Abstract
Background
The symptoms of long COVID, which include fatigue, breathlessness, dysregulated breathing, and exercise intolerance, have unknown mechanisms. These symptoms are also observed in heart failure and are partially driven by increased sensitivity of the carotid chemoreflex. As the carotid body has an abundance of ACE2 (the cell entry mechanism for SARS-CoV-2), we investigated whether carotid chemoreflex sensitivity was elevated in participants with long COVID.
Methods
Non-hositalised participants with long-COVID (n = 14) and controls (n = 14) completed hypoxic ventilatory response (HVR; the measure of carotid chemoreflex sensitivity) and cardiopulmonary exercise tests. Parametric and normally distributed data were compared using Student’s unpaired t-tests or ANOVA. Nonparametric equivalents were used where relevant. Peason’s correlation coefficient was used to examine relationships between variables.
Results
During cardiopulmonary exercise testing the VE/VCO2 slope (a measure of breathing efficiency) was higher in the long COVID group (37.8 ± 4.4) compared to controls (27.7 ± 4.8, P = 0.0003), indicating excessive hyperventilation. The HVR was increased in long COVID participants (−0.44 ± 0.23 l/min/ SpO2%, R2 = 0.77 ± 0.20) compared to controls (−0.17 ± 0.13 l/min/SpO2%, R2 = 0.54 ± 0.38, P = 0.0007). The HVR correlated with the VE/VCO2 slope (r = −0.53, P = 0.0036), suggesting that excessive hyperventilation may be related to carotid body hypersensitivity.
Conclusions
The carotid chemoreflex is sensitised in long COVID and may explain dysregulated breathing and exercise intolerance in these participants. Tempering carotid body excitability may be a viable treatment option for long COVID patients.
Plain language summary
Patients with long COVID suffer from breathlessness during exercise, leading to exercise intolerance. We know that SARS-CoV-2, the virus that causes COVID-19, can infect carotid bodies which is a small sensory organ that sends signals to the brain for regulating breathing and blood pressure. This is called the carotid chemoreflex. However, it is not clear if SARS-CoV-2 infection affects carotid chemoreflex. Here, we examine whether the normal functioning of carotid chemoreflex is disrupted in non-hospitalised patients with long COVID and if this is linked to excessive breathing during exercise. Our study shows that carotid chemoreflex is more sensitive in long COVID patients, who are otherwise healthy. The carotid bodies could be a good therapeutic target for treating breathlessness in patients with long COVID.
Similar content being viewed by others
Introduction
Long COVID (post-COVID-19 syndrome), is a multi-organ, often debilitating condition associated with a range of symptoms. The UK’s National Institute for Health Care and Excellence (NICE) defines long COVID as ongoing symptoms lasting for 12 or more weeks after initial SARS-CoV-2 infection, without alternative explanations1. The estimated incidence of long COVID varies, and is reported to be up to 41% of non-hospitalised cases2,3,4 and up to 76% of hospitalised cases5,6. The prevalence decreases in vaccinated populations7. In January 2023, 2 million people self-reported long COVID symptoms in the UK, with 77% experiencing adverse effects in their daily activities8. Persistent symptoms include chronic fatigue, ‘brain fog,’ cognitive impairment and memory loss, dyspnoea at rest and on exertion, exercise intolerance, orthostatic intolerance, inappropriate postural tachycardia, and episodic hyperadrenergic surges4,9,10. A meta-analysis of 63 studies worldwide, with a total COVID-19 population of 257,348, reported that between 3–6 and 9–12 months post-infection, fatigue and dyspnoea were the most reported symptoms, with a prevalence of 32–47% and 21–25% respectively11. Despite the prevalence of long COVID and severely disabling symptoms there are no treatment strategies available. Thus, it is crucial to identify the mechanisms involved in long COVID to inform urgently needed therapy. It is likely that mechanisms depend on the severity of the original infection and are different for hospitalised (e.g. long term sequalae from intensive care and intubation/ventilation) versus non-hospitalised patients who had mild to moderate initial symptoms.
Currently, the exact mechanisms driving long COVID in non-hospitalised patients remain unknown but are likely to be multiple12. Studies have shown that exercise intolerance and disorganised breathing or breathing inefficiency during exercise are key features of long COVID13,14,15,16 even in patients who have normal lung function and no evidence of gas exchange abnormalities15. The carotid bodies are key oxygen, carbon dioxide and pH sensing organs that control ventilation, dyspnoea, and the circulation at rest and during exercise in health and disease17,18. In fact, in chronic heart failure the carotid chemoreflex becomes chronically sensitised as a compensatory mechanism, and is associated with a worse prognosis19, exertional dyspnoea, dysfunctional or inefficient breathing, and poor exercise tolerance20, similar to symptoms in patients with long COVID who do not have heart failure.
SARS-CoV-2 infects host cells via binding of its receptor-binding domain to the membrane bound angiotensin-converting enzyme 2 (ACE2)21. ACE2 is abundant in the carotid bodies22. A role of the carotid chemoreflex in the acute phase of the infection is supported by reports of silent hypoxia22,23, SARS-CoV-2 invasion of glomus cells (main oxygen sensing cells) and microembolism within the small arteries suppling blood to the carotid body24,25. The carotid bodies express their own renin-angiotensin system26,27, where normal functioning is dependent on the balance of ACE1 and ACE226. Disruption of this local system causes increased carotid chemoreflex activity27,28. Thus, disturbances in the carotid body following SARS-CoV-2 infection; by viral invasion, blood flow disruption and local immune responses could cause chemoreceptive dysfunction, by increasing local ACE1/ACE2 imbalance (in favour of higher ACE1 expression versus ACE2) and angiotensin II receptor stimulation24,29.
We propose that carotid body dysfunction occurs in long COVID, which contributes to dysregulation of ventilation and cardiovascular control, especially during exercise. Therefore, we conducted a case-control study to determine whether carotid chemoreflex sensitivity is elevated in non-hospitalised patients with long COVID and whether this could help to explain impairments in exercise tolerance and dysregulated breathing reported during exercise in non-hospitalised patients with ongoing symptoms. We hypothesised that long COVID patients would exhibit increased hypoxic ventilatory responses at rest and poorer ventilatory efficiency during exercise compared to controls. Ventilatory efficiency is defined as ventilation relative to the CO2 production. The VE/VCO2 slope can be used to quantify the efficiency of ventilation in patient cohorts; where a steeper slope represents poorer efficiency30,31. There is an exception, however; the VE/VCO2 slope does not reflect ventilatory efficiency in patients with moderate to advanced chronic obstructive lung disease32. In the current study, since the long COVID patients recruited did not have COPD, the VE/VCO2 slope was used as the main measure of exercise ventilatory efficiency.
The findings of this study show that carotid chemoreflex sensitivity is amplified in non-hospitalised patients with long COVID (versus a control group) and that this correlates with hyperventilation and poor breathing efficiency during exercise. Elevated carotid chemoreceptor activity could explain several ongoing symptoms experienced by patients living with long COVID.
Methods
Design
This was a single-site case-control study.
Participants
Ethical approval for the study was granted by South Central Hampshire NHS Research Ethics Committee (21/SC/0260) and the Health Research Authority. Participants gave their written informed consent. All participants were asked to abstain from intense exercise and alcohol consumption 24 h before the study. All experimental protocols conformed to the Declaration of Helsinki. Inclusion criteria for all participants were: aged 18–80 years and a positive SARS-CoV-2 antibody test before vaccination, or a positive COVID-19 PCR antigen swab test. Long COVID participants had received a diagnosis of long COVID, where symptoms developed during or after an infection consistent with COVID-19 and continued for more than 12 weeks (and could not be explained by an alternative diagnosis) as per NHS (UK) National Institute for Health and Care Excellence guidelines33. Participants without long COVID had symptoms lasting less than 4 weeks after their initial infection. See the online-only Supplement Note for exclusion criteria. However, due to scheduling issues (limited by the availability of staff support rather than recruitment), data sets from six healthy participants from a previous study (NHS REC numbers: 17/SW/0171 and 18/SW/0241), completed before the SARS-CoV-2 pandemic using the same methods, equipment, and location (temperature controlled clinical room) were added to the control group.
Experimental protocol
Participants attended the NIHR Bristol Clinical Research facility for 2 studies, completed at the same time of day, and the laboratory conditions were at a set temperature (22 °C). In visit one, informed consent, in-depth medical history, clinic blood pressure assessment, sit-to-stand test for orthostatic intolerance, lung function tests (spirometry), 12-lead ECG, pregnancy tests, and a symptom-limited incremental cardiopulmonary exercise test were completed. At this visit, participants were asked to list their ongoing symptoms (if any) and were specifically asked about symptoms associated with long COVID that have been previously published9,10. The second visit involved resting ventilation and cardiovascular measurements followed by carotid chemoreflex assessment via the hypoxic ventilatory response.
Procedures
Clinic blood pressure
Participants rested in a chair for 10 min prior to clinic BP being assessed (Omron, 705IT, Omron Healthcare, Kyoto, Japan). Clinic BP was assessed in-line with European Hypertension Society Guidelines34.
Orthostatic intolerance was assessed using a sit-to-stand test using the HYVET protocol35 where BP was measured when sitting, immediately upon standing, and after 1, 2 and 3 minutes of standing.
Resting spirometry
Resting spirometry was used to assess lung function, to ensure no mechanical lung function abnormalities were present. Spirometry (Ergostick, CPET system, LoveMedical, UK) was completed in line with the joint American Thoracic and European Respiratory Society guidelines36. The Global Lung Function Initiative (GLI) network reference values were used to calculate the percentage of predicted values and z-scores37.
12-lead ECG: Resting 12-lead ECG was performed and checked by a Cardiologist at the Bristol Heart Institute for any ECG abnormalities, and to clear participants to exercise.
Cardiopulmonary exercise testing
After acclimatisation to the facemask and sitting on the cycle ergometer, participants completed a 5-min steady state resting period followed by 3 min of unloaded cycling. Participants then completed a continuous ramp incremental exercise test to volitional exhaustion where work rate increased by 15–30 W depending on their physical ability. Exercise tests were completed on an electronically braked cycle ergometer (Ergoselect 5; Ergoline, Germany). Cardiorespiratory data were recorded using a metabolic measurement system (Ergostik; LoveMedical, UK) with integrated 12-lead ECG and finger pulse oximetry for heart rate and SpO2% monitoring. Brachial arterial blood pressure was measured via an integrated automated auscultatory blood pressure cuff (LoveMedical, UK. Ratings of perceived exertion (6–20 Borg scale) and dyspnoea scores (modified Borg scale) were obtained at rest, and every minute during exercise, and at the end of exercise. Peak cardiopulmonary data were averaged over the last 30 seconds of exercise. The anaerobic threshold was measured via the V-slope method and Dual Criterion methods as recommended by the American Thoracic Society guidelines38.
Measurement of exercise ventilatory efficiency
The main measurement of ventilatory efficiency was the VE/VCO2 slope, calculated as the minute ventilation, and VCO2 values from initiation to peak exercise were used to measure VE/VCO2 slope via least squares linear regression31. Secondary measurements of ventilatory efficiency included, the VE/VCO2 nadir (calculated as the lowest VE/VCO2 ratio during exercise using 30 s of averaged breaths with 5-of-7 breath averaging30), and the VE/VCO2 at anaerobic threshold.
Carotid chemoreflex assessment
Resting carotid chemoreceptor sensitivity tests were completed with participants in a semi-supine position with continuous monitoring of beat-to-beat blood pressure (Finapres), SpO2 (ear-lobe pulse oximeter; Radical-7; Masimo Corp, USA), heart rate (lead II of 3-lead ECG, AD Instruments, Australia). Simultaneously, ventilation was measured via a facemask attached to a one-way non-rebreathing circuit (Hans Rudolph, Inc., USA). The inhalation part of the circuit delivered room air or 100% nitrogen gas (transiently) for carotid chemosensitivity testing. The exhalation arm of the circuit was connected to a gas analyser (Ad Instruments) and flow head (MLT3000L; AD Instruments) fitted with a differential pressure transducer (FE141 Spirometer; AD Instruments) for the measurement of inspired and expired fractions of O2 and CO2, tidal volume, breathing frequency and minute ventilation. All data were continuously monitored and recorded with a data acquisition system (Powerlab 16/30; AD Instruments) and stored for subsequent analyses using associated software (LabChart 8.0 Pro; AD Instruments).
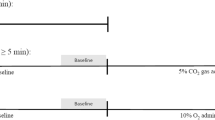
The transient hypoxic ventilatory response test was used to measure the sensitivity of the carotid chemoreflex20,39. After a period of quiet rest breathing room air (10 min baseline), the researcher added extra-nitrogen to the room air being delivered to the face-mask. Nitrogen gas administration was controlled silently using a high-pressure electric valve. The nitrogen blended into the room air was delivered for 2–8 breaths, followed by a 3-minute recovery period or until ventilation and haemodynamic variables returned to baseline levels. This was repeated 6–8 times to obtain a range of oxygen saturations (SpO2: ~70–100%). The average of the two largest consecutive breaths in the 1 min proceeding the nitrogen exposure was used to calculate the ventilatory response to reductions in SpO2%19. The hypoxic ventilatory response was evaluated as the slope of the linear regression relating the minute ventilation to the nadir of SpO2 for each nitrogen exposure19,39 (Fig. 1b). The response of tidal volume and breathing frequency to reductions in SpO2 were also evaluated in the same way as that for minute ventilation.
a Example carotid chemoreflex test in a control (n = 1, solid line) and long COVID (n = 1, dashed line) participants. Data shows the raw SpO2 response to nitrogen exposure (bottom panel) and the resultant tidal volume, breathing frequency, and minute ventilation responses. b The minute ventilation (average of the largest 2 consecutive breaths) is plotted against the nadir SpO2 for each nitrogen exposure. The resultant slope of the linear regression is the hypoxic ventilatory response. Data are from a control (black circles) and a long-COVID (open red circles) participants, c the minute ventilation (VE) plotted against the volume of expired carbon dioxide (VCO2) for each breath during exercise in the same 2 participants (control; grey circles and long-COVID; open red circles). The slope of the regression is the VE/VCO2 slope and is used as a measure of breathing efficiency.
The peak heart rate and blood pressure was determined following each hypoxic challenge using a 3-beat rolling average and plotted against the nadir oxygen saturation. The hypoxic heart rate (beats/min/%) and blood pressure (mmHg/%) were calculated as the slope of the simple linear regression obtained from baseline and the hypoxic challenges.
Statistics and reproducibility
Statistical analyses were completed in GraphPad Prism (V9.5.1). Participant demographics, resting spirometry, cardiopulmonary exercise and hypoxic ventilatory response data were analysed using an independent samples t-test or Mann Whitney U test if data were not normally distributed (Shapiro-Wilk test and visual inspection using Q-Q plots) or were non-parametric. Where data are compared across multiple time points between the groups, a mixed model ANOVA was used with a Bonferroni correction for pairwise comparisons. Data are reported as mean ± standard deviation (SD) or median (interquartile range) unless otherwise stated. A significance level of α < 0.05 was used for all analyses.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Participants
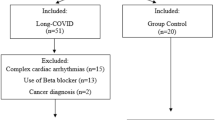
Supplementary Fig. 1 shows recruitment, screening, cases excluded and the final sample size. Sixty-four individuals contacted the group about the study, and all were sent a participant information sheet. Of these, 32 replied and completed phone screening. Six were excluded due to screen failure (met exclusion criteria). Twenty-six participants were recruited (long COVID n = 16 and controls n = 10). Two participants (1 long COVID and 1 control) did not complete the study since they could not tolerate the mask for spirometry, 1 long COVID participant was excluded during the study visit due to identification of cardiac disease (valve disease or non-benign arrhythmia), and 1 participant was excluded between visits due to diagnosis of gout (control). The final sample size was 14 participants with long COVID and 8 controls (recovered from initial viral infection within 4 weeks without ongoing symptoms). Data sets from six healthy participants from a previous study (NHS REC numbers: 17/SW/0171 and 18/SW/0241), completed before the SARS-CoV-2 pandemic using the same methods, equipment, and location (clinical room) were added to the control group. These participants were identified based on age, sex, and body mass index (BMI) so that they matched the participants in the long COVID study.
Participant demographics are shown in Table 1. Age, BMI, height, and body mass were similar between the control and long COVID groups (P > 0.05). Clinic systolic blood pressure (SBP), diastolic BP (DBP) and heart rate (HR), were not different between groups (P > 0.05).
All participants who had COVID-19 were first infected from April 2020 to December 2021, when the dominant variants of SARS-CoV-2 were the original virus, Alpha, Delta, and Omicron. The average number of days since first infection to the study visit, in the long COVID and control groups, were similar (Table 1, P = 0.1006). As per the exclusion criteria, none of the participants were hospitalised (admitted) for COVID-19 and had a previous positive PCR test at the time of the initial infection or were seropositive for SARS-CoV-2 antibodies (see inclusion/exclusion criteria in methods). The initial infection caused mild to moderate symptoms in all participants. Table 2 outlines the most common ongoing symptoms previously reported9,40,41 and the percentage of the long COVID participants in this study reporting these symptoms. Long COVID participants reported at least 3 symptoms, with the most common symptoms being dyspnoea at rest and on exertion, extreme fatigue, “brain fog” and chest pain.
Ten out of the 14 long COVID participants had been seen in a cardiology outpatient long COVID clinic at a UK based University Hospital NHS Trust, where structural cardiac disease (e.g., myocarditis, heart failure, ischaemia) had been excluded. All the participants reporting chest pain were reviewed in a cardiology clinic, which was diagnosed as non-cardiac in origin. None of the long COVID participants (or controls) had been diagnosed with pre-existing cardiovascular or pulmonary diseases. Four of the long COVID participants had been prescribed ivabradine in the cardiology outpatient long COVID clinic. These participants stopped taking ivabradine 48 h before their study visits (plasma half-life: 2 h and effective half-life: 11 h). Severe asthma was an exclusion criterion, however, 2 controls and 6 long COVID participants had been previously diagnosed with mild asthma (before COVID). All participants had a normal resting 12-lead ECG, which was completed as part of the study screening process. Twelve of the 14 long COVID participants self-reported having a chest x-ray, all of which were normal. Finally, Supplementary Table 1 provides information on drugs prescribed in both groups.
Resting spirometry
Spirometry data are summarised in Table 1 and are presented as z-scores with a normal z-score being between ± 1.6442. There were no differences between the two groups for FEV1 (forced expiratory volume in 1 s), FVC (forced vital capacity), FEV1/FVC and FEF75% (forced expiratory flow after 75% of the FVC has been exhaled; P > 0.05). No participants had an FEF75% smaller than -1.64 z-score, therefore excluding small airway disease. One control subject and 3 long COVID participants had a reduced FEV1 and FVC with a normal FEV1/FVC ratio, suggestive of a restrictive ventilatory defect. One long COVID participant had a reduced FEV1 and FVC, with an FEV1/FVC ratio consistent with an obstructive ventilatory defect (z-score = -3.25) indicating severe airflow obstruction (z-score = - 3.85)42.
Sit to standing blood pressure test
To check for orthostatic intolerance in both groups, a sit-to-standing test (like that used in the HYVET study35) was completed (3 mins of standing). Sit-to-stand was completed in all long COVID participants and 8 of the controls. BP was measured at seated rest, immediately after standing, 1 min, 2 min, and 3 min of standing. According to HYVET study guidelines, a fall in SBP > 15 and DBP > 7 mmHg was used as a diagnosis of orthostatic intolerance. During standing, the mean SBP and DBP did not fall by more than 15 and 7 mmHg in either group at any time point35 (Supplementary Fig. 2; Supplementary Table 2 for mean change data). Two control participants had a fall in SBP > 15 or DBP > 7 mmHg, and 1 participant from the long COVID group met this criterion (Supplementary Fig. 2). Mean data at each timepoint were analysed using a mixed model ANOVA. Interestingly, we found that the increase in HR was larger in the control group versus the long COVID group (main Time*Group effect; P = 0.0258, see Supplementary Data for ANOVA details and Fig. 2).
A more negative response indicates a greater HVR. Closed circles are control and open circles are long-COVID participants. The red dots denote control participants that did not have COVID-19 (n = 6). Panels a–c show the hypoxic ventilatory response based on minute ventilation, tidal volume and breathing frequency, respectively. There was a higher HVR based on minute ventilation and tidal volume in the participants with long COVID (n = 14) versus control (n = 14). Panels d–f show the heart rate, systolic and diastolic blood pressure hypoxic responses. Data are the slope of the linear regression where SpO2% was plotted against the heart rate, and BP during each hypoxic exposure. There was no difference in the HR or BP response to hypoxia between groups. Data are mean +/-SD.
Resting cardiopulmonary data
The resting HR, BP, minute ventilation, tidal volume and breathing frequency were similar between groups (Table 3, P > 0.05). Resting partial pressure of the end tidal CO2 (PETCO2) was lower in the long COVID group (range: 22 to 34 mmHg versus controls (range: 30–36 mmHg, Table 3, P = 0.0378). The minute ventilation was similar between groups despite a lower PETCO2 in the long COVID group. This was coupled with a higher ventilation for the volume of CO2 expired (VE/VCO2 ratio, P = 0.0246); suggesting that there is some hyperventilation occurring at rest in the long COVID group. There were no differences in the expiratory and inspiratory times, or the ratio of inspiration to expiratory times between groups (P > 0.05; Table 3 for exact P-values). Interestingly, the tidal volume/inspiratory time ratio (VT/Ti; an index of inspiratory flow43) was 16% higher in the long COVID group versus the controls (P = 0.0483) indicative of increased inspiratory drive.
Cardiopulmonary exercise testing
Cardiopulmonary exercise testing (CPET) was completed on a cycle ergometer to peak oxygen consumption (VO2 peak) to assess exercise tolerance and identify any breathing or cardiovascular abnormalities that might not be evident at rest. We also wanted to understand whether any dysfunctional breathing (defined here as inappropriate hyperventilation or poor breathing efficiency characterised by elevated minute ventilation/volume of CO2 expired (VE/VCO2) slopes16) during exercise could be linked to carotid chemoreflex hyperactivity in the long COVID group versus control.
To assess whether maximal effort was achieved and as an objective assessment of the quality of the test, a respiratory exchange ratio>1.15, maximum predicted HR > 85%, rating of perceived exertion (RPE; 6–20 Borg Scale) of 17–20 and a plateau in VO2 were used. Overall, 86% of the control group and 86% of the long COVID group achieved these criteria. Thus, differences between groups are likely not due to differences in effort or quality of the CPET. In fact, the control group reported a lower RPE at peak exercise (17; 17–18 (median, IQR)) versus the long COVID group (19; 18–19, P = 0.0081; Mann Whitney U). Table 4 shows the mean ± SD (range) for all CPET variables. None of the participants desaturated during the test (defined as SpO2%<95%, where peak SpO2% was similar between groups), and there were no exercise induced cardiac ischaemic changes observed on the 12-lead ECG.
The VO2 peak measured in L/min, mL/kg/min and as a % of peak predicted were lower in the long COVID group versus controls (Table 4; P < 0.05). Absolute VO2 at anaerobic threshold (AT) was also lower (P = 0.0025), but the % of peak VO2 at which the AT occurred was similar between groups (P = 0.6490), indicating normal metabolic function. Along these lines there were no other indications of defects in muscle metabolic pathways indicating no muscle metabolic limitations to VO2 peak in either group (i.e. normal peak respiratory exchange ratio (RER) and a normal VO2/work rate relationship44 (Table 4). Maximum HR was lower in the long COVID group versus controls, but there was no difference between groups when HR was measured as the % of predicted maximum (P = 0.1326). The slope of the HR plotted against the %VO2 peak was lower in the long COVID group (Table 4; P = 0.0114) versus controls indicating a blunted HR response to exercise and potentially some degree of chronotropic incompetence. Heart rate at AT was also lower in the long COVID group versus controls (P = 0.0453). Finally, the peak oxygen pulse (VO2/HR) was lower in the long COVID group versus controls, indicating a cardiovascular limitation to exercise. Despite this, the HR recovery at 1 min was similar between groups (P = 0.4653); indicating that parasympathetic engagement post-exercise in the long COVID group was similar to control.
The VCO2 at peak was lower in the long COVID group (Table 4; P = 0.0477), whereas minute ventilation, tidal volume and breathing frequency at peak were similar between groups (Table 4, P > 0.05). Supplementary Fig. 3 shows the minute ventilation versus the VCO2 and VO2 at rest, AT, and peak exercise in both groups. The mean breathing reserve (max minute ventilation/predicted maximal voluntary ventilation (calculated as FEV1 * 40) was similar between groups (P = 0.8708). Thus it is likely that the lower peak VO2 in the long COVID group is not a result of pulmonary mechanical limitations45.
The VE/VCO2 ratio at peak (ANOVA; P = 0.0051) and at AT (P = 0.0477) was higher in the long COVID group versus control (see Supplementary Fig. 3 for ANOVA details and VE/VCO2 ratio plotted against the VO2 at rest, AT, and peak exercise). The VE/VCO2 slope (Table 4, P = 0.0008) and the VE/VCO2 nadir (P = 0.0020) during exercise were also higher in the long COVID group versus controls indicating lower breathing efficiency (a higher minute ventilation to remove a given volume of CO2) indicating hyperventilation at any point during exercise (Fig. 1 for example slopes (raw data) in a control and long COVID participant). Thirteen percent of the control group had a VE/VCO2 slope higher than the normal range (20–3046) versus 88% in the long COVID group. Since VE/VCO2 has been shown to be higher in people with hypertension; we completed an ANCOVA with clinic SBP as a covariate to check that the slightly elevated SBP in the long COVID group was driving this relationship. The SBP did not adjust the relationship between VE/VCO2 and long COVID (SBP effect; P = 0.811) where VE/VCO2 remained elevated in the long COVID group versus controls (group effect, P = 0.002 (SPSS v28)). Finally, the PETCO2 was lower at the AT and at peak exercise in the long COVID group versus the control group (time*group effect; P = 0.0119, mixed model ANOVA), but the magnitude of rise of the PETCO2 from rest to AT and from rest to peak exercise was the same in both groups (P = 0.0904, Supplementary Fig. 4, mixed model ANOVA).
Hypoxic ventilatory response
Fig. 1a shows examples carotid chemoreflex tests in a control and a long COVID participants and the resultant hypoxic ventilatory responses (calculated from the minute ventilation) in these participants (Fig. 1b). The hypoxic ventilatory response (minute ventilation response to reductions in SpO2%) was elevated in the long COVID (−0.44 ± 0.23 l/min/ SpO2%, R2 = 0.77 ± 0.20) group compared to controls (−0.17 ± 0.13 l/min/SpO2%, R2 = 0.54 ± 0.38, Fig. 2a, P = 0.0007); thus, for a given decrease in SpO2, the participants with long COVID had a greater increase in minute ventilation. This was driven by a greater increase in tidal volume (Fig. 2b) in the participants with long COVID rather than a greater increase in breathing frequency compared to the controls (Fig. 2c). Taken together this indicates that the participants with long COVD have an elevated carotid chemoreflex sensitivity to hypoxia. Since elevated SBP is thought to be linked to heightened HVR – an ANCOVA was completed with clinic SBP as a covariate. The level of clinic SBP did not adjust the relationship between HVR and long COVID (SBP effect; P = 0.503), where having long COVID remained associated with an elevated HVR (group effect adjusted for SBP; P < 0.001 (completed in SPSS v28).
There was no difference in the HR response to hypoxia (Fig. 2d) between groups (control; -0.66 ± 0.35 beats/min/ SpO2% versus long COVID; -0.58 ± 0.60 beats/min/SpO2%, P = 0.5978). Additionally, there was no difference in the SBP or DBP response to hypoxia between groups (Figs. 2e, 2f). The BP response to hypoxia was variable between individuals with some participants showing a depressor response to hypoxia and others showing a pressor (most common, where the slope of the regression is negative in Figs. 2e, 2f) or biphasic response to hypoxia.
The hypoxic ventilatory response was correlated with the VE/VCO2 slope during exercise (r = -0.53, P = 0.0036) and the VE/VCO2 nadir (r = -0.45, P = 0.0159; supplementary Fig. 7); indicating that the high carotid chemoreflex sensitivity in the long COVID participants may partially explain the higher VE/VCO2 slope (poorer breathing efficiency) during exercise.
Discussion
The pathophysiological mechanisms driving ongoing symptoms in patients with long COVID, after an initial mild infection, are unclear. Here we show for the first time that carotid chemoreflex sensitivity is amplified in non-hospitalised patients with long COVID (versus a control group) and that this was correlated with hyperventilation and poor breathing efficiency during exercise. Elevated carotid chemoreceptor activity could explain several of the ongoing symptoms experienced by patients living with long COVID.
In this population of participants with long COVID, we show that despite similar lung function (resting spirometry and ventilatory reserve during exercise) to the control group, these participants hyperventilate at rest and during exercise. At rest, this is evidenced by a similar minute ventilation compared to control despite a lower resting PETCO2. Resting levels of arterial CO2 provides the key stimulus for respiratory drive, mainly via the central chemoreceptors47. Reductions in PETCO2 indicate decreases in PaCO248,49 which is a stimulus to lower ventilation; however, this has not occurred in the long COVID participants since their level of ventilation was the same as the controls suggesting a resetting of chemoreceptor set-point for breathing. Additionally, the VE/VCO2 ratio at rest is higher in the long COVID participants, showing that they are breathing more to remove the same volume of CO2 as controls. Altered VE/VCO2 ratio (and PETCO2) could result from changes to gas exchange between the alveoli and pulmonary circulation. However, we found that during exercise the magnitude of the rise in PETCO2 was the same in the long COVID participants versus the controls. During exercise (with a normal gas exchange) PETCO2 rises and peaks around anaerobic threshold. When gas exchange is impaired by abnormal ventilation or perfusion of the lung, the PETCO2 will show smaller rises, no rise, or even decrease as seen in COPD, heart failure and pulmonary hypertension50. As such, the similar rise in PETCO2 with exercise in long COVID patients and control participants suggests no differences in gas exchange between these groups. However, we acknowledge that this needs to be confirmed with gas diffusion studies. A recent study by Beaudry et al. 51 showed that gas diffusion was normal in long COVID participants versus controls.
We also found evidence of elevated resting VT/Ti, an index of inspiratory flow, in the long COVID group versus the control group. The VT/Ti is used as surrogate of primary neural drive for inspiration43 and thus could indicate a higher ‘inspiratory drive’ at rest in the long COVID participants which may contribute to their feelings of dyspnoea. High inspiratory flow can also indicate changes in airway mechanics52, however, this is unlikely in the long COVID group, since resting lung function (spirometry) was similar between groups. During exercise, hyperventilation or poor breathing efficiency was also evidenced by elevated VE/VCO2 slopes and an elevated VE/VCO2 ratio at anaerobic threshold and peak exercise. Since the carotid chemoreflex plays an important part in the control of breathing both at rest and exercise (including inspiratory drive53); it is possible that it is contributing to hyperventilation in long COVID. However, we did not directly measure PaCO2, thus the assumption that the lower PETCO2 and the higher VE for a given level of VCO2 during exercise indicates hyperventilation (and not increased dead space ventilation for example) should be taken with caution54.
The mammalian carotid chemoreflex is a protective reflex that contributes to ventilatory and cardiovascular control55. In addition to signalling the need for increased ventilation during hypoxia (and other stimuli18), the carotid body chemoreceptors contribute to resting ventilatory drive, as demonstrated by their tonic activity in humans and animal models53,56. It is well established that certain disease states exhibit exaggerated carotid chemoreflex sensitivity19 and tonicity17. Given that elevated carotid chemoreflex sensitivity measured by the hypoxic ventilatory response predicts symptom burden, exercise intolerance, dyspnoea, and hyperventilation in heart failure20,57, we aimed to assess carotid chemoreflex sensitivity in long COVID. The hypoxic ventilatory response was 145% higher in the long COVID group versus age-, BMI- and sex-matched controls indicating amplified carotid chemoreceptor sensitivity in the long COVID group. The higher hypoxic ventilatory response was observed despite a lower resting PETCO2 in the long COVID group even though hypocapnia normally depresses carotid chemoreflex sensitivity to hypoxia58. The hypoxic ventilatory response was in fact similar to that measured in a group of patients with heart failure reduced ejection fraction in our lab (-0.44 ± 0.23 L/min/SpO2% versus -0.48 ± 0.30 L/min/SpO2%; mean ± SD) using the same equipment before March 2020 (see Supplementary Fig. 5). The elevated hypoxic ventilatory response in the long COVID participants was driven by amplified responses in tidal volume rather than breathing frequency, which supports previous reports in humans where the increase in minute ventilation during mild hypoxia is driven mainly by increases in tidal volume59.
The hypoxic ventilatory response was correlated with the VE/VCO2 slope, so that a person with high chemoreflex sensitivity had a higher VE/VCO2 slope (or vice versa). This suggests that elevated carotid chemoreflex sensitivity may partially explain reduced breathing efficiency and hyperventilation during exercise in participants with long COVID13,14,16. In fact, breathing efficiency was poor in some of the participants in this study; 64% of the long COVID group had a VE/VCO2 slope >34 (versus only 7% (one person) in the control group), which is a powerful prognostic indicator of future outcomes for people with heart failure60. It is possible that in long COVID the carotid chemoreflex has a similar function as that described in heart failure, partially explaining the dysregulated breathing and feelings of breathlessness in patients with ongoing symptoms after their initial mild infection. Elevated VE/VCO2 slopes are also caused by increased dead space ventilation due to 1) inadequate aeration of the alveoli and 2) poor perfusion of the aerated lung spaces, affecting gas exchange. However, in this study there was no evidence of mechanical lung or small airway issues (due to a similar prevalence of normal breathing reserve in the two groups and similar resting spirometry between the groups). No obvious evidence of gas exchange issues were observed either, because the PETCO2 during exercise (to AT) increased by a similar magnitude in the participants with long COVID versus the controls.
Finally, the resting PETCO2 was <30 mmHg in 4 participants with long COVID. Low PETCO2 causes similar symptoms as those often experienced by patients with long COVID including feelings of brain fog (due to poor cerebral perfusion) and paraesthesia61. The level of PETCO2 however did not reach apnoeic threshold levels62 in any patients because there were no apnoeas at rest and no evidence of periodic breathing patterns63. Low resting PETCO2 and hyperventilation is a hallmark of hyperventilatory syndrome which has been cited as a cause of ongoing in symptoms in some cohorts of patients with long COVID64. It is possible that problems with breathing at rest and during exercise, leading to lower PETCO2 levels and manifesting as hyperventilation syndrome could be partially driven by the carotid chemoreflex in patients with long COVID.
Possible mechanisms of increased carotid chemoreceptor sensitivity after SARS-CoV-2 infection include local changes within the parenchyma of the carotid body and/or dysfunction occurring in medullary regions that process afferent sensory information and control efferent ventilatory responses.
In the acute phase of the infection, local viral and immune cell invasion of the carotid body24 could disrupt normal functioning, potentially via immune cells destroying infected glomus cells explaining silent hypoxia in some patients22. In the long-term these cells are likely replaced but there is the possibility of long-term local inflammation and disruption of ACE1 and ACE2 balance leading to elevated carotid chemoreceptor drive. Additionally, since the carotid bodies are highly sensitive to disturbances in perfusion, any blood flow disruption caused by microthrombi and endothelial dysfunction24 could elevate carotid body activity65. It is also possible that dysfunction of the petrosal ganglion, which carries afferent sensory input into the brainstem, causes hyper-reactivity in the carotid chemoreflex. Of note, the petrosal ganglion participates in mediating taste signals in the brain. However, only 7% of our long COVID participants reported ongoing impaired sense of taste and smell11. Additionally, inflammation or neuronal damage in the medullary regions mediating afferent signals from the carotid body could contribute to increased chemoreceptor drive. Along these lines there is evidence from animal models that the original SARS-CoV infiltrates medullary brain regions66, but there is no strong evidence of this occurring with existing variants of SARS-CoV-2.
Finally, some evidence suggests the SARS-CoV-2 may affect mitochondrial function67. The glomus cells in the carotid body have unique mitochondria, which are important for O2 sensing68. Any mitochondrial dysfunction in glomus cells could lead to augmented chemoreflex activity68. Since mitochondrial dysfunction could occur in any organ, the exercise intolerance and hyperventilation observed in long COVID could also be driven, for example, by the metaboreceptors in skeletal muscle due to poor mitochondrial function and this needs to be examined.
There are some limitations that need to be considered. Firstly, the control group are a combination of prospectively recruited participants who recovered from a SARS-CoV-2 infection in <4 weeks, and participants who were retrospectively identified from our previous studies (all measures were taken using the same equipment, procedures, and site). Thus 6 of the controls did not have a previous SARS-CoV-2 infection. We cannot rule out that SARS-CoV-2 infection has long lasting effects of the cardiopulmonary system even if individuals recover with no ongoing symptoms. However, our exploratory analyses (see Supplementary Fig. 6) indicates that even without the controls who did not have COVID-19, the carotid chemoreflex sensitivity and VE/VCO2 slope (and VE/VCO2 nadir) remained elevated in the long COVID patients versus the controls who did have COVID-19 (Supplementary Fig. 6). Secondly, we measured the carotid chemoreflex sensitivity using poikilocapnic hypoxia, thus PETCO2 decreased after each hypoxic exposure (due to ventilatory adjustments). However, it is unlikely that this affected the ventilatory response to hypoxia because 1) the PETCO2 decreased only after the breaths that were used as measurements for ventilation and thus could not affect the data and 2) we waited for the PETCO2 to return to baseline after each hypoxic exposure. Thirdly, this was a single site study with a small sample size and thus needs to be evaluated in a larger population of patients with long COVID. Forth, we did not take blood gases measurements, thus we cannot be sure that the low PETCO2 observed in our long COVID patients is a true reflection of the low PaCO2 and thus hyperventilation in these patients. Finally, although we see evidence of elevated carotid chemoreceptor sensitivity in patients with long COVID, we need to evaluate whether dampening down the hyperreflexia helps to improve symptoms.
Conclusions
Here, we show for the first time to the best of our knowledge that patients with long COVID have elevated carotid chemoreceptor sensitivity and that this is correlated with poor breathing efficiency or hyperventilation during exercise. Interventions that temper carotid body excitability could be explored as a treatment option for long COVID. Previously our group had shown that P2X3 receptors in the carotid body can be targeted to reduce carotid chemoreflex hyperreflexia in an animal model of hypertension17 and heart failure69, and could be a viable target in humans with long COVID. Gefapixant, an oral P2X3 receptor antagonist, has recently demonstrated efficacy and an acceptable safety profile in chronic cough in phase 3 clinical trials70. P2X3 receptors could therefore be a viable target in humans with long COVID.
Data availability
Source data underlying Figs. 1 and 2 are provided as Supplementary Data 1 and Supplementary Data 2 Excel files. The authors intend to share their underpinning research data to maximise reuse and evidence their findings. At the time these data were generated, participants were not asked for their permission to share data ‘openly’. The data will be deposited at the University of Bristol Research Data Repository (data.bris.ac.uk/data) where once published, they will be assigned the following https://doi.org/10.5523/bris.2w4fl5nhvpl782hhnybwsv3zw. A metadata record will be published openly by the repository and this record will clearly state how data can be accessed by bona fide researchers. Access requests will be directed to the Research Data team at Bristol, who will assess the motives of potential data re-users before granting access to the data. No authentic request for access will be refused and re-users will not be charged for any part of this process.
References
Venkatesan, P. NICE guideline on long COVID. Lancet Respir. Med. 9, 129 (2021).
Thompson, E. J. et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records.
Ahmad, I. et al. High prevalence of persistent symptoms and reduced health-related quality of life 6 months after COVID-19. Front Public Health 11, 1104267 (2023).
Augustin, M. et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg. Health Eur. 6, 100122 (2021).
O’Mahoney, L. L. et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. eClinicalMedicine 55, 101762 (2023).
Huang, C. et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397, 220–232 (2021).
Antonelli, M. et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect. Dis. 22, 43–55 (2022).
(ONS), O.f.N.S. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 2 February 2023. (ONS website, statistical bulletin, 2023).
Whitaker, M. et al. Persistent COVID-19 symptoms in a community study of 606,434 people in England.
Ballering, A. V., van Zon, S. K. R., Olde Hartman, T. C. & Rosmalen, J. G. M. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 400, 452–461 (2022).
Alkodaymi, M. S. et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin. Microbiol. Infect. 28, 657–666 (2022).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 (2023).
Durstenfeld, M. S. et al. Use of cardiopulmonary exercise testing to evaluate long COVID-19 symptoms in adults: a systematic review and meta-analysis. JAMA Netw Open 5, e2236057 (2022).
Singh, I. et al. Persistent exertional intolerance after COVID-19: insights from invasive cardiopulmonary exercise testing. Chest 161, 54–63 (2022).
van Voorthuizen, E. L., van Helvoort, H. A. C., Peters, J. B., van den Heuvel, M. M. & van den Borst, B. Persistent exertional dyspnea and perceived exercise intolerance after mild COVID-19: a critical role for breathing dysregulation? Phys. Ther. 102, pzac105 (2022).
Frésard, I. et al. Dysfunctional breathing diagnosed by cardiopulmonary exercise testing in ‘long COVID’ patients with persistent dyspnoea. BMJ Open Respir. Res. 9, e001126 (2022).
Pijacka, W. et al. Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat. Med. 22, 1151–1159 (2016).
Iturriaga, R., Alcayaga, J., Chapleau, M. W. & Somers, V. K. Carotid body chemoreceptors: physiology, pathology, and implications for health and disease. Physiol. Rev. 101, 1177–1235 (2021).
Ponikowski, P. et al. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation 104, 544–549 (2001).
Chua, T. P. et al. Clinical characteristics of chronic heart failure patients with an augmented peripheral chemoreflex. Eur. Heart J. 18, 480–486 (1997).
Lan, J. et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581, 215–220 (2020).
Villadiego, J. et al. Is carotid body infection responsible for silent hypoxemia in COVID-19 patients? Function (Oxf) 2, zqaa032 (2021).
Villadiego, J. et al. Is carotid body infection responsible for silent hypoxemia in COVID-19 Patients? Function 2, zqaa032 (2020).
Porzionato, A. et al. Case report: the carotid body in COVID-19: histopathological and virological analyses of an autopsy case series. Front Immunol. 12, 736529 (2021).
Lambermont, B., Davenne, E., Maclot, F. & Delvenne, P. SARS-CoV-2 in carotid body. Intensive Care Med. 47, 342–343 (2021).
Schultz, H. D. Angiotensin and carotid body chemoreception in heart failure. Curr. Opin. Pharmacol. 11, 144–149 (2011).
Allen, A. M. Angiotensin AT1 receptor-mediated excitation of rat carotid body chemoreceptor afferent activity. J. Physiol. 510, 773–781 (1998).
Li, Y. L. et al. Angiotensin II enhances carotid body chemoreflex control of sympathetic outflow in chronic heart failure rabbits. Cardiovasc. Res. 71, 129–138 (2006).
Rysz, S. et al. COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system. Nat. Commun. 12, 2417 (2021).
Nayor, M. et al. Clinical and hemodynamic associations and prognostic implications of ventilatory efficiency in patients with preserved left ventricular systolic function. Circ Heart Fail 13, e006729 (2020).
Arena, R. et al. Development of a ventilatory classification system in patients with heart failure. Circulation 115, 2410–2417 (2007).
Neder, J. A. et al. Exercise ventilatory inefficiency in mild to end-stage COPD. Eur. Respir. J. 45, 377–387 (2015).
Excellence, N.I.f.H.a.C. COVID-19 rapid guideline: managing the long-term effects of COVID-19. NG188 (2020).
Williams, B. et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC). Blood Press 27, 314–340 (2018).
Peters, R. et al. Orthostatic hypotension and symptomatic subclinical orthostatic hypotension increase risk of cognitive impairment: an integrated evidence review and analysis of a large older adult hypertensive cohort. Eur. Heart J. 39, 3135–3143 (2018).
Graham, B. L. et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J. Respir. Crit. Care Med. 200, e70–e88 (2019).
Quanjer, P. H. et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur. Respir. J. 40, 1324–1343 (2012).
ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 167, 211–277. (2003).
Chua, T. P. & Coats, A. J. The reproducibility and comparability of tests of the peripheral chemoreflex: comparing the transient hypoxic ventilatory drive test and the single-breath carbon dioxide response test in healthy subjects. Eur. J. Clin. Invest. 25, 887–892 (1995).
Shah, W., Hillman, T., Playford, E. D. & Hishmeh, L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ 372, n136 (2021).
Cervia, C. et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat. Commun. 13, 446 (2022).
Sylvester, K. P. et al. ARTP statement on pulmonary function testing 2020. BMJ Open Respir. Res. 7, e000575 (2020).
Morgan, B. J., Adrian, R., Bates, M. L., Dopp, J. M. & Dempsey, J. A. Quantifying hypoxia-induced chemoreceptor sensitivity in the awake rodent. J. Appl. Physiol. 117, 816–824 (2014).
Riley, M. S., Nicholls, D. P. & Cooper, C. B. Cardiopulmonary exercise testing and metabolic myopathies. Ann. Am. Thorac. Soc. 14, S129–S139 (2017).
Glaab, T. & Taube, C. Practical guide to cardiopulmonary exercise testing in adults. Respir. Res. 23, 9 (2022).
Balady, G. J. et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122, 191–225 (2010).
Guyenet, P. G. & Bayliss, D. A. Central respiratory chemoreception. Handb. Clin. Neurol. 188, 37–72 (2022).
Jones, N. L., Robertson, D. G. & Kane, J. W. Difference between end-tidal and arterial PCO2 in exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 47, 954–960 (1979).
Jones, N. L., Robertson, D. G. & Kane, J. W. Difference between end-tidal and arterial PCO2 in exercise. J. Appl. Physiol. 47, 954–960 (1979).
Hansen, J. E., Ulubay, G., Chow, B. F., Sun, X. G. & Wasserman, K. Mixed-expired and end-tidal CO2 distinguish between ventilation and perfusion defects during exercise testing in patients with lung and heart diseases. Chest 132, 977–983 (2007).
Beaudry, R. I. et al. Persistent dyspnea after COVID-19 is not related to cardiopulmonary impairment; a cross-sectional study of persistently dyspneic COVID-19, non-dyspneic COVID-19 and controls. Front. Physiol. 13, 917886 (2022).
Younes, M. Measurement and testing of respiratory drive, (Dekker, 1981).
Blain, G. M., Smith, C. A., Henderson, K. S. & Dempsey, J. A. Contribution of the carotid body chemoreceptors to eupneic ventilation in the intact, unanesthetized dog. J. Appl. Physiol. 106, 1564–1573 (2009).
Stickland, M. K., Neder, J. A., Guenette, J. A., O’Donnell, D. E. & Jensen, D. Using cardiopulmonary exercise testing to understand dyspnea and exercise intolerance in respiratory disease. Chest 161, 1505–1516 (2022).
Paton, J. F. et al. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61, 5–13 (2013).
Dejours, P. Chemoreflexes in breathing. Physiol. Rev. 42, 335–358 (1962).
Giannoni, A. et al. Combined increased chemosensitivity to hypoxia and hypercapnia as a prognosticator in heart failure. J. Am. Coll. Cardiol. 53, 1975–1980 (2009).
Smith, C. A., Harms, C. A., Henderson, K. S. & Dempsey, J. A. Ventilatory effects of specific carotid body hypocapnia and hypoxia in awake dogs. J. Appl. Physiol. 82, 791–798 (1997).
Tipton, M. J., Harper, A., Paton, J. F. R. & Costello, J. T. The human ventilatory response to stress: rate or depth? J. Physiol. 595, 5729–5752 (2017).
Gitt, A. K. et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 106, 3079–3084 (2002).
Gluck, S. L. Acid-base. Lancet 352, 474–479 (1998).
Skatrud, J. B. & Dempsey, J. A. Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 55, 813–822 (1983).
Xie, A., Skatrud, J. B., Puleo, D. S., Rahko, P. S. & Dempsey, J. A. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am. J. Respir. Crit. Care Med. 165, 1245–1250 (2002).
Taverne, J. et al. High incidence of hyperventilation syndrome after COVID-19. J. Thorac. Dis. 13, 3918–3922 (2021).
Ding, Y., Li, Y. L. & Schultz, H. D. Role of blood flow in carotid body chemoreflex function in heart failure. J. Physiol. 589, 245–258 (2011).
Netland, J., Meyerholz, D. K., Moore, S., Cassell, M. & Perlman, S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 82, 7264–7275 (2008).
Guntur, V. P. et al. Signatures of mitochondrial dysfunction and impaired fatty acid metabolism in plasma of patients with post-acute sequelae of COVID-19 (PASC). Metabolites 12, 1026 (2022).
Holmes, A. P., Turner, P. J., Buckler, K. J. & Kumar, P. Moderate inhibition of mitochondrial function augments carotid body hypoxic sensitivity. Pflugers Arch. 468, 143–155 (2016).
Lataro, R. M. et al. P2X3 receptor antagonism attenuates the progression of heart failure. Nat. Commun. 14, 1725 (2023).
McGarvey, L. P. et al. Efficacy and safety of gefapixant, a P2X(3) receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet 399, 909–923 (2022).
Acknowledgements
This work was supported by the Elizabeth Blackwell Institute, University of Bristol and the Bristol and Weston Hospitals Charity pump priming scheme. This is research was carried out at the National Institute for Health and Care Research (NIHR) Bristol Clinical Research Facility (CRF). Thank you to the participants who took part (despite their often-debilitating symptoms), the study would not have been possible without them. Thank you also to the cardiology research nurses who helped support this study.
Author information
Authors and Affiliations
Contributions
ECH, AKN, JFRP, HB, AEM and ZA conceived and designed the study. AEM, ZA, HB and ECH recruited participants, collected data, performed the bulk of the analysis and drafted the initial manuscript. JFRP, AKN, KH, AK and APA provided ongoing review of and feedback on analyses over the course of multiple meetings, and reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
This manuscript has been previously reviewed at another Nature Portfolio journal. Communications Medicine thanks Bruno M Silva and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Medany, A., Adams, Z.H., Blythe, H.C. et al. Carotid body dysregulation contributes to Long COVID symptoms. Commun Med 4, 20 (2024). https://doi.org/10.1038/s43856-024-00447-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-024-00447-5