Abstract
Background
Preclinical evidence suggests that certain antipsychotic medications may inhibit the development of lung cancer. This study aims to investigate the association between incident lung cancer and different cumulative exposure periods of flupentixol or any antipsychotics.
Methods
Using electronic health records from the Hospital Authority in Hong Kong, this nested case-control study included case participants aged 18 years or older with newly diagnosed lung cancer after initiating antipsychotics between January 1, 2003, and August 31, 2022. Each case was matched to up to ten controls of the same sex and age, who were also antipsychotic users. Multivariable conditional logistic regression models were conducted to quantify the association between lung cancer and different cumulative exposure times of flupentixol (0–365 days [ref]; 366–1825 days; 1826+ days) and any antipsychotics (1–365 days [ref]; 366–1825 days; 1826+ days), separately.
Results
Here we show that among 6435 cases and 64,348 matched controls, 64.06% are males, and 52.98% are aged 65–84 years. Compared to patients with less than 365 days of exposure, those with 366–1825 days of exposure to flupentixol (OR = 0.65 [95% CI, 0.47–0.91]) and any antipsychotics (0.42 [0.38–0.45]) have a lower risk of lung cancer. A decreased risk is observed in patients who have 1826+ days of cumulative use of any antipsychotics (0.54 [0.47–0.60]).
Conclusions
A reduced risk of lung cancer is observed in patients with more than one year of exposure to flupentixol or any antipsychotics. Further research on the association between lung cancer and other antipsychotic agents is warranted.
Plain language summary
Antipsychotic drugs are mainly used to treat mental illnesses. Certain antipsychotic medications, such as flupentixol, may help protect patients against lung cancer. Here, we investigated whether prolonged use of flupentixol or other antipsychotics could reduce the occurrence of lung cancer among antipsychotic users. We demonstrated that a smaller proportion of patients with one to five years and more than five years of exposure to any antipsychotics develop lung cancer compared to those with less than one year of exposure. Specifically, for flupentixol, we observed a smaller proportion of patients with one to five years of exposure develop lung cancer compared to those with less than one year. To substantiate our current findings, further studies examining other populations and specific antipsychotic agents are necessary for developing effective lung cancer prevention strategies among this high-risk population.
Similar content being viewed by others
Introduction
Lung cancer remains one of the leading causes of death worldwide despite decades of local and global collaborative efforts to restrict tobacco use1. Antipsychotic users, who typically live with severe mental illnesses, have a notably higher crude incidence of lung cancer than the general population2. This elevated risk can be attributed to lower socioeconomic status, poorer health awareness and self-care, and most importantly, a higher smoking rate3,4,5.
Interestingly, there is analytic evidence showing that people living with severe mental illnesses or using antipsychotics might have a lower risk of some cancers6,7,8. Apart from a potential direct protective effect from the medications, this may be attributed to several reasons. First, some genetic factors involved in the etiology of schizophrenia may act as protective agents against cancer9. For example, the tumor suppressor gene p53 can reduce the risk of cancer through apoptosis9. Second, long-term antipsychotic use is associated with negative physical consequences, leading to a shorter life expectancy in comparison to the general population10. This could contribute to lower cancer incidence. Furthermore, the stigma surrounding mental illnesses and antipsychotic prescriptions, coupled with low health literacy and the deprioritization of mental health care, have created obstacles for individuals seeking medical help11. Consequently, the underdiagnosis of diseases, including cancer, might result in an artificially lowered cancer incidence among those receiving antipsychotic treatments.
The use of second-generation antipsychotics has been increasingly common in recent years12,13. However, flupentixol, a first-generation antipsychotic agent, has maintained a stable presence in the clinical treatment of schizophrenia due to its efficacy against negative and affective symptoms and a lower reported rate of severe adverse drug reactions14,15. In terms of pharmacology, flupentixol demonstrates promising effects in reducing the risk of lung cancer16. In non-small-cell lung cancer, flupentixol interferes with the PI3K/AKT pathway, which is often hyperactivated in living cancer cells, angiogenesis, invasion, proliferation, and metastasis17. Specifically, the docking of flupentixol to the PI3Kα protein inhibits the PI3K/AKT pathway and the survival of lung cancer cells both in vitro and in vivo16. This reduces the phosphorylation of AKT protein in a concentration-dependent manner16, blocking the activation of AKT protein to regulate downstream components18.
Despite the speculation of this molecular mechanism, there has been little clinical research conducted to determine the association between flupentixol and lung cancer. There is only one case-control study showing reduced odds of lung cancer associated with antipsychotic use compared with non-use, which excluded flupentixol19. In addition, the authors of that study acknowledged the small number of antipsychotic users recruited, which could reduce the statistical power for ascertainment of association19. Hence, this current study aims to examine the association between flupentixol or any antipsychotic use and lung cancer among the population of antipsychotic users with varying degrees of exposure time. Given the aforementioned preclinical evidence and the unique mechanism of flupentixol, we hypothesize that there is an inverse association. Our study shows that patients exposed to any antipsychotics for 366–1825 days or 1826 + days have a decreased risk of lung cancer when compared to those exposed for less than 365 days. In particular, we note a reduced lung cancer risk in patients with 366–1825 days of flupentixol exposure compared to those with less than 365 days of exposure.
Methods
Study design and data source
We conducted a nested case-control study using de-identified electronic medical records from the Hong Kong Clinical Data Analysis and Reporting System (CDARS). CDARS is a territory-wide database developed by the Hospital Authority (HA), a statutory body managing all public hospitals and providing healthcare services for more than 7 million Hong Kong residents20. CDARS has collected information on demographics, clinical diagnoses, procedures, admission and discharge records, laboratory tests, and prescriptions from inpatient, outpatient, and accident & emergency departments since 199321. The quality and reliability of data from CDARS have been extensively validated by various pharmacoepidemiologic studies including studies on antipsychotic use as well as cancer22,23,24,25. For this study, we retrieved data from CDARS from September 01, 2022, to October 31, 2022. The data were reported using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline26.
Ethics approval
This study was approved by the Institutional Review Board of the University of Hong Kong/ Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB, reference number: UW 20-113). As our data were all anonymized without any personal identification information, no informed consent was required for the study.
Selection of cases and controls
We included patients who received prescriptions for any antipsychotics between January 1, 2001, and August 31, 2022. Specific antipsychotic agents were identified by the British National Formulary (BNF) chapter 4.2.1 (Antipsychotic drugs) and 4.2.2 (Antipsychotic depot injections). Patients who (1) had missing values on age, sex, and date of death; (2) had incorrect records (i.e., the death date was before the date of first prescription of antipsychotics); (3) had a diagnosis of lung cancer before or at the date of the first prescription of antipsychotics; or (4) had a diagnosis of lung cancer before January 01, 2003, were excluded. The years 2001 and 2002 were employed as the screening period to exclude non-incident lung cancer cases. Given the clinical information before 2001 was not available, the first prescription of antipsychotics between 2001 and 2022 was designated as the first prescription of antipsychotics during the study period for each patient.
The outcome of this study was the first diagnosis of lung cancer between January 01, 2003, and August 31, 2022. Lung cancer was identified by the ICD-9-CM codes 162.0–162.9.
Cases were patients aged 18 years or older with newly diagnosed lung cancer between January 01, 2003, and August 31, 2022. The index date for cases was the date of the first diagnosis of lung cancer.
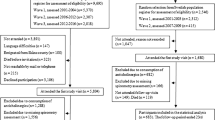
Up to ten controls who were antipsychotic users and without a diagnosis of lung cancer before or at the index date were randomly matched for each case by sex and age in years using the incidence density sampling approach27. The index date for controls was designated as the date of the first diagnosis of lung cancer for their matched cases. Each patient can contribute as a control for up to four different cases28, and thus, they could have up to four different assigned index dates. For each matching process, control candidates were excluded from the pool of controls if they did not have any prescription of antipsychotics before their assigned index date. Figure 1 shows the flowchart of cases and controls selection.
Exposure
All patients included in this study had at least one day of exposure to any antipsychotics before the index date. A patient might have multiple records of antipsychotic prescriptions. All antipsychotic prescriptions between the first antipsychotic use and the index date were extracted for each patient to calculate the cumulative drug exposure. The exposure period of a single record of the prescription was defined as the time between the start and the end dates of the prescription for that record. The cumulative exposure of all antipsychotics was calculated by summing up the periods of all records of any antipsychotic prescriptions before the index date. If multiple drugs were prescribed within the same period, the exposure period of all antipsychotics would be counted only once. The treatment period of flupentixol was calculated by summing up the periods of all records of flupentixol prescriptions before the index date. According to current clinical guidelines, which suggest the treatment duration of antipsychotics should be one to 5 years29, the cumulative exposure to any antipsychotics (including flupentixol) was categorized as 1–365 days (selected as the reference group), 366–1825 days, and 1826+ days. Since not all included antipsychotic users received flupentixol, the cumulative exposure of flupentixol was categorized as 0–365 days (selected as the reference group), 366–1825 days, and 1826+ days. Two analyses were conducted separately to investigate the association between the different cumulative exposure times of (1) flupentixol; and (2) any antipsychotics and the reduced risk of incident lung cancer.
More than 98% of records had the start and end dates of antipsychotic prescriptions. We used the dosage, and prescribed quantity and frequency to determine the prescription duration when the start or end dates were not available. We further used the median prescription value of each specific drug to impute the corresponding drug exposure period when the above information was missing.
Confounders
We adjusted the potential risk factors of lung cancer or indications for antipsychotic use as confounders to identify the independent association between antipsychotics and lung cancer, including the use of non-steroidal anti-inflammatory drugs (NSAIDs) (i.e., ibuprofen, diclofenac, indomethacin, ketoprofen, ketorolac, mefenamic acid, naproxen, piroxicam, celecoxib, and etoricoxib), statins (i.e., atorvastatin, fluvastatin, lovastatin, rosuvastatin, pravastatin, and simvastatin), aspirin, and metformin, and the diagnosis of tobacco use, diabetes, chronic obstructive pulmonary disease (COPD), hypertension, hyperlipidemia, cirrhosis, chronic kidney disease (CKD), peptic ulcer, pneumonia, schizophrenia, depressive disorders, anxiety disorders, bipolar disorders, personality disorders, delusional disorders, other nonorganic psychoses, and dementia19,30,31,32. All confounders were identified by using clinical information between January 01, 2001 and the index date. The coding details for these confounders are shown in Supplementary Table 1.
Statistical analysis
We tabulated sample characteristics at the index date for cases and controls. We conducted two multivariable conditional logistic regressions to estimate risks of lung cancer associated with the cumulative use of (1) flupentixol (analysis one); and (2) any antipsychotics (including flupentixol [analysis two]), separately. All confounding variables were adjusted in the models. For the analysis of flupentixol treatment (analysis one), the model was further adjusted for the cumulative use of antipsychotics except for flupentixol (categorized as 0–365 days [selected as the reference group], 366–1825 days, and 1826+ days). All parameters were expressed as odds ratios (ORs) with a 95% confidence interval (95% CI). We carried out subgroup analyses stratified by sex, as there is a sex difference in response to antipsychotic treatment33,34. In the secondary analysis, the outcome of lung cancer was further classified into seven subgroups according to the ICD-CM-9 codes, including malignant neoplasm of trachea (ICD-CM-9 code, 162.0), malignant neoplasm of main bronchus (162.2), malignant neoplasm of upper lobe, bronchus or lung (162.3), malignant neoplasm of middle lobe, bronchus or lung (162.4), malignant neoplasm of lower lobe, bronchus or lung (162.5), malignant neoplasm of other parts of bronchus or lung (162.8), and malignant neoplasm of bronchus and lung, unspecified (162.9). We fitted seven models using the leave-one-out approach, which removed one subtype of lung cancer from the outcome each time35. We did two sets of sensitivity analyses to test the robustness of study findings: (1) Excluding all patients who had any previous use of risperidone, pimozide, aripiprazole, olanzapine, lurasidone, brexpiprazole, trifluoperazine, clozapine, chlorpromazine, and haloperidol before the index date, as these drugs been suggested to be associated with lung cancer risk reduction;19,36,37,38,39,40,41,42,43,44 and (2) For the case-control matching procedure, each patient can be selected as a control for an unlimited number of cases.
We used statistical software R (version 4.1.2) for all analyses45. Two-sided P values of 0.05 or below were considered indicative of statistical significance.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Participant characteristics
We identified 445,015 patients with antipsychotic prescriptions between January 01, 2001, and August 31, 2022. After examining for eligibility, 6435 cases and 64,348 matched controls were included for the primary analysis. Of these, 341 patients contributed as both cases and controls and 13,113 patients contributed multiple times in the control groups. Table 1 shows the descriptive statistics for cases and controls. The majority of cases were males (N = 4122 [64.06%]), aged 65–84 years (3409 [52.98%]). The mean age of the case group was 74 (SD = 13) years. Compared to controls, cases were more likely to receive a previous prescription for NSAIDs and diagnosis of tobacco use, COPD, cirrhosis, and pneumonia.
The sample characteristics by exposure group of flupentixol and any antipsychotics are shown in Supplementary Table 2. Despite the exposure period, most patients in the flupentixol group were males. Compared to less than 365 days users, patients with longer exposure to flupentixol were younger, more likely to receive NSAIDS and metformin, and had a higher prevalence of cirrhosis, schizophrenia, depressive disorders, bipolar disorders, personality disorders, delusional disorders, and other nonorganic psychoses. The highest prevalence of comorbidity was found in schizophrenia in 366–1825 days (N = 666 [73.84%]) and 1826+ days (703 [85.94%]) groups. The descriptive statistics were similar for any antipsychotics.
Odds of lung cancer associated with antipsychotic use
Before the index date, 98.85% (N = 6361) of cases and 97.34% (62,634) of controls had no or less than 365 days of exposure to flupentixol (Fig. 2). The prevalence of less than 365 days of cumulative use of any antipsychotics in case and control groups was 74.48% (4793) and 50.30% (32,367), respectively. Controls were more likely to have longer exposure to flupentixol (i.e., 1.42% of controls versus 0.59% of cases for 366–1825 days of exposure; 1.24% of controls versus 0.56% of cases for 1826+ days of exposure) and any antipsychotics (i.e., 27.87% of controls versus 13.13% of cases for 366–1825 days of exposure; 21.83% of controls versus 12.39% of cases for 1826+ days of exposure). Compared to less than 365 days of exposure, patients with 366–1825 days of exposure to flupentixol (OR = 0.65 [95% CI, 0.47–0.91]) and any antipsychotics (0.42 [0.38–0.45]) carried a significantly decreased risk of lung cancer. The risk was also lower for longer-term use of any antipsychotics, with the OR of 0.54 (0.47–0.60) for 1826+ days of exposure (Fig. 2).
Error bars represent the 95% confidence intervals of these odd ratios. Analysis one is to investigate the association between the lung cancer and different cumulative exposure time of flupentixol, adjusting for other antipsychotics and all interested covariates. Analysis two is to investigate the association between the lung cancer and different cumulative exposure time of all antipsychotics (including flupentixol), adjusting for all interested covariates.
For sex-stratified analysis, the association between cumulative use of flupentixol and lung cancer was only significant among the female 366–1825 days users (0.50 [0.27–0.91]). A significantly lower risk of lung cancer was found in 366–1825 days (males: OR = 0.41 [95% CI, 0.36–0.45]; females: 0.43 [0.38–0.49]) and 1826+ days (males: OR = 0.52 [95% CI, 0.46–0.59]; females: 0.57 [0.49–0.67]) of exposure to any antipsychotics for males and females. Estimates from the full analysis are shown in Supplementary Table 3.
Secondary and sensitivity analyses
Results from secondary analyses (Table 2) showed a similar risk pattern to the main analysis. For instance, for the total sample, compared to the 0–365 days of exposure to flupentixol, the ORs for 366–1825 days use of flupentixol ranged from 0.60 (95 CI, 0.39–0.91) (excluding malignant neoplasm of upper lobe, bronchus or lung) to 0.69 (0.48–1.00) (excluding malignant neoplasm of lower lobe, bronchus or lung). Regarding any antipsychotics, a significantly decreased risk was observed in patients with both 365–1825 days and 1826+ days of exposure.
Results from sensitivity analyses were consistent with the main findings (Supplementary Tables 4 and 5). For example, for the first sensitivity analysis (Supplementary Table 4), a significantly decreased risk of lung cancer was observed in 366–1825 days and 1826+ days of exposure to antipsychotics. However, the lower risk was only found in patients with 1826+ days of flupentixol exposure compared to their flupentixol-free counterparts.
Discussion
In this study, we identified a lower risk of lung cancer in patients with more than 1 year of exposure to any antipsychotics. For flupentixol, the significantly reduced risk was only observed in the 366–1825 days exposure group. Similar findings were supported by results from any antipsychotics in both males and females, and flupentixol in females with 0–365 days of exposure. Comparable findings were identified in the secondary analysis of different locations of the tumor.
Previous laboratory results indicate that using A549 cell lines, flupentixol has been shown to reduce the level of Bcl-2 expression, a pro-apoptotic protein located on the mitochondrial membrane which is upregulated by the overactivated AKT protein when PI3K/AKT pathway is hyperactivated16,46. AKT inhibits BAD and BAX, the pro-apoptotic Bcl-2 family members inducing cytochrome C leakage and apoptosis. The loss of the Bcl-2 signal reduces the survival of lung cancer A549 cell lines16. This current study is among one of the large-scale, real-world studies that investigated the inhibitory effect of flupentixol on lung cancer, which introduced evidence of the unexplored potentials of antipsychotics for cancer prevention. The inverse association of flupentixol or any antipsychotics use with lung cancer observed in this study is consistent with preclinical evidence from in vitro and in vivo studies showing the potential mechanisms of lung cancer growth suppression on lung cancer16,47,48. We showed that in addition to the benefits regarding severe mental illness management on lung cancer, a lung cancer inhibitory effect may also be found in the use of flupentixol and other antipsychotics. Our study added empirical evidence on the choice of different specific antipsychotics to be further explored for potential drug repurposing among people living with severe mental illnesses.
The findings are consistent with a previous real-world case-control study in Mainland China showing an overall protective effect of antipsychotic use on lung cancer compared with non-users19. As previous research has suggested a growing trend in antipsychotic prescription worldwide, the risk-benefit analysis of antipsychotic use should indeed be closely updated according to newly generated evidence32,49. Similar to flupentixol, reduced risks have been found in other antipsychotics, including trifluoperazine and clozapine, some of which have also been suggested to suppress cancer cells in preclinical studies42,50. Indeed, with the increase in accessibility of various antipsychotics, flupentixol, a typical antipsychotic agent, might not be an obvious choice in more recent years, considering the cost and tolerability differences between individuals. Hence, other antipsychotic agents with promising mechanisms for reducing lung cancer risk should be further explored.
Conversely, there was previous research showing an increased risk of cancer and cancer-related deaths in patients with severe mental illnesses6,51,52,53. However, these studies used the healthy general population as the reference group. In this study, the comparison of lung cancer risk was restricted to patients with a history of antipsychotic medication. The observed reduced risk of lung cancer was a relative risk, determined by comparing antipsychotic users with different exposure times. In addition to antipsychotics, there are many confounders that have a much greater impact on lung cancer incidence, including smoking status, previous radiation therapy, and family history of lung cancer54. These lifestyle factors and clinical characteristics can greatly differ between antipsychotic users in this study and the healthy general population in previous research. It is plausible that patients with severe mental illnesses may still have a higher risk of cancer and cancer-related deaths compared to the general population, even considering the potential protective effects of antipsychotics.
There are clear strengths to this study. First, this research investigates the potential of flupentixol in inhibiting lung cancer development within a real-world context, providing supplementary evidence on the properties of antipsychotics in cancer prevention. Second, CDARS serves more than 80% of the entire population in Hong Kong, ensuring the representativeness of our study sample20. Third, the diagnoses and prescription records were made by registered doctors using a comprehensive, tertiary-wide electronic health record system. The accuracy and completeness of the diagnostic record in this system have been well validated, and the potential biases introduced by the coding system are thus minimum55.
This study has some limitations. Firstly, the current dataset only covers patients who used public health care services in Hong Kong. Patients who only presented in the private sector cannot be captured by this study. Secondly, we did not consider different drug dosages in our analyses, which is a possible factor contributing to the magnitude of the association. Thirdly, the under-recording of diagnoses is a common issue in observational studies using electronic health records. For this study, data on diagnosis and medications were not available before 2001. Hence, some estimations, such as the prevalence of comorbidities and the exposure duration of antipsychotics, might be underestimated. Moreover, mental health conditions were associated with surrounded stigma and health seeking behaviors (i.e., bipolar disorder vs. schizophrenia), which might result in the underdiagnosis issue of other health conditions, including the lung cancer. The incomplete capture of comorbidities, especially the mental health problems, may further influence the results. Fourthly, patients with short- and long-term use of flupentixol or antipsychotic showed different demographic and clinical profiles. Thus, the observed differences in risk profiles for lung cancer and potentially other physical health conditions among patients with varying drug exposure times may be subject to indication bias. Fifthly, the number of patients using flupentixol was modest, which limited the statistical power for a more precise estimate. Sixthly, smoking status and cessation are not routinely recorded in the current dataset56. The tobacco use status in this study was identified using ICD-9-CM codes instead of universally self-reported smoking habits, which could lead to an underestimation of the true prevalence of smoking. It is also possible that patients with lung cancer are more likely to be given an ICD-9-CM diagnosis of tobacco use in clinical practice due to more frequent presentations to hospitals. However, given the low observed prevalence of this covariate, any potential bias would be minimal. Furthermore, the lack of smoking cessation data introduces suspicion as to whether the lower risk is attributable to smoking cessation. Nevertheless, a previous study reported an increased smoking rate following the initiation of antipsychotic use, possibly suggesting the use of tobacco as a coping strategy for the side effects of the medication57. As such, it is improbable that the observed risk reduction of lung cancer was a result of smoking cessation. Finally, regarding flupentixol, a significantly reduced risk of lung cancer was only observed in certain exposure groups of flupentixol, regardless of the whole sample or the sex-specific groups. Caution is required in the interpretation of the results.
In conclusion, this study showed a decreased risk of lung cancer in patients who used flupentixol or any antipsychotics for more than 1 year. Further research involving other populations and other specific antipsychotic agents should be conducted to validate the findings and identify more promising antipsychotic agents for potential repurposing in lung cancer prevention.
Data availability
Data are not available as the data custodians (the Hospital Authority and the Department of Health of Hong Kong SAR) have not given permission for sharing due to patient confidentiality and privacy concerns. Local academic institutions, government departments, or non-governmental organizations may apply for the access to data through the Hospital Authority’s data-sharing portal (https://www3.ha.org.hk/data). The numerical data underlying Fig. 2 are shown in tables in Fig. 2. All other data are available from the corresponding author (or other sources, as applicable) on reasonable request.
Code availability
The code used for this study is available on Zenodo58 (https://doi.org/10.5281/zenodo.8009776).
References
Ebrahimi, H. et al. Global, regional, and national burden of respiratory tract cancers and associated risk factors from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Respir. Med. 9, 1030–1049 (2021).
Arffman, M. et al. The impact of severe mental illness on lung cancer mortality of patients with lung cancer in Finland in 1990-2013: a register-based cohort study. Eur. J. Cancer 118, 105–111 (2019).
Ohi, K. et al. Smoking rates and number of cigarettes smoked per day in schizophrenia: a large cohort meta-analysis in a Japanese population. Int. J. Neuropsychopharmacol. 22, 19–27 (2019).
Gur, S. et al. Mortality, morbidity and medical resources utilization of patients with schizophrenia: a case-control community-based study. Psychiatry Res. 260, 177–181 (2018).
Grassi, L. et al. Mortality from cancer in people with severe mental disorders in Emilia Romagna Region, Italy. Psycho-Oncology 30, 2039–2051 (2021).
Li, H. et al. The incidence rate of cancer in patients with schizophrenia: a meta-analysis of cohort studies. Schizophr. Res. 195, 519–528 (2018).
Tahir, R. et al. Antipsychotic treatment in breast cancer patients. Am. J. Psychiatry 171, 616–621 (2014).
Correll, C. U., Detraux, J., De Lepeleire, J. & De Hert, M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 14, 119–136 (2015).
Gal, G. et al. Cancer in parents of persons with schizophrenia: Is there a genetic protection? Schizophr. Res. 139, 189–193 (2012).
Basciotta, M. et al. Antipsychotics and the risk of mortality or cardiopulmonary arrest in hospitalized adults. J. Am. Geriatr. Soc. 68, 544–550 (2020).
Muhorakeye, O. & Biracyaza, E. Exploring barriers to mental health services utilization at Kabutare District Hospital of Rwanda: perspectives from patients. Front. Psychol. 12, 638377 (2021).
Park, Y. et al. Antipsychotic medication use among publicly insured pregnant women in the United States. Psychiatric Serv. 68, 1112–1119 (2017).
Matone, M. et al. The relationship between mental health diagnosis and treatment with second-generation antipsychotics over time: a national study of U.S. Medicaid-enrolled children. Health Serv. Res. 47, 1836–1860 (2012).
Grohmann, R. et al. Flupentixol use and adverse reactions in comparison with other common first- and second-generation antipsychotics: data from the AMSP study. Eur. Arch. Psychiatry Clin. Neurosci. 264, 131–141 (2014).
Ruhrmann, S. et al. Efficacy of flupentixol and risperidone in chronic schizophrenia with predominantly negative symptoms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 31, 1012–1022 (2007).
Dong, C. et al. The antipsychotic agent flupentixol is a new PI3K inhibitor and potential anticancer drug for lung cancer. Int. J. Biol. Sci. 15, 1523–1532 (2019).
Martini, M., De Santis, M. C., Braccini, L., Gulluni, F. & Hirsch, E. PI3K/AKT signaling pathway and cancer: an updated review. Ann. Med. 46, 372–383 (2014).
Fumarola, C., Bonelli, M. A., Petronini, P. G. & Alfieri, R. R. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem. Pharmacol. 90, 197–207 (2014).
Li, J., Tang, F., Si, S. & Xue, F. Association between antipsychotic agents and risk of lung cancer: a nested case-control study. Cancer Commun. 42, 175–178 (2022).
Food and Health Bureau. Report of the Strategic Review on Healthcare Manpower Planning and Professional Development (Food and Health Bureau, 2017).
Food and Health Bureau. Mental Health Review Report (Food and Health Bureau, 2017).
Chai, Y. et al. Antidepressant use and risk of self-harm among people aged 40 years or older: a population-based cohort and self-controlled case series study. Lancet Regional Health Western Pacific 27, 100557 (2022).
Lai, F. T. T. et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine: a case-control study. Ann. Intern. Med. 175, 362–370 (2022).
Wei, Y. et al. Association of long-acting injectable antipsychotics and oral antipsychotics with disease relapse, health care use, and adverse events among people with schizophrenia. JAMA Netw. Open 5, e2224163 (2022).
Yu, S.-Y. et al. Low-dose aspirin and incidence of lung carcinoma in patients with chronic obstructive pulmonary disease in Hong Kong: a cohort study. PLoS Med. 19, e1003880 (2022).
Elm, E. V. et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335, 806–808 (2007).
Vandenbroucke, J. P. & Pearce, N. Case-control studies: basic concepts. Int. J. Epidemiol. 41, 1480–1489 (2012).
Hitchings, M. D. T. et al. Change in covid-19 risk over time following vaccination with CoronaVac: test negative case-control study. BMJ 377, e070102 (2022).
Takeuchi, H., Suzuki, T., Uchida, H., Watanabe, K. & Mimura, M. Antipsychotic treatment for schizophrenia in the maintenance phase: a systematic review of the guidelines and algorithms. Schizophr. Res. 134, 219–225 (2012).
de Groot, P. & Munden, R. F. Lung cancer epidemiology, risk factors, and prevention. Radiol. Clin. 50, 863–876 (2012).
Akhtar, N. & Bansal, J. G. Risk factors of lung cancer in nonsmoker. Current Problems Cancer 41, 328–339 (2017).
Lao, K. S. et al. Prescribing trends and indications of antipsychotic medication in Hong Kong from 2004 to 2014: general and vulnerable patient groups. Pharmacoepidemiol. Drug Safety 26, 1387–1394 (2017).
Ceskova, E., Prikryl, R., Libiger, J., Svancara, J. & Jarkovsky, J. Gender differences in the treatment of first-episode schizophrenia: results from the European First Episode Schizophrenia Trial. Schizophr. Res. 169, 303–307 (2015).
Hoekstra, S. et al. Sex differences in antipsychotic efficacy and side effects in schizophrenia spectrum disorder: results from the BeSt InTro study. NPJ Schizophr. 7, 39 (2021).
Cheng, H., Garrick, D. J. & Fernando, R. L. Efficient strategies for leave-one-out cross validation for genomic best linear unbiased prediction. J. Anim. Sci. Biotechnol. 8, 38 (2017).
Goncalves, J. M., Silva, C. A. B., Rivero, E. R. C. & Cordeiro, M. M. R. Inhibition of cancer stem cells promoted by Pimozide. Clin. Exp. Pharmacol. Physiol. 46, 116–125 (2019).
Suzuki, S. et al. Aripiprazole, an antipsychotic and partial dopamine agonist, inhibits cancer stem cells and reverses chemoresistance. Anticancer Res. 36, 5153–5161 (2016).
Sanomachi, T. et al. Olanzapine, an atypical antipsychotic, inhibits survivin expression and sensitizes cancer cells to chemotherapeutic agents. Anticancer Res. 37, 6177–6188 (2017).
Suzuki, S. et al. Lurasidone sensitizes cancer cells to osimertinib by inducing autophagy and reduction of survivin. Anticancer Res. 41, 4321–4331 (2021).
Sanomachi, T. et al. Brexpiprazole reduces survivin and reverses EGFR tyrosine kinase inhibitor resistance in lung and pancreatic cancer. Anticancer Res. 39, 4817–4828 (2019).
Jeong, J. Y. et al. Trifluoperazine and its analog suppressed the tumorigenicity of non-small cell lung cancer cell; applicability of antipsychotic drugs to lung cancer treatment. Biomedicines 10, 1046 (2022).
Yin, Y. C. et al. Clozapine induces autophagic cell death in non-small cell lung cancer cells. Cell Physiol. Biochem. 35, 945–956 (2015).
Fujiwara, R. et al. Chlorpromazine cooperatively induces apoptosis with tyrosine kinase inhibitors in EGFR-mutated lung cancer cell lines and restores the sensitivity to gefitinib in T790M-harboring resistant cells. Biochem. Biophys. Res. Commun. 626, 156–166 (2022).
MotieGhader, H. et al. Drug repositioning in non-small cell lung cancer (NSCLC) using gene co-expression and drug-gene interaction networks analysis. Sci. Rep. 12, 9417 (2022).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2022).
Kim, S. Y., Yoo, S. J., Ronnett, G. V., Kim, E. K. & Moon, C. Odorant stimulation promotes survival of rodent olfactory receptor neurons via PI3K/Akt activation and Bcl-2 expression. Mol Cells 38, 535–539 (2015).
Xue, Q. et al. Penfluridol: an antipsychotic agent suppresses lung cancer cell growth and metastasis by inducing G0/G1 arrest and apoptosis. Biomed. Pharmacother. 121, 109598 (2020).
Song, Y. et al. Thioridazine hydrochloride: an antipsychotic agent that inhibits tumor growth and lung metastasis in triple-negative breast cancer via inducing G0/G1 arrest and apoptosis. Cell Cycle 19, 3521–3533 (2020).
Verdoux, H., Tournier, M. & Begaud, B. Antipsychotic prescribing trends: a review of pharmaco‐epidemiological studies. Acta Psychiatrica Scandinavica 121, 4–10 (2010).
Yeh, C. T. et al. Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer. Am. J. Respir. Crit. Care Med. 186, 1180–1188 (2012).
Dalton, S. O. et al. Cancer risk among users of neuroleptic medication: a population-based cohort study. Br. J. Cancer 95, 934–939 (2006).
Zhuo, C., Zhuang, H., Gao, X. & Triplett, P. T. Lung cancer incidence in patients with schizophrenia: meta-analysis. Br. J. Psychiatry 215, 704–711 (2019).
Ni, L. et al. Mortality of site-specific cancer in patients with schizophrenia: a systematic review and meta-analysis. BMC Psychiatry 19, 323 (2019).
Jyoti, M., Matteo, M., Eva, N., Carlo, L. V. & Paolo, B. Risk factors for lung cancer worldwide. Eur. Respir. J. 48, 889 (2016).
Wong, M. et al. Health services research in the public healthcare system in Hong Kong: an analysis of over 1 million antihypertensive prescriptions between 2004–2007 as an example of the potential and pitfalls of using routinely collected electronic patient data. BMC Health Serv. Res. 8, 1–9 (2008).
Lau, W. C. Y. et al. Association between dabigatran vs warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. J. Am. Med. Assoc. 317, 1151–1158 (2017).
Matthews, A. M., Wilson, V. B. & Mitchell, S. H. The role of antipsychotics in smoking and smoking cessation. CNS Drugs 25, 299–315 (2011).
Chai, Y. Association between cumulative exposure periods of flupentixol or any antipsychotics and risk of lung cance. [Source code]. https://doi.org/10.5281/zenodo.8009776 (2023).
Acknowledgements
We gratefully acknowledge the generous provision of data by the Hospital Authority. F.L. and I.W. are partially supported by the Laboratory of Data Discovery for Health (D24H) funded by the by AIR@InnoHK administered by Innovation and Technology Commission.
Author information
Authors and Affiliations
Contributions
Y.C. and F.L. conceptualized and designed the study. I.W. supervised the whole project. Y.H. collected the data. Y.C. made the analysis plan and conducted the statistical analysis. Y.C., R.C., Y.H., I.L., F.C., H.L., M.W., S.C., E.C., I.W., and F.L. interpreted the data. Y.C. and R.C. drafted the manuscript. Y.C., R.C., Y.H., I.L., F.C., H.L., M.W., S.C., E.C., I.W., and F.L. revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work. Y.C., R.C., and Y.H. are co-first authors and I.W. and F. L. share the senior authorship.
Corresponding authors
Ethics declarations
Competing interests
E.C. reports grants from Research Grants Council of Hong Kong, Research Fund Secretariat of the Food and Health Bureau of Hong Kong, National Natural Science Fund of China, Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, and Narcotics Division of the Security Bureau of Hong Kong; honorarium from Hospital Authority; outside the submitted work. I.W. receives research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong Research Grants Council, the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, National Institute for Health Research in England, European Commission, and the National Health and Medical Research Council in Australia; has received speaker fees from Janssen and Medice in the previous 3 years; and is an independent non-executive director of Jacobson Medical in Hong Kong. F.L. has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, outside the submitted work. The remaining authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chai, Y., Chu, R.Y.K., Hu, Y. et al. Association between cumulative exposure periods of flupentixol or any antipsychotics and risk of lung cancer. Commun Med 3, 126 (2023). https://doi.org/10.1038/s43856-023-00364-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-023-00364-z