of the PTPN22 and INS risk genotypes on the progression to clinical type 1 diabetes after the initiation of β-cell autoimmunity. Diabetes 61, 963–966 (2012).
Redondo, M. J. et al. A type 1 diabetes genetic risk score predicts progression of islet autoimmunity and development of type 1 diabetes in individuals at risk. Diabetes Care 41, 1887–1894 (2018).
Bender, C., Schlosser, M., Christen, U., Ziegler, A. G. & Achenbach, P. GAD autoantibody affinity in schoolchildren from the general population. Diabetologia 57, 1911–1918 (2014).
Siljander, H. T. et al. Insulin secretion and sensitivity in the prediction of type 1 diabetes in children with advanced β-cell autoimmunity. Eur. J. Endocrinol. 169, 479–485 (2013).
Xu, P. et al. Prognostic accuracy of immunologic and metabolic markers for type 1 diabetes in a high-risk population: receiver operating characteristic analysis. Diabetes Care 35, 1975–1980 (2012).
Fouts, A. et al. Do electrochemiluminescence assays improve prediction of time to type 1 diabetes in autoantibody-positive TrialNet subjects? Diabetes Care 39, 1738–1744 (2016).
Sosenko, J. M. et al. The use of electrochemiluminescence assays to predict autoantibody and glycemic progression toward type 1 diabetes in individuals with single autoantibodies. Diabetes Technol. Ther. 19, 183–187 (2017).
Jia, X. et al. High-affinity ZnT8 autoantibodies by electrochemiluminescence assay improve risk prediction for type 1 diabetes. J. Clin. Endocrinol. Metab. 106, 3455–3463 (2021).
Bollyky, J. B. et al. Heterogeneity in recent-onset type 1 diabetes—a clinical trial perspective. Diabetes Metab. Res. Rev. 31, 588–594 (2015).
Luo, S. et al. Distinct two different ages associated with clinical profiles of acute onset type 1 diabetes in Chinese patients. Diabetes Metab. Res. Rev. 36, e3209 (2020).
Leslie, R. D. et al. Adult-onset type 1 diabetes: current understanding and challenges. Diabetes Care 44, 2449–2456 (2021).
Vicinanza, A., Messaaoui, A., Tenoutasse, S. & Dorchy, H. Diabetic ketoacidosis in children newly diagnosed with type 1 diabetes mellitus: role of demographic, clinical, and biochemical features along with genetic and immunological markers as risk factors. A 20-year experience in a tertiary Belgian center. Pediatr. Diabetes 20, 584–593 (2019).
Hameed, S. et al. Persistently autoantibody negative (PAN) type 1 diabetes mellitus in children. Pediatr. Diabetes 12, 142–149 (2011).
Stoupa, A. & Dorchy, H. HLA-DQ genotypes—but not immune markers—differ by ethnicity in patients with childhood onset type 1 diabetes residing in Belgium. Pediatr. Diabetes 17, 342–350 (2016).
Marino, K. R. et al. A predictive model for lack of partial clinical remission in new-onset pediatric type 1 diabetes. PLoS ONE 12, e0176860 (2017).
Ludvigsson, J. et al. Decline of C-peptide during the first year after diagnosis of type 1 diabetes in children and adolescents. Diabetes Res. Clin. Pract. 100, 203–209 (2013).
Greenbaum, C. et al. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. J. Am. Med. Assoc. 318, 1891–1902 (2017).
Wherrett, D. K. et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 378, 319–327 (2011).
Ludvigsson, J. et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N. Engl. J. Med. 366, 433–442 (2012).
Christie, M. R., Molvig, J., Hawkes, C. J., Carstensen, B. & Mandrup-Poulsen, T. IA-2 antibody-negative status predicts remission and recovery of C-peptide levels in type 1 diabetic patients treated with cyclosporin. Diabetes Care 25, 1192–1197 (2002).
Herold, K. C. et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N. Engl. J. Med. 381, 603–613 (2019).
Pescovitz, M. D. et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 361, 2143–2152 (2009).
Yu, L. et al. Rituximab selectively suppresses specific islet antibodies. Diabetes 60, 2560–2565 (2011).
Elsayed, N. A. et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care 46, S19–S40 (2023).
So, M. et al. Advances in type 1 diabetes prediction using islet autoantibodies: beyond a simple count. Endocr. Rev. 42, 584–604 (2021).
So, M. et al. Characterising the age-dependent effects of risk factors on type 1 diabetes progression. Diabetologia 65, 684–694 (2022).
Arif, S. et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes 63, 3835–3845 (2014).
Viisanen, T. et al. Circulating CXCR5+PD-1+ICOS+ follicular T helper cells are increased close to the diagnosis of type 1 diabetes in children with multiple autoantibodies. Diabetes 66, 437–447 (2017).
Andrade Lima Gabbay, M., Sato, M. N., Duarte, A. J. S. & Dib, S. A. Serum titres of anti-glutamic acid decarboxylase-65 and anti-IA-2 autoantibodies are associated with different immunoregulatory milieu in newly diagnosed type 1 diabetes patients. Clin. Exp. Immunol. 168, 60–67 (2012).
Spanier, J. A. et al. Increased effector memory insulin-specific CD4+ T cells correlate with insulin autoantibodies in patients with recent-onset type 1 diabetes. Diabetes 66, 3051–3060 (2017).
Chen, Y. G. et al. Molecular signatures differentiate immune states in type 1 diabetic families. Diabetes 63, 3960–3973 (2014).
Anand, V. et al. Islet autoimmunity and HLA markers of presymptomatic and clinical type 1 diabetes: Joint analyses of prospective cohort studies in Finland, Germany, Sweden, and the U.S. Diabetes Care 44, 2269 (2021).
Frohnert, B. I. et al. Late-onset islet autoimmunity in childhood: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 60, 998–1006 (2017).
Hanna, S. J. et al. Slow progressors to type 1 diabetes lose islet autoantibodies over time, have few islet antigen-specific CD8+ T cells and exhibit a distinct CD95hi B cell phenotype. Diabetologia 63, 1174–1185 (2020).
Pöllänen, P. M. et al. Dynamics of islet autoantibodies during prospective follow-up from birth to age 15 years. J. Clin. Endocrinol. Metab. 105, e4638–e4651 (2020).
So, M., O’Rourke, C., Bahnson, H. T., Greenbaum, C. J. & Speake, C. Autoantibody reversion: changing risk categories in multiple-autoantibody-positive individuals. Diabetes Care 43, 913–917 (2020).
Chung, W. K. et al. Precision medicine in diabetes: a Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 63, 1671–1693 (2020).
Bingley, P. J. & Williams, A. J. K. Validation of autoantibody assays in type 1 diabetes: workshop programme. Autoimmunity 37, 257–260 (2009).
Oram, R. A. et al. Utility of diabetes type-specific genetic risk scores for the classification of diabetes type among multiethnic youth. Diabetes Care 45, 1124–1131 (2022).
Tarn, A. C. et al. Predicting insulin-dependent diabetes. Lancet 1, 845–850 (1988).
Bonifacio, E. & Achenbach, P. Birth and coming of age of islet autoantibodies. Clin. Exp. Immunol. 198, 294–305 (2019).
Weiss, A. et al. Progression likelihood score identifies substages of presymptomatic type 1 diabetes in childhood public health screening. Diabetologia 65, 2121–2131 (2022).
Frohnert, B. I. et al. Refining the definition of stage 1 type 1 diabetes: an ontology-driven analysis of the heterogeneity of multiple islet autoimmunity. Diabetes Care https://doi.org/10.2337/dc22-1960 (2023).
Ghalwash, M. et al. Two-age islet-autoantibody screening for childhood type 1 diabetes: a prospective cohort study. lancet. Diabetes Endocrinol. 10, 589–596 (2022).
Kwon, B. C. et al. Progression of type 1 diabetes from latency to symptomatic disease is predicted by distinct autoimmune trajectories. Nat. Commun. 13, 1514 (2022).
Ng, K. et al. Islet autoantibody type-specific titer thresholds improve stratification of risk of progression to type 1 diabetes in children. Diabetes Care 45, 160–168 (2022).
Nielsen, L. B. et al. Relationship between ZnT8Ab, the SLC30A8 gene and disease progression in children with newly diagnosed type 1 diabetes. Autoimmunity 44, 616–623 (2011).
Andersen, M. L. M. et al. Association between autoantibodies to the Arginine variant of the Zinc transporter 8 (ZnT8) and stimulated C-peptide levels in Danish children and adolescents with newly diagnosed type 1 diabetes. Pediatr. Diabetes 13, 454–462 (2012).
Sorensen, J. S. et al. Islet autoantibodies and residual beta cell function in type 1 diabetes children followed for 3-6 years. Diabetes Res. Clin. Pract. 96, 204–210 (2012).
Chao, C. et al. Change of glutamic acid decarboxylase antibody and protein tyrosine phosphatase antibody in Chinese patients with acute-onset type 1 diabetes mellitus. Chin. Med. J. 126, 4006–4012 (2013).
Pecheur, A. et al. Characteristics and determinants of partial remission in children with type 1 diabetes using the insulin-dose-adjusted A1C definition. J. Diabetes Res. 2014, 851378 (2014).
Camilo, D. S. et al. Partial remission in Brazilian children and adolescents with type 1 diabetes. Association with a haplotype of class II human leukocyte antigen and synthesis of autoantibodies. Pediatr. Diabetes 21, 606–614 (2020).
Steck, A. K. et al. Factors associated with the decline of C-peptide in a cohort of young children diagnosed with type 1 diabetes. J. Clin. Endocrinol. Metab. 106, E1380–E1388 (2021).
Gale, E. A. M., Bingley, P. J., Emmett, C. L. & Collier, T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 363, 925–931 (2004).
JS, S. et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the diabetes prevention trial-type 1. Diabetes Care 28, 1068–1076 (2005).
Näntö-Salonen, K. et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 372, 1746–1755 (2008).
Herold, K. C. et al. Teplizumab (Anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 62, 3766–3774 (2013).
Aronson, R. et al. Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care 37, 2746–2754 (2014).
Demeester, S. et al. Preexisting insulin autoantibodies predict efficacy of otelixizumab in preserving residual β-cell function in recent-onset type 1 diabetes. Diabetes Care 38, 644–651 (2015).
Krischer, J., Schatz, D., Bundy, B., Skyler, J. & Greenbaum, C. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA 318, 1891–1902 (2017).
Acknowledgements
We thank Krister Aronsson and Maria Bjorklund from Lund University for assistance with database searches and Russell de Souza from McMaster University for advice on critical appraisal. The ADA/EASD Precision Diabetes Medicine Initiative, within which this work was conducted, has received the following support: The Covidence license was funded by Lund University (Sweden) for which technical support was provided by Maria Björklund and Krister Aronsson (Faculty of Medicine Library, Lund University, Sweden) and Russell de Souza from McMaster University for advice on critical appraisal. Administrative support was provided by Lund University (Malmö, Sweden), University of Chicago (IL, USA), and the American Diabetes Association (Washington D.C., USA). The Novo Nordisk Foundation (Hellerup, Denmark) provided grant support for in-person writing group meetings (PI: L Phillipson, University of Chicago, IL). J.L.F.: DiabDocs K12 program 1K12DK133995-01 (DiMeglio, Maahs PIs), The Leona M. & Harry B. Helmsley Charitable Trust Grant #2307-06126 (Felton PI); M.J.R.: NIH NIDDK R01DK124395; R.A.O.: R.A.O. had a UK MRC confidence in concept award to develop a type 1 diabetes GRS biochip with Randox R&D and has ongoing research funding from Randox; and has research funding from a Diabetes UK Harry Keen Fellowship (16/0005529), National Institute of Diabetes and Digestive and Kidney Diseases grants (NIH R01 DK121843–01 and U01DK127382–01), JDRF (3-SRA-2019–827-S-B, 2-SRA-2022–1261-S-B, 2-SRA-2002–1259-S-B, 3-SRA-2022–1241-S-B, and 2-SRA-2022–1258-M-B), and The Larry M and Leona B Helmsley Charitable Trust; and is supported by the National Institute for Health and Care Research Exeter Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the National Institutes for Health Research or the Department of Health and Social Care; S.A.L.: NIH NIAID R01 AI141952 (PI), NIH NCI R01 CA231226 (Other support), NIH NIAID 1 R01HL149676 (Other support), NIH NIDDK 1UC4DK117483 (subaward), JDRF 3-SRA-2019-851-M-B; S.O.G.: NIH R01 DK121843–01; S.R.: R01 DK122586, The Leona M and Harry B Helmsley Charitable Trust 2204-05134; C.E.M.: R01DK093954, R01DK127236, U01DK127786, R01DK127308, and UC4DK104166, U54DK118638, P30 P30 DK097512), a US Department of Veterans Affairs Merit Award (I01BX001733), grants from the JDRF (3-IND-2022-1235-I-X) and Helmsley Charitable Trust (2207-05392), and gifts from the Sigma Beta Sorority, the Ball Brothers Foundation, and the George and Frances Ball Foundation; L.A.D.: NIH for TrialNet U01DK106993/6163-1082-00-BO, DiabDocs K12 program 1K12DK133995-01, CTSI UL1TR001108-01; H.I.: K23DK129799; R.J.: NIH R03-DK127472 and The Leona M. and Harry B. Helmsley Charitable Trust (2103-05094); J.W.: JDRF 2-SRA-2022-1282-M-X, 3-SRA-2022-1095-M-B, 4-SRA-2022-1246-M-N, 3-SRA-2023-1374-M-N; K.G.: The Leona M. and Harry B. Helmsley Charitable Trust and Sanford Health. E.K.S.: R01DK121843; R01DK121929A1, R01DK133881, U01DK127786, U01 DK127382 Effort from this grant (to E.K.S., H.I., J.F.) is also supported by Grant 2021258 from the Doris Duke Charitable Foundation through the COVID-19 Fund to Retain Clinical Scientists collaborative grant program and was made possible through the support of Grant 62288 from the John Templeton Foundation.
Author information
Authors and Affiliations
Consortia
Contributions
J.L.F., K.J.G., R.A.O., M.J.R. and E.K.S. designed the project, performed systematic review, interpreted results, and wrote and edited the manuscript. C.S., S.A.L., S.O.G., S.S.R., G.S.F.M., A.H.K., D.P., Z.S., B.H., R.J., C.E.M., L.A.D., H.M.I., D.D., R.K.J., M.U. and J.M.W. contributed to design of the project, performed systematic review, and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
E.K.S. has received compensation for educational lectures from Medscape, ADA, and MJH Life Sciences and as a consultant for DRI Healthcare. C.E.M. reported serving on advisory boards for Provention Bio, Isla Technologies, MaiCell Technologies, Avotres, DiogenyX, and Neurodon; receiving in-kind research support from Bristol Myers Squibb and Nimbus Pharmaceuticals; and receiving investigator initiated grants from Lilly Pharmaceuticals and Astellas Pharmaceuticals. L.A.D. reports research support to institution from Dompe, Lilly, Mannkind, Provention, Zealand and consulting relationships with Abata and Vertex. R.A.O. had a UK MRC Confidence in concept grant to develop a T1D GRS biochip with Randox Ltd, and has ongoing research funding from Randox R&D. No other authors report any relevant conflicts of interest.
Peer review
Peer review information
Communications Medicine thanks Yang Xiao, Riitta Veijola and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Felton, J.L., Redondo, M.J., Oram, R.A. et al. Islet autoantibodies as precision diagnostic tools to characterize heterogeneity in type 1 diabetes: a systematic review. Commun Med 4, 66 (2024). https://doi.org/10.1038/s43856-024-00478-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-024-00478-y