Abstract
Responsive neurostimulation (RNS) is an effective therapy for people with drug-resistant focal epilepsy. In clinical trials, RNS therapy results in a meaningful reduction in median seizure frequency, but the response is highly variable across individuals, with many receiving minimal or no benefit. Understanding why this variability occurs will help improve use of RNS therapy. Here we advocate for a reexamination of the assumptions made about how RNS reduces seizures. This is now possible due to large patient cohorts having used this device, some long-term. Two foundational assumptions have been that the device’s intracranial leads should target the seizure focus/foci directly, and that stimulation should be triggered only in response to detected epileptiform activity. Recent studies have called into question both hypotheses. Here, we discuss these exciting new studies and suggest future approaches to patient selection, lead placement, and device programming that could improve clinical outcomes.
Similar content being viewed by others
Introduction
Epilepsy is a common neurological disorder that afflicts one in 26 people during their lives1. People with epilepsy experience sporadic seizures, in which electrical activity is abnormal in the brain. These seizures usually start in the same parts of an individual’s brain, described as the seizure foci. Despite many anti-seizure medications being available, one-third of people with epilepsy continue to have uncontrolled seizures2. For these individuals, described as having drug-resistant epilepsy, surgical removal (i.e., resection) of seizure-producing brain tissue offers the greatest chance of seizure control3 (see Box 1 for a glossary of specialized terms used in this article). Benefits of resective surgery are immediate, and 50–80% of well-selected patients will be seizure-free4. Whilst generally safe and potentially curative, resective surgery has limitations. Removal of brain tissue can result in permanent neurological deficits5. Precise localization of the seizure focus is required when planning surgery to ensure the correct tissue is removed, but this is not always possible6. Effects of resection may not last long term7,8. Also, resection is typically not an option when seizures arise from brain regions involved in language or muscle control.
Responsive neurostimulation (RNS) is an alternative to resective surgery in which a device that is implanted into the skull delivers electrical stimulation through electrodes that are inserted directly into the seizure focus or foci9,10. RNS is designed to be a “closed-loop” therapy, which means the device continuously senses neural activity and delivers stimulation only in response to detection of particular patterns of brain activity that are known to precede the occurrence of seizures in a particular person11. RNS does not require removal of brain tissue and is reversible (i.e., the device can be removed). It can also be used when resection is not suitable, such as when there are bitemporal seizure foci (i.e., seizures arising from the temporal lobe on each side of the brain) or when there are concerns removal of brain tissue will be problematic10. Randomized controlled trials have established the efficacy of RNS for drug-resistant epilepsy involving one or two seizure foci, with patients experiencing an average reduction in seizure frequency of 75% after nine years of therapy12.
However, RNS also has limitations. Patients seldom become seizure-free, thus RNS is often a palliative therapy where patients have to live with their disease rather than be cured. Maximal seizure reduction can take years to occur, with ongoing seizure-related morbidity and frequent clinic visits required for device tuning to optimize effectiveness. Although the long-term responder rate (i.e., the proportion of patients with ≥50% reduction in seizure frequency) is high (73%)12, over a quarter of patients do not respond well to treatment. There are no established methods to determine which patients will benefit from RNS, which means that one in four patients currently undergo a costly, invasive surgical procedure and years of follow-up appointments with little ultimate benefit.
Fortunately, increased experience from users of RNS, advanced neuroimaging techniques, and analysis of data stored by the RNS device13 are providing more information about how the device works. In this Perspective, we begin by outlining the current model that proposes how RNS reduces seizures. We then discuss recent studies that highlight limitations of this model, and we describe how this model should be revised to better describe the mechanism of action of RNS. We conclude by discussing the implications of this revised model for current clinical practice and how it can inform the design of next-generation neurostimulation devices.
Current model for RNS seizure reduction
Nearly 70 years ago, pioneering work by Penfield and Jasper14 showed that direct electrical stimulation of human cortex could attenuate spontaneous epileptiform discharges, laying the foundation for research into using neurostimulation to treat epilepsy. Decades later, evidence that stimulation of the seizure focus suppresses afterdischarges15,16 informed development of an external RNS that involved a battery-operated desktop device17. RNS devices were further refined to develop a more compact internal RNS that was approved for clinical use in 2013 following clinical trials18. Thus, RNS was developed to be a “seizure stopper,” similar to the rationale for the use of automated defibrillators during cardiac arrhythmias19. While the idea that seizures are aborted through acute, targeted electrical counter-stimulation of spiking occurring during seizures (ictal activity) is intuitive, limited experimental evidence supports it20. RNS stimulations are associated with acute reductions of spectral power21 and with high-frequency desynchronization22, effects reflecting decreased energy in brain waves. These effects could be expected to disrupt seizures, and, in cats, closed-loop stimulation of subcortical structures has been shown to suppress spiking between seizures (interictal spiking) more effectively than random stimulation23.
This mechanistic model for RNS assumes that RNS is most effective when stimulation is delivered as close as possible to the seizure focus and as early as possible after seizure onset. Thus, clinicians aim to localize seizures precisely (to inform RNS lead placement) and refine detection algorithms on the RNS device iteratively (to optimize sensitivity and specificity for seizures).
Clinical reality following RNS
However, many of the clinical observations made during use of RNS are not compatible with the above model being the only mechanism by which RNS works. Application of the above model would predict that RNS should reduce the impact of seizures quickly, yet any improvement in outcome tends to be slow and steady over many years12. This contrasts with the impact of defibrillators, which immediately treat cardiac arrhythmias. RNS devices store records of brain waves as chronic electrocorticograms (ECoGs). Unequivocal examples of stimulation-induced seizure termination are uncommon in RNS ECoGs, even when accounting for preferential storage of long-duration epileptiform activity by the device24, and, when present, tend not to be associated with clinical outcomes25. Most patients treated with RNS receive hundreds to thousands of brief stimulations each day, far exceeding the expected number of seizures; thus, most stimulation occurs in the interictal state, not during seizures. Although precise delineation of the seizure focus is thought to be required for RNS, patients with the most well-localized seizures (e.g., bilateral hippocampal sclerosis, which is typically treated by RNS) do not necessarily have the best outcomes, and treatment response in bitemporal epilepsy does not depend on whether stimulating electrodes are placed within or outside of the hippocampi26. Conversely, patients with poorly demarcated, spatially extensive (regional) neocortical seizure foci can do well with RNS even though the sizes of electrical stimulation fields near intracranial electrodes are smaller than the area of seizure foci27. Finally, long-duration, low-frequency RNS stimulation paradigms can be more effective than conventional short-duration, high-frequency ones, which suggests acute seizure disruption is not the only mechanism of action of RNS28.
Rationale for observed additional actions of RNS
Recent studies exploring structural and functional network connectivity within the brain, the timing of stimulation relative to dynamic brain states, and markers of neural plasticity provide some explanation for these unexpected clinical observations. Although the RNS clinical trials were not powered for subgroup comparisons based on clinical or imaging features, there is emerging evidence that some brain networks may be intrinsically more responsive to RNS stimulation than others. Retrospective analysis of intracranial electroencephalography (EEG) in patients who were later treated with RNS revealed that ictal synchronizability, a metric reflecting the ease by which neural activity propagates through a functionally connected brain network, is inversely related to the degree of seizure reduction with RNS therapy29. Thus, RNS responders and non-responders can be distinguished prior to device implantation based on a biomarker derived from electrographic features of their seizures. Another recent study using pre-RNS magnetoencephalography (MEG) found that interictal global functional connectivity in certain frequency bands was lower in RNS non-responders compared with responders30. Taken together, it would seem the effectiveness of RNS therapy depends on intrinsic neurophysiological properties of seizures and the brain networks that give rise to them. For example, a speculative possibility is that interictal RNS stimulation can more readily diffuse through networks with high functional connectivity, which could potentiate its therapeutic effects, and that seizures less able to synchronize widespread networks are those most readily reduced by RNS stimulation.
If RNS efficacy depends on network characteristics, therapy could be expected to be more effective if the tissue activated by stimulating electrodes includes key nodes within these networks. Multiple studies of hippocampal neurostimulation have found no link between the precise anatomical location of electrodes and patient outcomes26,31,32. However, outcomes can be predicted when the specific brain circuit(s) being stimulated is known. In a study of RNS patients with leads extending into the hippocampus, seizure reduction was greatest when diffusion imaging revealed that the activated tissue was structurally connected to medial prefrontal cortex, anterior cingulate, and precuneus, nodes which tended to connect with more posterior regions of the hippocampus31. This study suggests that the current strategy of placing RNS leads based on anatomic landmarks should be revised to also include a consideration of patient-specific networks. These networks could potentially be delineated preoperatively and information about them used to target convergence points of white matter tracts implicated in the seizure. This approach is already being adopted when brain stimulation is used to treat movement disorders33 and psychiatric disorders34. Another study that highlighted the importance of patient-specific functional connectivity for determining RNS efficacy used cortico-cortical evoked potentials to define “receiver” and “projection” nodes, areas of greater inward or outward connectivity, respectively, during intracranial EEG monitoring in patients who were later treated with RNS35. Clinical outcomes were significantly better when RNS electrodes were placed near receiver nodes. The findings suggest that epileptic networks may have points of vulnerability where targeted stimulation can exert high network controllability36,37 and so might suppress seizures as well or better than stimulation directly at the seizure focus. Identification of these critical points, conceptually, the “Achilles’ heels” of epileptic networks, could enable RNS leads to be better placed for optimal efficacy. This strategy is also in line with a larger body of evidence38,39,40 that indicates that precise lead targeting within networks that involve thalamic nuclei is critical for the efficacy of deep brain stimulation (DBS), another treatment for epilepsy.
As the structural and functional network determinants of RNS efficacy are better understood, it is also becoming clear that temporal variables play a significant role. For a long time, seizures were thought to occur at random, but studies of RNS ECoG and other datasets have revealed the existence of a cyclical temporal structure in epilepsy41. This has led to the development of contemporary models of seizure timing which propose that there are alternating high and low states of seizure likelihood, which coincide with cycles in the rate of interictal epileptiform activity42. Cycles of interictal epileptiform activity exist over multiple timescales, from circadian to multidien (multi-day)43,44,45, and seizures preferentially occur at certain phases of these cycles. Since cycles of epileptiform activity could be indicative of resting-state dynamics of the interictal network46, the effects of RNS stimulation could depend on the specific network state at the time of stimulation. Indeed, a recent study found that the effects of changing RNS stimulation parameters (e.g., frequency, burst duration, and charge density) depend on the initial seizure risk state47, with parameter changes effective at reducing seizures in one seizure risk state being less effective during another risk state. Consistent with this, a recent study examining how stimuli were distributed across these low- and high-risk states found improved outcomes when stimulations were delivered preferentially in low-risk states, i.e., those states less disrupted by ongoing epileptiform activity48. RNS stimulation parameters are typically adjusted every few months, remaining constant in the interim. Thus, observed outcomes may underestimate the potential impact of RNS therapy by being the compositive of potentially opposing effects during network state cycling49.
The slow time course of seizure reduction with RNS therapy provides some of the strongest evidence for a long-term neuromodulatory effect on brain networks that generate seizures50. Instead of acute stimulation arresting seizures, chronic stimulation could render the network less prone to initiating seizures. This hypothesis has garnered recent support from clinical data. Analysis of stimulation effects on electrographic seizure patterns in RNS ECoGs showed that immediate inhibition of these patterns was not associated with clinical outcomes but that “indirect” effects, defined as those occurring before or at some latency (>10 s) from stimulations, were associated with clinical outcomes25. This suggests that the beneficial effects of stimulation may not be a consequence of direct involvement in seizures. Subsequently, analysis of chronic interictal RNS ECoGs revealed differential plasticity in functional network connectivity between RNS responders and non-responders51. Patients with the best outcomes from RNS are those with the greatest ability to reorganize functional network connectivity. Stimuli inducing this plasticity may be more effective when delivered during periods with less epileptiform activity48, when endogenous neuroplasticity mechanisms are more active52,53.
These effects may not be unique to RNS. An alternative neurostimulation therapy for epilepsy is DBS of the anterior thalamic nuclei. This uses scheduled intermittent stimulation (open-loop) to modulate functional network connectivity54 and also takes years to maximally reduce seizures55. Chronic neuromodulatory effects could help explain the similarity in outcomes between open-loop neurostimulation modalities55,56,57 and closed-loop RNS12. This also calls into question whether the effectiveness of RNS depends on its responsive, feedback activity. In principle, to determine whether stimulation at the start of seizures is necessary for seizure reduction, RNS detection settings could be tuned to patterns of neural activity that are not present at seizure onset, though this would necessarily compromise seizure quantification from the device13.
Emerging model for RNS seizure reduction
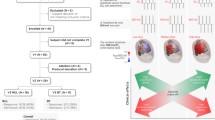
Multidimensional network determinants of RNS efficacy help explain observed variability in clinical outcomes. Ictal and interictal network connectivity, lead location in relation to key structural and functional network nodes, cyclical network states, and long-term functional network reorganization collectively influence the likelihood that RNS will benefit a given individual (Fig. 1). Since current practice parameters do not generally consider these factors, we think it is remarkable that RNS works as well as it does. Hyperacute termination of ictal patterns may still play a role in seizure reduction with RNS, but chronic neuromodulatory effects are probably more important. Recent studies have revealed that one size does not fit all for epilepsy neurostimulation therapies, which need to be as diverse and dynamic as brain networks themselves. The implication of this new conceptual framework is that the clinical approach to virtually every step of RNS management, including patient selection, lead placement, and device programming, needs to be reconsidered. Some currently non-responding patients might benefit from the consideration of dynamic network features when using RNS and other neurostimulation devices, such as thalamic DBS.
Although RNS was originally conceived as a seizure stopper that works by acutely terminating seizures at their point(s) of origin, recent evidence reveals that this may not be its primary mechanism of action. Features of individual brain networks, including connectivity patterns, key structural and functional nodes, cyclical seizure risk states, and long-term plasticity collectively determine the extent of seizure reduction with RNS therapy.
Future directions
Understanding of network-guided neuromodulation50 might enable further personalization and better inform the choice and management of neurostimulation devices. Pre-surgical evaluations should focus on defining functional networks, in addition to identifying anatomical lesions and margins of seizure foci. Patient-specific models that use advanced neuroimaging techniques and integrate multi-dimensional network characteristics are already being employed40 and are likely to become the standard of care. However, challenges remain that, if addressed, could accelerate clinical improvement. It is not yet known whether there are interventions that could catalyze network reorganization, reducing the time required for patients to wait for meaningful seizure reduction. The appeal of palliative neuromodulation therapies will increase if seizure-free outcomes become similar to or exceed those currently attainable with resection. Indeed, as our ability to decode and manipulate brain networks increases, we might be able to help our patients’ brains stop initiating seizures. Next-generation devices that possess enhanced capabilities will be essential to achieve this outcome. Such devices could benefit from a greater number of leads that interface with more brain networks and on-board artificial intelligence that enables real-time state analysis and adaptive stimulation58. We hope and anticipate that a future version of RNS may finally prove to be a seizure stopper.
References
England, M. J., Liverman, C. T., Schultz, A. M. & Strawbridge, L. M. Epilepsy across the spectrum: promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy Behav. 25, 266–276 (2012).
Brodie, M. J., Barry, S. J., Bamagous, G. A., Norrie, J. D. & Kwan, P. Patterns of treatment response in newly diagnosed epilepsy. Neurology 78, 1548–1554 (2012).
Kwan, P., Schachter, S. C. & Brodie, M. J. Drug-resistant epilepsy. N. Engl. J. Med. 365, 919–926 (2011).
Wiebe, S. & Jette, N. Pharmacoresistance and the role of surgery in difficult to treat epilepsy. Nat. Rev. Neurol. 8, 669–677 (2012).
Rolston, J. D., Englot, D. J., Knowlton, R. C. & Chang, E. F. Rate and complications of adult epilepsy surgery in North America: analysis of multiple databases. Epilepsy Res. 124, 55–62 (2016).
Jehi, L. et al. Comparative effectiveness of stereotactic electroencephalography versus subdural grids in epilepsy surgery. Ann. Neurol. 90, 927–939 (2021).
de Tisi, J. et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 378, 1388–1395 (2011).
Mohan, M. et al. The long-term outcomes of epilepsy surgery. PLoS ONE 13, e0196274 (2018).
Jarosiewicz, B. & Morrell, M. The RNS System: brain-responsive neurostimulation for the treatment of epilepsy. Expert Rev. Med. Devices 18, 129–138 (2021).
Ma, B. B. & Rao, V. R. Responsive neurostimulation: candidates and considerations. Epilepsy Behav. 88, 388–395 (2018).
Inaji, M., Yamamoto, T., Kawai, K., Maehara, T. & Doyle, W. K. Responsive neurostimulation as a novel palliative option in epilepsy surgery. Neurol. Med. Chir. 61, 1–11 (2021).
Nair, D. R. et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology 95, e1244–e1256 (2020). Clinical trial showing slow improvement in patient outcomes with RNS therapy over nine years.
Rao, V. R. Chronic electroencephalography in epilepsy with a responsive neurostimulation device: current status and future prospects. Expert Rev. Med. Devices 18, 1093–1105 (2021).
Penfield, W. & Jasper, H. Epilepsy and the Functional Anatomy of the Human Brain (Little, Brown and Company, 1954).
Lesser, R. P. et al. Brief bursts of pulse stimulation terminate afterdischarges caused by cortical stimulation. Neurology 53, 2073–2081 (1999).
Motamedi, G. K. et al. Optimizing parameters for terminating cortical afterdischarges with pulse stimulation. Epilepsia 43, 836–846 (2002).
Kossoff, E. H. et al. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia 45, 1560–1567 (2004).
Morrell, M. J. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 77, 1295–1304 (2011).
Morrell, M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr. Opin. Neurol. 19, 164–168 (2006).
Salam, M. T., Perez Velazquez, J. L. & Genov, R. Seizure suppression efficacy of closed-loop versus open-loop deep brain stimulation in a rodent model of epilepsy. IEEE Trans. Neural Syst. Rehabil. Eng. 24, 710–719 (2016).
Rønborg, S. N. et al. Acute effects of brain-responsive neurostimulation in drug-resistant partial onset epilepsy. Clin. Neurophysiol. 132, 1209–1220 (2021).
Sohal, V. S. & Sun, F. T. Responsive neurostimulation suppresses synchronized cortical rhythms in patients with epilepsy. Neurosurg. Clin. N. Am. 22, 481–488 (2011).
Psatta, D. M. Control of chronic experimental focal epilepsy by feedback caudatum stimulations. Epilepsia 24, 444–454 (1983).
Sisterson, N. D. et al. A rational approach to understanding and evaluating responsive neurostimulation. Neuroinformatics 18, 365–375 (2020).
Kokkinos, V., Sisterson, N. D., Wozny, T. A. & Richardson, R. M. Association of closed-loop brain stimulation neurophysiological features with seizure control among patients with focal epilepsy. JAMA Neurol. 76, 800–808 (2019).
Geller, E. B. et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia 58, 994–1004 (2017).
Ma, B. B. et al. Responsive neurostimulation for regional neocortical epilepsy. Epilepsia 61, 96–106 (2020).
Alcala-Zermeno, J. L., Starnes, K., Gregg, N. M., Worrell, G. & Lundstrom, B. N. Responsive neurostimulation with low-frequency stimulation. Epilepsia 64, e16–e22 (2023).
Scheid, B. H. et al. Intracranial electroencephalographic biomarker predicts effective responsive neurostimulation for epilepsy prior to treatment. Epilepsia 63, 652–662 (2022).
Fan, J. M. et al. Network connectivity predicts effectiveness of responsive neurostimulation in focal epilepsy. Brain Commun. 4, fcac104 (2022). Magnetoencephalography study linking brain network connectivity to effectiveness of RNS therapy.
Charlebois, C. M. et al. Patient-specific structural connectivity informs outcomes of responsive neurostimulation for temporal lobe epilepsy. Epilepsia 63, 2037–2055 (2022).
Bondallaz, P. et al. Electrode location and clinical outcome in hippocampal electrical stimulation for mesial temporal lobe epilepsy. Seizure 22, 390–395 (2013).
Middlebrooks, E. H. et al. Neuroimaging advances in deep brain stimulation: review of indications, anatomy, and brain connectomics. AJNR Am. J. Neuroradiol. 41, 1558–1568 (2020).
Riva-Posse, P. et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol. Psychiatry 23, 843–849 (2018).
Kobayashi, K. et al. Effective connectivity relates seizure outcome to electrode placement in responsive neurostimulation. Brain Commun. in press (2023).
Khambhati, A. N. et al. Functional control of electrophysiological network architecture using direct neurostimulation in humans. Netw. Neurosci. 3, 848–877 (2019).
Scheid, B. H. et al. Time-evolving controllability of effective connectivity networks during seizure progression. Proc. Natl Acad. Sci. USA 118, e2006436118 (2021).
Gross, R. E., Fisher, R. S., Sperling, M. R., Giftakis, J. E. & Stypulkowski, P. H. Analysis of deep brain stimulation lead targeting in the stimulation of anterior nucleus of the thalamus for epilepsy clinical trial. Neurosurgery 89, 406–412 (2021).
Schaper, F. et al. Mapping lesion-related epilepsy to a human brain network. JAMA Neurol. 80, 891–902 (2023).
Warren, A. E. L. et al. The optimal target and connectivity for deep brain stimulation in Lennox-Gastaut Syndrome. Ann. Neurol. 92, 61–74 (2022).
Karoly, P. J. et al. Cycles in epilepsy. Nat. Rev. Neurol. 17, 267–284 (2021).
Baud, M. O., Proix, T., Rao, V. R. & Schindler, K. Chance and risk in epilepsy. Curr. Opin. Neurol. 33, 163–172 (2020).
Leguia, M. G. et al. Seizure cycles in focal epilepsy. JAMA Neurol. 78, 454–463 (2021).
Baud, M. O. et al. Multi-day rhythms modulate seizure risk in epilepsy. Nat. Commun. 9, 88 (2018). Seizures tend to occur at certain phases of cycles of interictal epileptiform activity, providing evidence that brain state influences seizure likelihood.
Karoly, P. J. et al. Circadian and circaseptan rhythms in human epilepsy: a retrospective cohort study. Lancet Neurol. 17, 977–985 (2018).
Ojemann, W. K. S. et al. Resting-state background features demonstrate multidien cycles in long-term EEG device recordings. Brain. Stimul. in press (2023).
Chiang, S. et al. Evidence of state-dependence in the effectiveness of responsive neurostimulation for seizure modulation. Brain Stimul. 14, 366–375 (2021).
Anderson, D. N. et al. Closed-loop stimulation in periods with less epileptiform activity drives improved epilepsy outcomes. Brain https://doi.org/10.1093/brain/awad343 (2023). Stimulation during brain states with less epileptiform activity enables long-term neuroplasticity observed in RNS responders.
Frauscher, B. et al. Stimulation to probe, excite, and inhibit the epileptic brain. Epilepsia https://doi.org/10.1111/epi.17640 (2023).
Piper, R. J. et al. Towards network-guided neuromodulation for epilepsy. Brain 145, 3347–3362 (2022).
Khambhati, A. N., Shafi, A., Rao, V. R. & Chang, E. F. Long-term brain network reorganization predicts responsive neurostimulation outcomes for focal epilepsy. Sci. Transl. Med. 13, eabf6588 (2021). Network connections change over time in RNS responders, providing evidence for neuromodulatory effects of RNS therapy.
Beck, H., Goussakov, I. V., Lie, A., Helmstaedter, C. & Elger, C. E. Synaptic plasticity in the human dentate gyrus. J. Neurosci. 20, 7080–7086 (2000).
Naik, A. A. et al. Mechanism of seizure-induced retrograde amnesia. Prog. Neurobiol. 200, 101984 (2021).
Silva, A. B., Khambhati, A. N., Speidel, B. A., Chang, E. F. & Rao, V. R. Effects of anterior thalamic nuclei stimulation on hippocampal activity: chronic recording in a patient with drug-resistant focal epilepsy. Epilepsy Behav. Rep. 16, 100467 (2021).
Salanova, V. et al. The SANTÉ study at 10 years of follow-up: effectiveness, safety, and sudden unexpected death in epilepsy. Epilepsia 62, 1306–1317 (2021).
Cukiert, A., Cukiert, C. M., Burattini, J. A., Mariani, P. P. & Bezerra, D. F. Seizure outcome after hippocampal deep brain stimulation in patients with refractory temporal lobe epilepsy: a prospective, controlled, randomized, double-blind study. Epilepsia 58, 1728–1733 (2017).
Lundstrom, B. N., Gompel, J. V., Khadjevand, F., Worrell, G. & Stead, M. Chronic subthreshold cortical stimulation and stimulation-related EEG biomarkers for focal epilepsy. Brain Commun. 1, fcz010 (2019).
Worrell, G. A. & Kremen, V. in Neurostimulation for Epilepsy (ed. Rao, V. R.) Ch. 9 (Academic Press, 2023).
Acknowledgements
We thank Hargunbir Singh, MBBS for assistance with creating the infographic. V.R.R. is supported by an endowed professorship from the Ernest Gallo Foundation. J.D.R. is supported by the National Institutes of Health K23 (NS114178) and UH3 (NS109557) grants.
Author information
Authors and Affiliations
Contributions
V.R.R. and J.D.R. were both involved in the conception, writing, and approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: both authors are prior, but not current, consultants for NeuroPace, Inc., manufacturer of the RNS System, and are currently investigators in the NIH-funded RNS System Lennox-Gastaut Syndrome (LGS) Feasibility Study (ClinicalTrials.gov Identifier: NCT05339126). The authors declare no targeted compensation for this work.
Peer review
Peer review information
Communications Medicine thanks David Burdette and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rao, V.R., Rolston, J.D. Unearthing the mechanisms of responsive neurostimulation for epilepsy. Commun Med 3, 166 (2023). https://doi.org/10.1038/s43856-023-00401-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-023-00401-x