Abstract
Background
A precision medicine approach in type 2 diabetes requires the identification of clinical and biological features that are reproducibly associated with differences in clinical outcomes with specific anti-hyperglycaemic therapies. Robust evidence of such treatment effect heterogeneity could support more individualized clinical decisions on optimal type 2 diabetes therapy.
Methods
We performed a pre-registered systematic review of meta-analysis studies, randomized control trials, and observational studies evaluating clinical and biological features associated with heterogenous treatment effects for SGLT2-inhibitor and GLP1-receptor agonist therapies, considering glycaemic, cardiovascular, and renal outcomes. After screening 5,686 studies, we included 101 studies of SGLT2-inhibitors and 75 studies of GLP1-receptor agonists in the final systematic review.
Results
Here we show that the majority of included papers have methodological limitations precluding robust assessment of treatment effect heterogeneity. For SGLT2-inhibitors, multiple observational studies suggest lower renal function as a predictor of lesser glycaemic response, while markers of reduced insulin secretion predict lesser glycaemic response with GLP1-receptor agonists. For both therapies, multiple post-hoc analyses of randomized control trials (including trial meta-analysis) identify minimal clinically relevant treatment effect heterogeneity for cardiovascular and renal outcomes.
Conclusions
Current evidence on treatment effect heterogeneity for SGLT2-inhibitor and GLP1-receptor agonist therapies is limited, likely reflecting the methodological limitations of published studies. Robust and appropriately powered studies are required to understand type 2 diabetes treatment effect heterogeneity and evaluate the potential for precision medicine to inform future clinical care.
Plain language summary
This study reviews the available evidence on which patient features (such as age, sex, and blood test results) are associated with different outcomes for two recently introduced type 2 diabetes medications: SGLT2-inhibitors and GLP1-receptor agonists. Understanding what individual characteristics are associated with different response patterns may help clinical providers and people living with diabetes make more informed decisions about which type 2 diabetes treatments will work best for an individual. We focus on three outcomes: blood glucose levels (raised blood glucose is the primary symptom of diabetes and a primary aim of diabetes treatment is to lower this), heart disease, and kidney disease. We identified some potential factors that reduce effects on blood glucose levels, including poorer kidney function for SGLT2-inhibitors and lower production of the glucose-lowering hormone insulin for GLP1-receptor agonists. We did not identify clear factors that alter heart and kidney disease outcomes for either medication. Most of the studies had limitations, meaning more research is needed to fully understand the factors that influence treatment outcomes in type 2 diabetes.
Similar content being viewed by others
Introduction
Two of the most recently introduced anti-hyperglycaemic drug classes, SGLT2-inhibitors (SGLT2i) and GLP1-receptor agonists (GLP1-RA), have been shown in randomized clinical trials not only to reduce glycaemia1 but also to lower the risk of renal and cardiovascular disease (CVD) outcomes among high-risk individuals with type 2 diabetes (T2D)2,3,4,5. Based on average treatment effects reported in placebo-controlled trials, current T2D clinical consensus guidelines recommend a stratified approach to treatment selection, preferentially recommending these drug classes independent of their glucose lowering effect for individuals with cardiovascular or renal comorbidity. Specifically, people with heart failure and/or chronic kidney disease are recommended to initiate SGLT2i and people with prior CVD or high risk for CVD are recommended to initiate either an SGLT2i or a GLP1-RA. In addition, these drugs are recommended as second-line glucose lowering medications to be added after metformin6.
A limitation of the current stratified approach to SGLT2i and GLP1-RA treatment in clinical guidelines is that it is informed by selective trial recruitment strategies, and consequential accumulation of evidence of treatment benefits only for specific subgroups with or at high risk of cardiorenal disease, rather than from an understanding of how the benefits and risks of each drug class vary across the whole spectrum of T2D. A more comprehensive approach to treatment selection would require recognition of the extreme heterogeneity in the demographic, clinical, and biological features of people with T2D, and the impact of this heterogeneity on drug-specific clinical outcomes. Identification of robust and reproducible patterns of heterogenous treatment effects is plausible as, at the individual patient level, responses to the same drug treatment appear to vary greatly7. A greater understanding of population-wide heterogenous treatment effects and enhanced capacity to predict individual treatment responses is needed to advance towards the central goal of precision type 2 diabetes medicine—using demographic, clinical, biological, or other patient-level features to match individuals to their optimal anti-hyperglycaemic regimen as part of routine T2D clinical care.
To assess the evidence base for treatment effect heterogeneity for SGLT2i and GLP1-RA, we undertook a systematic literature review to summarize key findings from studies that specifically examined interactions between individual-level biomarkers and the effects of these drug classes on clinical outcomes. Although biomarkers may connote laboratory-based measurements in traditional contexts, herein we broadly conceptualized biomarkers as individual-level demographic, clinical, and biological features, including both laboratory measures as well as genetic and genomic markers. We focused on three categories of outcomes relevant to T2D care: (1) glycaemic response (as measured by hemoglobin A1c; HbA1c); (2) CVD outcomes; and (3) renal outcomes. Our review was guided by the following research question: In a population with T2D, treated with SGLT2i or GLP1-RA, what are the biomarkers associated with heterogenous treatment effects in glycaemic, CVD, and renal outcomes? Each of the three outcomes were evaluated separately for each of the two drug classes for a total of 6 sub-studies. Given the heterogeneity of the T2D population, we anticipated that we would find one or more biomarkers modifying the effects of SGLT2i and GLP1-RA.
The Precision Medicine in Diabetes Initiative (PMDI) was established in 2018 by the American Diabetes Association (ADA) in partnership with the European Association for the Study of Diabetes (EASD). The ADA/EASD PMDI includes global thought leaders in precision diabetes medicine who are working to address the burgeoning need for higher quality, individualized diabetes prevention and care through precision medicine8. This systematic review is written on behalf of the ADA/EASD PMDI as part of a comprehensive evidence evaluation in support of the 2nd International Consensus Report on Precision Diabetes Medicine9.
We find that a majority of the papers identified by our review have methodological limitations precluding robust assessment of treatment effect heterogeneity. For SGLT2-inhibitors, multiple observational studies suggest lower renal function as a predictor of lesser glycaemic response, while markers of reduced insulin secretion predict lesser glycaemic response with GLP1-receptor agonists. For both therapies, multiple post-hoc analyses of randomized control trials (including trial meta-analysis) identify minimal clinically relevant treatment effect heterogeneity for cardiovascular and renal outcomes.
Methods
We conducted a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines10. The protocol was pre-registered (PROSPERO registration number: CRD42022303236). As above, our review was guided by the following research question: In a population with T2D, treated with SGLT2i and GLP1-RA, what are the biomarkers associated with heterogenous treatment effects in glycaemic, CVD, and renal outcomes?
Search strategy
The search strategy for this review was developed for each drug class (SGLT2i and GLP1-RA) and outcome (glycaemic, cardiovascular, and renal) to capture studies specifically evaluating treatment effect heterogeneity associated with demographic, clinical, and biological features in people with type 2 diabetes. Terms for drug class (SGLT2i or GLP1-RA) and individual generic names of licensed drugs within each class (e.g. ‘empagliflozin’) were included. Potential effect modifiers of interest comprised age, sex, ethnicity, clinical features, routine blood tests, metabolic markers, and genetics; all search terms were based on medical subject sub-headings (MeSH) terms and are reported in Supplementary Note 1. SGLT2i and GLP1-RA were evaluated at drug class level, and we did not aim to identify within-class heterogeneity in treatment effects. Electronic searches were performed in PubMed and Embase by two independent academic librarians in February 2022. Forwards and backwards citation searching was conducted but grey literature and white papers were not searched.
Inclusion criteria
To be included, studies were required to meet the following criteria: full-text English-language publications of RCTs, meta-analyses, post-hoc analyses of RCTs, pooled cohort analyses, prospective and retrospective observational analyses published in peer-reviewed journals; adult populations with type 2 diabetes taking at least one of either SGLT2i or GLP1-RA with sample size >100 for the active drug of interest; at least a 4-month potential follow up period (chosen pragmatically as a suitable time length over which changes in glycaemic response could be assessed) after initiation of the drug class of interest; randomized control trials (RCTs) required a comparison against placebo or an active comparator anti-hyperglycaemic drug (observational studies did not require a comparator group); a pre-specified aim of the study must be to examine heterogeneity in treatment outcome, such as biomarker-treatment interactions, stratified analyses, or heterogeneity-focused machine learning approaches; and the study must report differential effects of the drug class on an outcome of interest (see Outcomes section below) with respect to a biomarker. All individual trial or observational cohorts included in a meta-analysis or pooled cohort analysis must have met the inclusion criteria stated above.
We further excluded studies based on the following criteria: studied type 1 or other forms of non-type 2 diabetes; included children/minors; inpatient studies; conference proceeding abstracts, editorials, opinions papers, book chapters, clinical trial registries, case reports, commentaries, narrative reviews, or non-peer reviewed studies; did not adequately adjust for confounders (individual RCTs and observational studies only, this criteria was not applied for meta-analyses and pooled cohort analyses); did not address the question of treatment response heterogeneity for biomarkers of interest.
Titles and abstracts were independently screened by pairs of research team members to identify potentially eligible studies; these were then independently evaluated for inclusion in the full-text review. Any discrepancies were discussed with a third author until reaching consensus. Discrepancies were discussed as part of larger group meetings to ensure consistency in decisions across reviewer pairs.
Data extraction and quality assessment
Pairs of authors independently reviewed the main reports and supplementary materials and extracted the following data for each of the included papers: publication (PMID, journal, publication year, first author, title, study type); study (setting and region, study time period, follow up period); population (overall characteristics, ethnicity); intervention (drug class, specific therapies, treatment/comparator arm sizes); statistical analysis (outcome, outcome measurement, subgroups/predictors analysed with respect to biomarkers, statistical model, covariate set); and results (relevant figures and tables, main findings, methodology, quality). Covidence systematic review software11 was used for data extraction.
After data were extracted, information was synthesized by drug class and outcome and further examined by biomarkers or subgroups analyzed within each study. Results were extracted within these subsections and summarized for each paper, where general trends in results for each subsection were outlined.
Risk of bias evaluations were conducted alongside the data extraction by each pair of authors, using the Joanna Briggs Institute (JBI) Critical Appraisal Tool for Cohort Studies12 for all included research papers. This was used to determine the extent of bias within the study’s design, execution, and analysis, specifically within the population, outcome measurements, and statistical modelling. The Cohort studies tool was applied for all studies as we did not identify any individual RCTs designed to specifically examine treatment effect heterogeneity, and all included RCT meta-analyses represent post-hoc rather than pre-specified analyses. Further detail on the risk of bias can be seen in Supplementary Figs. 1 and 2. Additionally, the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework13,14 was applied at the outcome level for each drug class to determine the quality of evidence and certainty of effects for these subsections; an overall GRADE evaluation for all evidence was also provided.
Outcomes
Three outcome categories were assessed in the included studies: (1) changes in HbA1c from baseline associated with treatment; (2) CVD outcomes limited to cardiovascular (CV)-related death, non-fatal myocardial infarction, non-fatal stroke, hospitalization for angina, coronary artery bypass graft, percutaneous coronary intervention, hospitalization for heart failure, carotid endarterectomy, and peripheral vascular disease; and (3) renal outcomes including development of chronic kidney disease (including end-stage renal disease, ESRD), and longitudinal changes in markers of renal function including eGFR/creatinine and albuminuria. Specific measurements and procedures for each category of outcome varied across the included studies. Summaries of the included papers assessing each outcome for each drug class are reported in Supplementary Tables 1-8.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Literature search and screening results
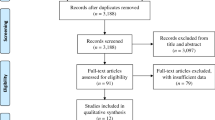
Figures 1 and 2 depict the outcomes of the study screening processes for SGLT2i (Fig. 1) and GLP1-RA (Fig. 2).
For SGLT2i, a total of 3415 unique citations underwent title and abstract screening. A total of 3076 were determined to not meet the pre-defined eligibility criteria. The remaining 339 full-text articles were screened, through which process 238 articles were excluded. The most common reasons for exclusion were: studies did not report on the heterogeneity of treatment response (126 studies), studies reported only univariate or unadjusted associations (41 studies), and studies did not meet inclusion criteria (64 studies). In total, 101 studies were identified for inclusion based on the systematic search.
For GLP1-RA, a total of 2270 unique citations underwent title and abstract screening. 2109 were determined to not meet the pre-defined eligibility criteria. The remaining 161 full-text articles were screened, through which process 86 articles were excluded. The most common reasons for exclusion were: studies did not meet inclusion criteria (39 studies), studies reported only univariate or unadjusted associations (26 studies), and studies did not report on the heterogeneity of treatment response (17 studies). In total, 75 studies were identified for inclusion.
Description of included studies
Included studies for CVD and renal outcomes were predominantly secondary analyses of industry-funded placebo-controlled trials (RCT), or meta-analyses of these trials, with a smaller number of observational studies. For glycaemic outcomes, most studies were observational. Supplementary Tables 1-8 show all included studies for GLP1-RA and SGLT2i, split by glycaemic, CVD, and renal outcomes, and including information on study population size, examined biomarkers, and notable findings. Summaries of the major individual RCTs that were included in meta-analyses are detailed in Supplementary Tables 9 and 10.
SGLT2i, GLP1-RA, and glycaemic outcomes
Study quality for assessment of heterogenous treatment effects of both drug classes was variable with strong methodological limitations for the study of predictors of glycaemia treatment response common. A core weakness with many studies was a lack of head-to-head comparisons between therapies, which is required to separate broader prognostic factors (that predict response to any glucose-lowering therapy) from drug-specific factors that are associated with differential treatment response. Put otherwise, even when data suggested that a biomarker was associated with glycaemic response, it was not clear if this factor was helpful for choosing between therapies due to the lack of an active comparator.
Other common methodological weaknesses included the use of arbitrary subgroups (rather than the assessment of continuous predictors), small numbers in comparator subgroups that limited statistical power, dichotomized outcomes (responder analysis), multiple testing, and lack of adjustment for key potential confounders.
SGLT2i
Of 27 studies that met our inclusion/exclusion criteria, 9 observational studies (usually retrospective analysis of healthcare records), 5 post-hoc analysis of individual RCTs, 10 pooled analyses of individual data from multiple RCTs, and 3 RCT meta-analyses were included (Supplementary Table 7). All included studies assessed routine clinical characteristics and routinely measured clinical biomarkers (Table 1). No pharmacogenetic, or, with the exception of one study of HOMA-B15, non-routine biomarker studies were identified.
A key finding across multiple studies including appropriately adjusted analysis of RCT and observational data was that HbA1c reduction with SGLT2i is substantially reduced with lower eGFR16,17,18,19,20,21,22. In pooled RCT data for canagliflozin 300 mg, 6-month HbA1c reduction was estimated to be 11.0 mmol/mol for participants with eGFR ≥90 mL/min/1.73 m2, compared to 6.7 mmol/mol for those with eGFR 45-6022. With empagliflozin 25 mg, 6-month HbA1c reduction was 9.6 mmol/mol at eGFR ≥90, and 4.3 mmol/mol at eGFR 30-6019.
A further finding confirmed by multiple robust studies is that in keeping with other glucose-lowering agents, higher baseline HbA1c is associated with greater HbA1c lowering with SGLT2 inhibitors, including verses placebo15,21,23,24,25,26,27. Active comparator studies suggested that higher baseline HbA1c may predict greater relative HbA1c response to SGLT2i therapy in comparison to DPP4i and sulfonylurea therapy15,25,26. Notably, an individual participant data meta-analysis of two RCTs showed greater improvement with empagliflozin (6-month HbA1c decline per unit higher baseline HbA1c [HbA1c slope] −0.49% [95%CI −0.62, −0.37] compared to sitagliptin (6-month HbA1c slope −0.29% [95%CI −0.42, −0.15]) and glimepiride (12-month HbA1c slope: empagliflozin -0.52% [95%CI −0.59, −0.44]; glimepiride −0.32% [95%CI −0.39, −0.25])25.
A number of studies assessing differences in glycaemic response to SGLT2i by ethnicity suggest that initial glycaemic response to this medication class does not vary by ethnicity28,29,30,31,32. Similarly, many studies also showed that response did not vary meaningfully by sex. Some studies suggested older age may be associated with reduced glycaemia response; however, analyses usually did not adjust for eGFR which may confound this association, as eGFR declines with age17,23,32,33,34,35.
GLP1-RA
Of 49 studies that met our inclusion/exclusion criteria, 24 observational studies, 6 post-hoc analysis of individual RCTs, and 19 meta-analyses were included (Supplementary Table 8). The majority of included studies assessed routine clinical characteristics and routinely measured clinical biomarkers, although 3 studies evaluated genetic variants, and 15 studies evaluated non-routine biomarkers (Table 1).
Studies consistently identified baseline HbA1c as a predictor of greater HbA1c response. For other clinical features, the strongest evidence was that, in many observational studies, markers of lower insulin secretion (including longer diabetes duration [or proxies such as insulin treatment], lower fasting C-peptide, lower urine C-peptide-to-creatinine ratio, and positive glutamic acid decarboxylase (GAD) or islet antigen 2 (IA2) islet autoantibodies) were associated with lesser glycaemic response to GLP1-RA36,37,38,39,40,41,42,43,44,45,46,47,48,49. One large prospective study (n=620) observed clinically relevant reductions in HbA1c response with GLP1-RA in individuals with GAD or IA2 autoantibodies (mean HbA1c reduction −5.2 vs. −15.2 mmol/mol without autoantibodies) or C-peptide <0.25 nmol/L (mean HbA1c reduction −2.1 vs. −15.3 mmol/mol with C-peptide >0.25 nmol/L). In contrast, post-hoc RCT analyses have found T2D duration50 and beta-cell function51,52 do not modify glycaemic outcomes. This may reflect trial inclusion criteria as included participants had relatively higher beta-cell function, and were less-commonly insulin-treated, compared with the observational cohorts51.
Few studies contrasted HbA1c outcome for GLP1-RA versus a comparator drug. One meta-analysis showed a greater HbA1c reduction with the GLP1-RA liraglutide compared to other antidiabetic drugs (sitagliptin, glimepiride, rosiglitazone, exenatide, and insulin glargine) across all baseline HbA1c categories (n = 1804)53, a finding supported for the GLP1-RA dulaglutide compared to glimepiride and insulin glargine54.
Overall, there was no consistent evidence for effect modification by body mass index (BMI), sex, age or kidney function, with studies reporting contrasting, or null, associations for these clinical features39,40,44,45,46,50,54,55,56,57,58,59,60,61,62,63,64. In comparative analysis, one large observational study found that markers of insulin resistance (including higher HOMA-IR, BMI, fasting triglycerides, and HDL) do not alter GLP1-RA response, but are associated with lesser DPP4-inhibitor response57.
There was limited evidence for differences by ethnicity. One large pooled RCT analysis (N = 2355) suggested greater HbA1c response in Asian participants compared to those of other ethnicities, but other studies have not identified differences in response across ethnic groups65,66,67,68. Similarly, limited studies evaluated pharmacogenetics, although two small studies suggest variants rs163184 and rs10305420, but not rs3765467, may be associated with lesser response in Chinese patients43,69.
SGLT2i, GLP1-RA and cardiovascular outcomes
SGLT2i: Evidence from clinical trials
Of 65 studies, 58 were post-hoc meta-analysis of RCTs or meta-analysis of multiple RCTs. Heart failure was common as a secondary outcome. The majority of studies were derived from EMPA-REG70 and the CANVAS program71, although more recent meta-analyses included up to 12 cardiovascular outcome trials (CVOTs) with different inclusion criteria, treatments, primary outcomes, and follow-up duration (Supplementary Table 9). Most studies included only participants with established CVD or elevated cardiovascular risk, although some studies were restricted to patients with pre-existing heart failure or chronic kidney disease. While most CVOTs and meta-analyses included only patients with type 2 diabetes, some meta-analyses also included data from patients without diabetes in the EMPEROR-P72, EMPEROR-R73, DAPA-HF74 and DAPA-CKD75 RCTs. Studies primarily focused on relative rather than absolute treatment effects and one of two primary outcomes: 3-point MACE which was a composite of cardiovascular death, non-fatal MI, and non-fatal stroke; or composite heart failure outcomes including hospitalized heart failure and cardiovascular death. The longest duration of follow-up was in the CANVAS CVOT with a median follow-up of 5.7 years, while most other included CVOTs had durations of 1 to 4 years.
On average, in relative terms, SGLT2i reduce the risk of cardiovascular disease (MACE) by 10% (HR 0.90 [95%CI 0.85, 0.95]), and heart failure hospitalization by 32% (HR 0.68 [95%CI 0.61, 0.76]) in individuals with or at high-risk of CVD2. The majority of meta-analyses of CVOTs found no significant interactions for MACE or heart failure outcomes across a variety of biomarkers (Table 2; Supplementary Table 1). Several meta-analyses found no interactions by age, sex, and adiposity for MACE or heart failure outcomes. Four meta-analyses examined interactions by race for MACE outcomes and found no interactions. Three meta-analyses consistently identified a greater relative heart failure benefit of SGLT2i in people of Black and Asian ethnicity76,77,78 (HR SGLT2i versus placebo 0.60 [95% CI 0.47, 0.74]) compared to White individuals (HR 0.82 [95% CI 0.73, 0.92])76, however, one meta-analysis reported no difference between White and non-White individuals79.
Contemporary meta-analysis incorporating the CREDENCE and VERTIS-CV trials alongside EMPA-REG, CANVAS, and DECLARE suggests history of CVD does not modify the efficacy of SGLT2i for MACE2,80. One meta-analysis suggests heart failure severity modifies the efficacy of SGLT2i’s for heart failure outcome (composite outcome of cardiovascular death or hospitalization for heart failure) with greater efficacy in patients with NYHA heart failure class II (HR SGLT2i versus placebo 0.66 [95%CI 0.59, 0.74]) than class III or IV (HR 0.86 [95%CI 0.75, 0.99])77. Other meta-analyses that examined treatment effect heterogeneity using heart failure history as a binary predictor did not find significant interactions2,81.
A recent meta-analysis82 that included 6 CVOTs of patients with diabetes and 4 CVOTs of patients with and without diabetes found that eGFR did not alter the relative benefit of SGLT2 inhibitors for MACE and heart failure outcomes;2,77,81,83,84,85 however, a greater relative benefit was reported for MACE in those with higher baseline albuminuria (ACR>300 mg/g HR 0.74 [95%CI 0.66, 0.84]; ACR 30-300 mg/g HR 0.95 [95%CI 0.82, 1.10]) ACR<30 mg/g HR 0.87 [95%CI 0.77, 0.98]).
We identified many secondary analyses of single CVOTs, which largely found no interactions by biomarkers (Supplementary Table 1). Single studies identified potential effect modification for MACE by history of CVD86, and obesity87, and history of heart failure for heart failure outcome88, but these associations were not replicated across the other studies or in multi-RCT meta-analyses. In a secondary analysis of CANVAS, participants with higher levels of biomarkers of cardiovascular stress (high-sensitivity cardiac troponin T (hs-cTnT), soluble suppression of tumorigenesis-2 (sST2), and insulin-like growth factor binding protein 7 (IGFBP7)) had greater relative benefit for MACE; for a multimarker score summing high levels of these 3 biomarkers, the relative benefit of SGLT2i for no abnormal biomarkers was HR: 0.99 [95% CI: 0.66–1.49], 1 abnormal biomarker HR: 1.34 [95% CI: 0.94–1.89), 2 abnormal biomarkers HR: 0.61 [95% CI: 0.45–0.82]), and 3 abnormal biomarkers HR: 0.46 [95% CI:0.18–1.17]; Pinteraction trend =0.005)89. Unlike meta-analyses, studies based on single RCTs typically performed multivariable adjustment for potential confounders.
GLP1-RA: Evidence from clinical trials
Of the 35 studies that investigated heterogeneity in the effect of GLP1-RAs on cardiovascular health and met our inclusion criteria, 15 were meta-analyses of RCTs or pooled analyses of multiple RCTs, 15 were post-hoc analyses of RCTs, and 5 were observational studies (Supplementary Table 2). Most studies used data collected from the LEADER, SUSTAIN 6, and EXSCEL trials, however in total the data from 7 CVOTs were used (Supplementary Table 10). The majority of these CVOTs investigated the effect of us CVD on the cardiovascular efficacy of GLP1-RAs using 3-point MACE as a primary outcome, and with heart failure being a common secondary outcome, focusing on relative rather than absolute benefit. The population of 6 of the 7 CVOTs had established CVD or high CVD risk. The CVOT with the longest median follow-up was REWIND with a median follow-up of 5.4 years, and the median follow-up of the other CVOTs ranged from 1 to 4 years.
Contemporary meta-analysis data suggests GLP1-RA reduces the relative risk of cardiovascular disease (MACE) by 14% (HR 0.86 [95%CI 0.80-0.93]), and heart failure hospitalization by 11% (HR 0.89 [95%CI 0.82, 0.98]) compared to placebo3. Several large meta-analyses examining heterogenous treatment effects in placebo-controlled CVOTs have been conducted for GLP1-RA76,83,84,90,91,92,93,94,95,96,97, with the majority of studies focusing on whether prior established CVD modifies the relative effect of GLP1-RA on MACE and/or heart failure. Two meta-analyses reported the relative MACE benefit of GLP-RA may be restricted to those with established CVD83,90, the largest of which included 7 RCTs and reported a 14% relative risk reduction with GLP1-RA specific to individuals with established CVD (with CVD: HR 0.86 [95%CI 0.80, 0.93]; at high-risk of CVD: HR 0.94 [95% CI 0.82, 1.07])83. However, this risk difference is not conclusive and has not been replicated in other meta-analyses and pooled RCT analyses91,92,93,98,99, including an individual participant level re-analysis of the SUSTAIN and PIONEER RCTs which evaluated baseline CVD risk as a continuous rather than subgroup-level variable100.
Differential relative effects of GLP1-RAs on MACE have been reported by ethnicity in two out of three meta-analyses:76,83,90 one showed a benefit of GLP1-RA treatment compared to placebo in Asian (HR 0.76 [95%CI 0.61, 0.96]) and Black (HR 0.77 [95%CI 0.59, 0.99]) individuals, but not in White individuals (HR 0.95 [95%CI 0.88, 1.02]);90 the second showed a significantly greater benefit of GLP1-RA for MACE in Asian compared to White individuals (HR Asian 0.68 [95%CI 0.53, 0.84]; White 0.87 [95% 0.81, 0.94])76. For other clinical features including sex, BMI/obesity, baseline kidney disease, duration of diabetes, baseline HbA1c, background glucose lowering medications, and prior history of microvascular disease, the overall body of evidence from meta-analyses does not provide robust evidence to support differential effects of GLP1-RA on CVD outcomes (Table 2).
SGLT2i and GLP1-RA: Evidence from observational studies
10 observational studies met our inclusion criteria, with studies primarily reporting relative rather than absolute risk differences101,102,103,104,105,106,107,108,109,110. These studies comparing SGLT2i and GLP1-RA individually with other oral therapies (predominantly DPP4i) generally reported average relative benefits for CVD and heart failure outcomes in-line with placebo-controlled trials, with no consistent pattern of subgroup level differences across studies (Supplementary Tables 1 and 2).
A few observational studies compared SGLT2i and GLP1-RA CVD outcomes. In a US claims-based study with follow-up to two years (n = 47,343), Htoo et al. 106 reported a higher relative risk of MACE with SGLT2i compared to GLP1-RA specific to individuals without CVD and heart failure (Relative risk [RR] 1.31 [95% CI 1.09, 1.56]), and a higher risk of stroke with SGLT2i versus GLP1-RA specific to individuals without CVD (No CVD without heart failure: RR 1.62 [95%CI 1.10, 2.38]; No CVD with heart failure: RR 3.30 [95%CI 1.22, 8.97]). In contrast, over a median follow-up of 7 months, Patorno et al. 105 reported a lower relative risk of myocardial infarction with SGLT2i compared to GLP1-RA in US claims data specific to individuals with a history of CVD (n=156,825; HR 0.83 [95%CI 0.74, 0.93] with history of CVD; HR 1.13 [95%CI 1.00, 1.28] without history of CVD), with no differences in stroke outcomes irrespective of CVD status. Both studies reported a consistent benefit of SGLT2i over GLP1-RA for heart failure. Raparelli et al. 102 identified potential differences by sex in the Truven Health MarketScan database (n=167,341): compared to sulfonylureas and over a median follow-up of 4.5 years, there was a greater relative reduction with GLP1-RA for females (HR 0.57 [95%CI 0.48, 0.68]) compared to males (HR 0.82 [95%CI 0.71, 0.95]), but a similar benefit for both sexes with SGLT2i (females HR 0.58 [95%CI 0.57, 0.83]; males HR 0.69 [95%CI 0.57, 0.83]).
SGLT2i, GLP1-RA, and renal outcomes
SGLT2i: Evidence from clinical trials
A total of 29 studies met our inclusion criteria. These included 20 post-hoc analyses of individual RCTs, 7 trial meta-analyses (Supplementary Table 4), and 2 analyses of observational data. All of the post-hoc RCT analyses and all but 1 of the meta-analyses used only data from the 12 SGLT2i cardiovascular/renal RCTs shown in Supplementary Table 9, which therefore provided most of the evidence in our review. These trials included people with type 2 diabetes with and without pre-existing cardiovascular disease, and had composite renal endpoints incorporating two or more of the following (which differed between trials): changes in eGFR/serum creatinine, end-stage renal disease, changes in urine albumin:creatinine ratio (ACR), and/or death from renal causes. Most studies assessed routine clinical characteristics, especially renal function as measured by eGFR or urine ACR or a combination of both. In addition, 4 post-hoc RCT analyses examined non-routine plasma biomarkers. We found no genetic studies (Table 3).
On average, SGLT2i have a relative benefit for a number of renal outcomes including kidney disease progression (HR 0.63, 95%CI 0.58,0.69) and acute kidney injury (HR 0.77, 95%CI 0.70, 0.84)4. Placebo-controlled trial meta-analyses of subgroups found no evidence for heterogeneity of SGLT2i treatment effects on relative renal outcomes by age79, use of other glucose-lowering drugs79, use of blood pressure/cardiovascular medications79,111, blood pressure79, BMI79, diabetes duration79, White race79, history of cardiovascular disease or heart failure2,80 or sex79.
For baseline eGFR, an early meta-analysis that included EMPA-REG, CANVAS, and DECLARE reported greater effect of SGLT2i on renal outcomes in those with higher eGFR112 but both a later meta-analysis that added CREDENCE111 and a recent meta-analysis that added two further studies (SCORED and DAPA-CKD, including some participants without diabetes)82 showed no effect of baseline eGFR on renal outcomes with SGLT2i. For urine ACR, meta-analyses of subgroups found no evidence for greater SGLT2i effect with higher UACR2,82,111,113. Single RCTs found no heterogeneity of treatment effect by eGFR and UACR, or subgroups defined by the combination of these two114,115,116,117,118, with the exception of Neuen et al. 119 which showed a greater SGLT2i effect in preventing eGFR decline relative to placebo for those with higher UACR, and heterogeneity in a composite renal outcome by UACR. Overall, there was limited or no evidence to support modifying effects of baseline eGFR or UACR on the effect of SGTL2i on renal function outcomes.
A few post-hoc analyses of the CANVAS RCT considered non-routine biomarkers, with most showing no interaction with SGLT2i treatment and renal outcomes. Two RCTs studied the effect of SGLT2i on renal outcomes at differing plasma IGFBP7 levels. One study reported an interaction of IGFBP7 with SGLT2i treatment for progression of albuminuria (>96.5 ng/ml HR 0.64; <=96.5 ng/ml HR 0.95, Pinteraction = 0.003)120 but no effect was seen for the composite renal endpoint in two studies89,120. The biomarker panel (sST2, IGFBP7, hs-cTnT) that showed a strong interaction with SGLT2i for MACE outcomes (see above) did not show any interaction for renal outcomes89.
GLP1-RA: Evidence from clinical trials
7 studies met our inclusion criteria: all post-hoc RCT analyses, 6 of individual trials (or multiple trials analysed separately) and 1 pooled analysis of two RCTS (Supplementary Table 5). These studies used data from 5 of the 7 GLP1-RA cardiovascular outcome trials shown in Supplementary Table 10, with renal outcomes only a secondary endpoint. Most of these trials had composite renal endpoints as per the SGLT2i cardiovascular/renal trials, while some examined changes in either eGFR or urine ACR only. All studies assessed routine clinical characteristics, especially renal function as measured by eGFR or urine ACR. No studies of genetics or non-routine biomarkers were identified (Table 3). The overall sample sizes were small and subgroup analyses underpowered to show a subgroup by treatment interaction for renal outcomes.
Overall, GLP1-RA reduce the relative risk of albuminuria over 2 years by 24% versus placebo (HR 0.76 [95% CI 0.73-0.80; P < 0.001), and similarly reduce the relative risk of a 40% reduction in eGFR (HR, 0.86 [95% CI 0.75-0.99]; P = 0.039)5. Studies found no heterogeneity of GLP1-RA relative treatment effect by age121, blood pressure122,123, diabetes duration124, history of cardiovascular disease/heart failure122,125 or use of RAS inhibitors122. For BMI, a post-hoc analysis of EXSCEL (Exenatide) found a greater GLP1-RA effect on reducing rate of eGFR decline in those with lower BMI (BMI ≤ 30 kg/m2 treatment difference 0.26 mL/min/1.73m2/year [95% CI 0.04, 0.48] vs BMI > 30 kg/m2 −0.12 [-0.26, 0.03], Pinteraction = 0.005)122. However, Verma et al.126 found no significant interaction by BMI subgroup with GLP1-RA treatment for a composite renal outcome in LEADER (Liraglutide) or SUSTAIN 6 (Semaglutide).
For baseline eGFR, a pooled analysis of LEADER and SUSTAIN-6 reported a significant interaction, with lower eGFR associated with greater GLP1-RA effect in reducing eGFR decline: Semaglutide 1.0 mg vs placebo, eGFR < 60 difference in decline 1.62 ml/min/1.73m2/year vs eGFR> = 60 difference in decline 0.64 ml/min/1.73 m2/year, Pinteraction = 0.057; Liraglutide 1.8 mg vs placebo, eGFR < 60 difference in decline 0.67 ml/min/1.73m2/year vs 0.15 ml/min/1.73 m2/year, Pinteraction = 0.008)5. However, a study of Exenatide LAR found no treatment heterogeneity for this same outcome by eGFR category122, and in a further analysis of LEADER, the renal composite endpoint was used with no interaction reported by baseline eGFR category127. The overall evidence does not support an effect of baseline eGFR on the relative renal benefit for GLP1-RA as an overall drug class.
For baseline UACR, a pooled analysis of LEADER and SUSTAIN-65 and EXSCEL122 showed a greater benefit of GLP1-RA on eGFR reduction or eGFR slope with higher UACR; however, there was either no significant interaction5 or no formal interaction test was reported122. For ELIXA, Muskiet et al. 128 did not find a significant interaction of UACR category on eGFR decline. A further study found no association between UACR and GLP1-RA effect on reducing a composite renal outcome127.
Two studies found that GLP1-RAs more effectively reduced UACR in those with higher UACR. In a pooled analysis of LEADER and SUSTAIN-6, those with normal albuminuria had a 20% (95%CI 15%, 25%) reduction in UACR compared to placebo; those with microalbuminuria had a 31% (95%CI 25–37%) reduction; those with macroalbuminuria had a 19% (95%CI 7–30%); Pinteraction = 0.0215. In ELIXA, least-squares mean percentage change in UACR was –1·69% (SE 5·10; 95% CI –11·69 to 8·30; p = 0·7398) in participants with normoalbuminuria, –21.10% (10.79; –42.25 to 0·04; p = 0.0502) in participants with microalbuminuria, and –39·18% (14·97; –68·53 to –9·84; p = 0·0070) in participants with macroalbuminuria in favour of lixisenatide; a formal test for interaction was not reported128. A third study found no treatment heterogeneity for this same outcome122.
In summary, the included studies showed conflicting results for renal outcomes of GLP1-RA, though the majority were underpowered to detect heterogenous treatment effects. The most consistent finding was that a higher UACR is associated with greater GLP1-RA reduction in UACR relative to placebo, but this does not translate to benefits in eGFR-defined measures of renal function. There were no other biomarkers that robustly predicted benefit from GLP1-RA for the renal outcomes examined.
SGLT2i and GLP1-RA: Evidence from observational studies
There were no observational studies for GLP1-RA and renal outcomes included, and no comparison studies between people treated with GLP1-RA and SGLT2i. Observational studies comparing SGLT2i to other glucose-lowering drugs confirmed the lack of treatment effect heterogeneity associated with age129,130, use of blood pressure/cardiovascular medications127, blood pressure (Koh 2021), history of cardiovascular disease129 and sex129, but one study in a Korean population found greater SGLT2i benefit on progression to end stage renal impairment with higher BMI (BMI < 25 kg/m2, HR 0.80 (95%CI 0.51, 1.25); BMI ≥ 25 kg/m2 HR 0.27 (0.16, 0.44), Pinteraction = 0.002) and with abdominal obesity compared to without129. This is not consistent with results from meta-analysis of RCTs.
Summary of quality assessment
To evaluate risk of bias, we used the JBI critical appraisal tool for cohort studies as the best flexible tool for the range of studies included. Due to our screening criteria, no manuscripts that passed full text screening were excluded due to risk of bias. The checklist results for the 11 points in the appraisal checklist are shown as a heatmap in Supplementary Figure 1(SGLT2i) and 2(GLP1-RA).
Additionally, the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework was applied at the outcome level for each drug class to determine the quality of evidence and certainty of effects (Table 4)13. Overall certainty of evidence was rated as moderate for all outcomes except glycaemia with GLP1-RA which was rated low certainty. This reflects that a larger proportion of the studies included for evaluation of GLP1-RA glycaemia outcomes were observational (24/49). By contrast, for SGLT2i glycaemia outcomes there were 18 RCT/meta-analyses and 9 observational studies. For CVD and renal outcomes, observational studies were limited and the majority of evidence came from industry-funded CVOTs (RCT designs), including post-hoc analyses of individual trials as well as meta-analyses.
Discussion
This systematic review provides a comprehensive review of observational and RCT-based studies of people with type 2 diabetes, specifically examining heterogenous treatment effects for SGLT2i and GLP1-RA therapies on glycaemic, cardiovascular, and renal outcomes. We assessed evidence for treatment effect modification for a wide range of demographic, clinical and biological features, including pharmacogenetic markers. Each of the three clinical outcomes were evaluated separately for each drug class for a total of 6 sub-studies. Overall, our review identified limited evidence for treatment effect heterogeneity for glycaemia, cardiovascular, and renal outcomes for the two drug classes. We summarize the key findings below.
For glycaemic response, there was high certainty that reduced renal function is associated with lower efficacy of SGLT2i. For GLP1-RA there was moderate certainty that markers of reduced insulin secretion, either directly measured (e.g. c-peptide or HOMA-B) or proxy measures, such as diabetes duration, were associated with reduced glycaemic response to GLP1-RA, although the majority of evidence was from observational studies. As with other glucose-lowering drug classes, a greater glycaemic response with both SGLT2i and GLP1-RA was seen at higher baseline HbA1c. We did not identify any studies examining whether the relative efficacy of SGLT2i compared to GLP1-RA is altered by baseline HbA1c levels. Of note, many of the included studies for HbA1c outcome were observational, meaning findings could potentially reflect biases from differential prescribing behaviour, or regression to the mean, although we did attempt to account for the latter by including adjustment for baseline HbA1c as one of our study inclusion criteria.
For both CVD and heart failure outcomes, RCT meta-analyses do not support differences in the relative efficacy of either GLP1-RA or SGLT2i based on an individuals’ prior CVD status. However, this finding should be interpreted cautiously as all RCTs to-date have predominantly included participants with, or at high-risk of, CVD, thereby excluding the majority of the wider T2D population at lower risk. However, meta-analyses suggest (with moderate certainty) that the relative effects of both drug classes may be greater in people of non-White ethnicity. In particular, those of Asian and African ethnicity (compared to Whites) have been shown to have a greater relative benefit for hospitalization for heart failure/CV death (but not MACE) with SGLT2i, and MACE for GLP1-RA.
When evaluating renal outcomes, there was no consistent evidence of treatment heterogeneity for SGLT2i, but for GLP1-RA, there was greater reduction in proteinuria in those with higher baseline proteinuria.
This limited evidence could reflect a true lack of heterogenous treatment effects, but it more likely reflects an absence of clinical studies that were well designed or sufficiently powered to robustly identify and characterise treatment effect heterogeneity. Although five of the six sub-studies we evaluated were evaluated at GRADE B, there were methodological concerns with many of the included studies. As individual RCTs are by design powered only for the main effect of treatment131, our primary focus when reporting were meta-analyses of post-hoc subgroup analyses of RCTs. However, we found the subgroup analyses in these studies primarily focused on stratification by baseline risk for the outcome in question e.g. baseline HbA1c on glycaemic response, CKD stage or albuminuria on renal outcomes, and CVD risk or established CVD for CVD outcomes. Other common subgroups included those defined by BMI, age, sex or other routinely collected clinical characteristics, with very few studies evaluating non-routine biomarkers or pharmacogenetic markers (as highlighted in Tables 1–3). A major limitation was that studies predominantly focused on conventional approaches to subgroup analysis, with very few studies assessing continuous features (such as BMI) on a continuous scale which is required to maximize power to detect treatment effect heterogeneity131,132.
It is also important to recognize that almost all the studies evaluating cardiovascular and renal endpoints included in our systematic review focused on the relative effect of a biomarker/stratifier on the outcome, as most studies reported a hazard ratio compared with a placebo arm for the outcome of interest (e.g. MACE, incident renal disease). This does not recognize that baseline absolute risk of these endpoints is likely to differ substantially across these strata; so although, for example, there was no difference in relative benefit of an SGLT2i by age, this means that on the absolute scale, benefit will increase with age (as underlying absolute risk increases), and it is this absolute benefit that should be considered when deciding on whether to initiate SGLT2i treatment.
An important finding of our review is the lack of robust comparative effectiveness studies directly examining treatment effect heterogeneity for these two major drug classes, either head-to-head or compared with other major anti-hyperglycaemic therapies. Insight into effect modification for a single drug class is not sufficient to support the clinical translation of a precision medicine approach. The lack of direct comparisons between therapies obscures the interpretation of biomarkers with regards to whether they function as broad prognostic factors, which may be relevant to any (or at least multiple) drug class, or as markers of heterogenous treatment effects specific to a particular drug class. An evidence base that includes more high-quality studies on heterogeneity in the comparative effectiveness of SGLT2i, GLP1-RA, and other drug classes is needed to advance the field towards clinically useful precision diabetes medicine. For cardiovascular and renal outcomes, these studies need to incorporate both absolute outcome risk and relative estimates of treatment effects in order to usefully inform clinical decision-making. Only when this evidence is available can precision medicine support more individualised treatment decisions, allowing providers to select an optimal therapy from a set of multiple options informed by each medication’s risk/benefit profile specific to the characteristics of an individual patient.
We identified the following additional, high-level evidence gaps in our review: (1) Limited head-to-head comparative effectiveness studies examining treatment effect heterogeneity; (2) A lack of robust studies integrating multiple clinical features and biomarkers. The majority of studies only tested single biomarkers one at a time in subgroup analysis; (3) Few studies focused on pharmacogenetics or non-routine biomarkers; (4) Few studies conducted in low-middle income countries, required for an equitable global approach to precision type 2 diabetes medicine; (5) Few RCT meta-analyses based on individual-level participant data, precluding robust evaluation of between-trial heterogeneity and individual-level confounders; (6) An absence of confirmatory studies. We identified no prospective studies testing a priori hypotheses of potential treatment effect modifiers, or studies conducting independent validation of previously described heterogenous treatment effects; (7) A lack of population-based data representing individuals treated in routine care. As cardiovascular and renal trials have focused on high-risk participants, the benefits of SGLT2i and GLP1-RA for primary prevention is a major unanswered question; (8) Few cardiovascular and renal outcome studies considering treatment effect modification on the absolute as well as relative risk scale; (9) A focus on short-term glycaemic outcomes, with limited studies investigating durability of glycaemic response or time to glycaemic failure.
Of note, several studies published since our data extraction was completed in February 2022 which fill some of the evidence gaps identified in our review, and highlight the clear potential for a precision medicine approach to T2D treatment: the TriMaster study—a precision medicine RCT of SGLT2i, DPP4i and thiazolidinediones (TZD) that established that individuals with higher renal function (eGFR >90 ml/min/1.73 m2) have a greater HbA1c response with SGLT2i vs DPP4i relative to those with eGFR 60–90 ml/min/1.73 m2 133, a result concordant with our finding that reduced renal function is associated with lower efficacy of SGLT2i; a similarly designed two-way crossover trial in New Zealand which identified a greater relative benefit of TZD therapy compared to DPP4i in people with obesity and/or hypertriglyceridemia;134 a study using large-scale observational data and post-hoc analysis of individual participant-level data from 14 RCTs that specifically investigated differential treatment effects with SGLT2i and DPP4i, and developed a treatment selection model to predict HbA1c response on the two therapies based on an individuals’ routine clinical characteristics;135 and a robust study across observational and multiple RCTs identifying pharmacogenetic markers of differential glycaemic response to GLP1-RA136. In addition, three large trials (AMPLITUDE-O investigating cardiovascular and renal outcomes in 4076 participants with T2D for the GLP-RA efpeglenatide137, DELIVER investigating worsening heart failure or cardiovascular death in 3131 participants [45% with T2D] for the SGLT2i Dapagliflozin138, and EMPA-KIDNEY investigating progression of kidney disease or cardiovascular death in 6609 participants [44% with T2D]139) have recently been published. Although all three are primary RCTs examining average treatment effects rather than treatment effect heterogeneity, and thus would have been ineligible for our review, future meta-analysis studies integrating the results of these and other ongoing SGLT2i and GLP1-RA trials may add to the evidence we have presented.
As our aim was to provide a comprehensive review of these treatments, we did not conduct quantitative analysis of specific biomarkers due to the range of different biomarkers, methodologies, and outcomes evaluated in the included studies. However, this review provides guidance for where future targeted quantitative meta-analysis could be most insightful. In addition, different methods for synthesising the current available evidence, such as conducting an umbrella review, may offer further insights into the current state-of-play of precision Type 2 diabetes treatment.
This review highlights the need for several research priorities to advance our limited understanding of heterogenous treatment effects among individuals with type 2 diabetes. We outline priorities for research to advance the field towards a translational model of evidence-based, empirical precision diabetes medicine (Fig. 3), and highlight the recent Predictive Approaches to Treatment effect Heterogeneity (PATH) Statement to guide this research132. In the future, with a greater understanding of heterogenous treatment effects and enhanced capacity to predict individual treatment responses, precision treatment in type 2 diabetes may be able to integrate demographic, clinical, biological, or other patient-level features to match individuals to their optimal anti-hyperglycaemic regimen.
Conclusions
There is limited evidence of treatment effect heterogeneity with SGLT2i and GLP1-RA for glycaemic, cardiovascular, and renal outcomes in people with type 2 diabetes. This lack of evidence likely reflects the methodological limitations of the current evidence base. Robust future studies to fill the research gaps identified in this review are required for precision medicine in type 2 diabetes to translate to clinical care.
References
Tsapas, A. et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann. Int. Med. 173, https://doi.org/10.7326/M20-0864 (2020).
McGuire, D. K. et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 6, 148–158 (2021).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diab. Endocrinol. 9, https://doi.org/10.1016/S2213-8587(21)00203-5 (2021).
Group, N. D. o. P. H. R. S. & Consortium, S. i. M.-A. C.-R. T. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet (London, England) 400, https://doi.org/10.1016/S0140-6736(22)02074-8 (2022).
Shaman, A. M. et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation 145, 575–585 (2022).
Davies, M. J. et al. Management of hyperglycemia in type 2 diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 45, 2753–2786 (2022).
Dennis J. M. Precision medicine in type 2 diabetes: using individualized prediction models to optimize selection of treatment. Diabetes 69, https://doi.org/10.2337/dbi20-0002 (2020).
Nolan, J. J. et al. ADA/EASD precision medicine in diabetes initiative: an international perspective and future vision for precision medicine in Diabetes. Diabetes Care 45, 261–266 (2022).
Tobias, D. et al. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat. Med. https://doi.org/10.1038/s41591-023-02502-5 (2023).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339, b2700 (2009).
Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia).
Joanna Briggs Institute. Critical Appraisal Checklist for Cohort Studies, <https://jbi.global/sites/default/files/2021-10/Checklist_for_Cohort_Studies.docx> (2017).
Guyatt, G. et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 64, https://doi.org/10.1016/j.jclinepi.2010.04.026 (2011).
Santesso, N. et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 119, 126–135 (2020).
Matthews, D. R., Zinman, B., Tong, C., Meininger, G. & Polidori, D. Glycaemic efficacy of canagliflozin is largely independent of baseline β-cell function or insulin sensitivity. Diabetic Medicine 33, 1744–1747 (2016).
Wanner, C. et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 137, 119–129 (2018).
Scheerer, M. F., Rist, R., Proske, O., Meng, A. & Kostev, K. Changes in HbA1c, body weight, and systolic blood pressure in type 2 diabetes patients initiating dapagliflozin therapy: a primary care database study. Diab. Metab. Syndrom. Obes. 9, 337–345 (2016).
Cherney, D. Z. I. et al. The differential effects of ertugliflozin on glucosuria and natriuresis biomarkers: prespecified analyses from VERTIS CV. Diab. Obes. Metab. 24, 1114–1122 (2022).
Cherney, D. Z. I. et al. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 93, 231–244 (2018).
Yamout, H. et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am. J. Nephrol. 40, 64–74 (2014).
Lee, J. Y. et al. Predictors of the therapeutic efficacy and consideration of the best combination therapy of sodium-glucose co-transporter 2 inhibitors. Diab. Metab. J. 43, 158–173 (2019).
Gilbert, R. E. et al. Impact of age and estimated glomerular filtration rate on the glycemic efficacy and safety of canagliflozin: a pooled analysis of clinical studies. Can. J. Diab. 40, 247–257 (2016).
Wilding, J. et al. Dapagliflozin therapy for type 2 diabetes in primary care: Changes in HbA1c, weight and blood pressure over 2 years follow-up. Primary Care Diab. 11, 437–444 (2017).
Chen, J. F. et al. Use and effectiveness of dapagliflozin in patients with type 2 diabetes mellitus: a multicenter retrospective study in Taiwan. PeerJ 8, e9998 (2020).
DeFronzo, R. A. et al. Slope of change in HbA1c from baseline with empagliflozin compared with sitagliptin or glimepiride in patients with type 2 diabetes. Endocrinol. Diab. Metab. 1, https://doi.org/10.1002/edm2.16 (2018).
Del Parigi, A., Tang, W., Liu, D., Lee, C. & Pratley, R. Machine learning to identify predictors of glycemic control in type 2 diabetes: an analysis of target HbA1c reduction using Empagliflozin/Linagliptin data. Pharmaceut. Med. 33, 209–217 (2019).
Inzucchi, S. E. et al. Empagliflozin treatment effects across categories of baseline HbA1c, body weight and blood pressure as an add-on to metformin in patients with type 2 diabetes. Diab. Obes. Metab. 23, 425–433 (2021).
Scheen, A. J. SGLT2 inhibitors as add-on therapy to metformin for people with type 2 diabetes: a review of Placebo-controlled trials in Asian versus Non-Asian Patients. Diab. Metab. Syndrom. Obes. 13, 2765–2779 (2020).
Cai, X. et al. No disparity of the efficacy and all-cause mortality between Asian and non-Asian type 2 diabetes patients with sodium-glucose cotransporter 2 inhibitors treatment: a meta-analysis. J. Diab. Investig. 9, 850–861 (2018).
Montvida, O., Verma, S., Shaw, J. E. & Paul, S. K. Cardiometabolic risk factor control in black and white people in the United States initiating sodium-glucose co-transporter-2 inhibitors: a real-world study. Diab. Obes. Metab. 22, 2384–2397 (2020).
Liu, J. et al. Efficacy and safety of ertugliflozin across racial groups in patients with type 2 diabetes mellitus. Curr. Med. Res. Opin. 36, 1277–1284 (2020).
Frías, J. P. et al. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly alone, or dapagliflozin alone added to metformin monotherapy in subgroups of patients with type 2 diabetes in the DURATION-8 randomized controlled trial. Diab. Obes. Metab. 20, 1520–1525 (2018).
Wang, Y., Shao, X. & Liu, Z. Efficacy and safety of sodium-glucose co-transporter 2 inhibitors in the elderly versus non-elderly patients with type 2 diabetes mellitus: a meta-analysis. Endocr. J. https://doi.org/10.1507/endocrj.EJ21-0616 (2022).
Pratley, R. et al. Efficacy and safety of ertugliflozin in older patients with type 2 diabetes: a pooled analysis of phase III studies. Diab. Obes. Metab. 22, 2276–2286 (2020).
Sinclair, A. et al. Efficacy and safety of canagliflozin compared with placebo in older patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. BMC Endocr. Disord. 14, 37 (2014).
Jones, A. G. et al. Markers of β-cell failure predict poor glycemic response to GLP-1 receptor agonist therapy in type 2 diabetes. Diab. Care 39, 250–257 (2016).
Lapolla, A. et al. Correlation between baseline characteristics and clinical outcomes in a large population of diabetes patients treated with liraglutide in a real-world setting in Italy. Clin. Therap. 37, 574–584 (2015).
Gomez-Peralta, F. et al. Interindividual differences in the clinical effectiveness of liraglutide in Type 2 diabetes: a real-world retrospective study conducted in Spain. Diab. Med. 35, 1605–1612 (2018).
Simioni, N. et al. Predictors of treatment response to liraglutide in type 2 diabetes in a real-world setting. Acta Diabetol. 55, 557–568 (2018).
Thong, K. Y. et al. The association between postprandial urinary C-peptide creatinine ratio and the treatment response to liraglutide: a multi-centre observational study. Diab. Med. 31, 403–411 (2014).
Wang, T. et al. Predictive factors associated with glycaemic response to exenatide in Chinese patients with type 2 diabetes mellitus. J. Clin. Pharm. Therap. 45, 1050–1057 (2020).
Thong, K. Y. et al. Insulin treatment and longer diabetes duration both predict poorer glycaemic response to liraglutide treatment in type 2 diabetes: The Association of British Clinical Diabetologists Nationwide Liraglutide Audit. Br. J. Diab. Vas. Dis. 15, 169–172 (2015).
Yu, M., Wang, K., Liu, H. & Cao, R. GLP1R variant is associated with response to exenatide in overweight Chinese Type 2 diabetes patients. Pharmacogenomics 20, 273–277 (2019).
Feng, P. et al. Liraglutide reduces the body weight and waist circumference in Chinese overweight and obese type 2 diabetic patients. Acta Pharmacol. Sin. 36, 200–208 (2015).
Di Dalmazi, G. et al. Exenatide once weekly: effectiveness, tolerability, and discontinuation predictors in a real-world setting. Clin. Therap 42, 1738–1749.e1731 (2020).
Blonde, L. et al. Predictors of outcomes in patients with type 2 diabetes in the lixisenatide GetGoal clinical trials. Diab. Obes. Metab. 19, 275–283 (2017).
Berra, C. C. et al. Clinical efficacy and predictors of response to dulaglutide in type-2 diabetes. Pharmacol. Res. 159, 104996 (2020).
Anichini, R. et al. Gender difference in response predictors after 1-year exenatide therapy twice daily in type 2 diabetic patients: a real world experience. Diab. Metab Syndrom. Obes. 6, 123–129 (2013).
Seufert, J., Bailey, T., Barkholt Christensen, S. & Nauck, M. A. Impact of diabetes duration on achieved reductions in glycated haemoglobin, fasting plasma glucose and body weight with liraglutide treatment for up to 28 weeks: a meta-analysis of seven phase III trials. Diab. Obes. Metab. 18, 721–724 (2016).
Gallwitz, B. et al. Effect of once-weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diab. Obes. Metab. 20, 409–418 (2018).
Mathieu, C. et al. Effect of once weekly dulaglutide by baseline beta-cell function in people with type 2 diabetes in the AWARD programme. Diab. Obes. Metab. 20, 2023–2028 (2018).
Bonadonna, R. C. et al. Lixisenatide as add-on treatment among patients with different β-cell function levels as assessed by HOMA-β index. Diab./Metab. Res. Rev. 33, https://doi.org/10.1002/dmrr.2897 (2017).
Henry, R. R. et al. Efficacy of antihyperglycemic therapies and the influence of baseline hemoglobin A(1C): a meta-analysis of the liraglutide development program. Endocrine Practice 17, 906–913 (2011).
Yoo, J. H. et al. Clinical efficacy and parameters affecting the response to dulaglutide treatment in patients with type 2 diabetes: a retrospective, real-world data study. Diab. Ther. 10, 1453–1463 (2019).
Berkovic, M. C. et al. Long-term effectiveness of liraglutide in association with patients’ baseline characteristics in real-life setting in croatia: an observational, retrospective, multicenter study. Diab. Ther. 8, 1297–1308 (2017).
Petri, K. C. C., Ingwersen, S. H., Flint, A., Zacho, J. & Overgaard, R. V. Exposure-response analysis for evaluation of semaglutide dose levels in type 2 diabetes. Diab. Obes. Metab. 20, 2238–2245 (2018).
Dennis, J. M. et al. Precision medicine in type 2 diabetes: clinical markers of insulin resistance are associated with altered short- and long-term glycemic response to DPP-4 inhibitor therapy. Diab. Care 41, 705–712 (2018).
Chitnis, A. S., Ganz, M. L., Benjamin, N., Langer, J. & Hammer, M. Clinical effectiveness of liraglutide across body mass index in patients with type 2 diabetes in the united states: a retrospective cohort study. Adv. Therapy 31, 986–999 (2014).
Shaw, J. E., Gallwitz, B., Han, J., Hardy, E. & Schernthaner, G. Variability in and predictors of glycaemic responses after 24 weeks of treatment with exenatide twice daily and exenatide once weekly. Diab. Obes. Metab. 19, 1793–1797 (2017).
Wolffenbuttel, B. H., Van Gaal, L., Durán-Garcia, S. & Han, J. Relationship of body mass index with efficacy of exenatide twice daily added to insulin glargine in patients with type 2 diabetes. Diab. Obes. Metab. 18, 829–833 (2016).
Yale, J. F. et al. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: pooled analysis of data from four SURE studies by baseline characteristic subgroups. BMJ Open Diabetes Res Care 10, https://doi.org/10.1136/bmjdrc-2021-002619 (2022).
Lee, J. et al. Dulaglutide as an add-on to insulin in type 2 diabetes; clinical efficacy and parameters affecting the response in real-world practice. Diab. Metab. Synd. Obes. 12, 2745–2753 (2019).
Wysham, C., Guerci, B., D’Alessio, D., Jia, N. & Botros, F. T. Baseline factors associated with glycaemic response to treatment with once-weekly dulaglutide in patients with type 2 diabetes. Diab. Obes. Metab. 18, 1138–1142 (2016).
Davidson, J. A., Brett, J., Falahati, A. & Scott, D. Mild renal impairment and the efficacy and safety of liraglutide. Endocrine Practice 17, 345–355 (2011).
DeSouza, C. et al. Efficacy and safety of semaglutide for type 2 diabetes by race and ethnicity: a post hoc analysis of the SUSTAIN trials. J. Clin. Endocrinol. Metab. 105, https://doi.org/10.1210/clinem/dgz072 (2020).
Shomali, M. E., Ørsted, D. D. & Cannon, A. J. Efficacy and safety of liraglutide, a once-daily human glucagon-like peptide-1 receptor agonist, in African-American people with Type 2 diabetes: a meta-analysis of sub-population data from seven phase III trials. Diab. Med. 34, 197–203 (2017).
Nunes, A. P. et al. Tolerability and effectiveness of exenatide once weekly relative to basal insulin among type 2 diabetes patients of different races in routine care. Diab. Therapy 8, 1349–1364 (2017).
Davidson, J. A., Ørsted, D. D. & Campos, C. Efficacy and safety of liraglutide, a once-daily human glucagon-like peptide-1 analogue, in Latino/Hispanic patients with type 2 diabetes: post hoc analysis of data from four phase III trials. Diab. Obes. Metab. 18, 725–728 (2016).
Geng, Z. et al. KCNQ1 variant rs163184 is a potential biomarker of glycemic response to exenatide. Pharmacogenomics 23, 355–361 (2022).
Zinman, B. et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. https://doi.org/10.1056/NEJMoa1504720, NJ201511263732207 (2015).
Neal, B. et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. https://doi.org/10.1056/NEJMoa1611925, NJ201708173770708 (2017).
Anker, S. D. et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. https://doi.org/10.1056/NEJMoa2107038, NJ202110143851606 (2021).
Packer, M. et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. https://doi.org/10.1056/NEJMoa2022190, NJ202010083831507 (2020).
McMurray, J. J. V. et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. https://doi.org/10.1056/NEJMoa1911303, NJ201911213812105 (2019).
Heerspink, H. J. L. et al. Dapagliflozin in Patients with Chronic Kidney Disease. https://doi.org/10.1056/NEJMoa2024816, NJ202010083831509 (2020).
Lee, M. M. Y., Ghouri, N., McGuire, D. K., Rutter, M. K. & Sattar, N. Meta-analyses of results from randomized outcome trials comparing cardiovascular effects of SGLT2is and GLP-1RAs in asian versus white patients with and without type 2 diabetes. Diabetes Care 44, 1236–1241 (2021).
Qiu, M., Ding, L. L. & Zhou, H. R. Factors affecting the efficacy of SGLT2is on heart failure events: a meta-analysis based on cardiovascular outcome trials. Cardiovas. Diagnos. Therapy 11, 699–706 (2021).
Bhattarai, M. et al. Association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcomes in patients with type 2 diabetes and other risk factors for cardiovascular disease: a meta-analysis. JAMA Netw. Open 5, e2142078 (2022).
Chang, R., Liu, S. Y. & Zhao, L. M. Impact of demographic characteristics and antihyperglycemic and cardiovascular drugs on the cardiorenal benefits of SGLT2 inhibitors in patients with type 2 diabetes mellitus: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 100, e27802 (2021).
Giugliano, D. et al. Sodium-glucose co-transporter-2 inhibitors for the prevention of cardiorenal outcomes in type 2 diabetes: an updated meta-analysis. Diab. Obes. Metab. 23, 1672–1676 (2021).
Bhatia, K. et al. Prevention of heart failure events with sodium-glucose co-transporter 2 inhibitors across a spectrum of cardio-renal-metabolic risk. Eur. J. Heart Fail. 23, 1002–1008 (2021).
Chun, K. J. & Jung, H. H. SGLT2 inhibitors and kidney and cardiac outcomes according to estimated GFR and albuminuria levels: a meta-analysis of randomized controlled trials. Kidney Med. 3, 732–744.e731 (2021).
D’Andrea, E. et al. Heterogeneity of antidiabetic treatment effect on the risk of major adverse cardiovascular events in type 2 diabetes: a systematic review and meta-analysis. Cardiovas. Diabetol. 19, 154 (2020).
Kawai, Y. et al. Comparison of effects of SGLT-2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in type 2 diabetes mellitus patients with/without albuminuria: a systematic review and network meta-analysis. Diab. Res. Clin. Pract. 183, 109146 (2022).
Li, N. et al. Effects of SGLT2 inhibitors on cardiovascular outcomes in patients with stage 3/4 CKD: a meta-analysis. PLoS ONE 17, e0261986 (2022).
Furtado, R. H. M. et al. DapaglifLozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation 139, 2516–2527 (2019).
Oyama, K. et al. Obesity and effects of dapagliflozin on cardiovascular and renal outcomes in patients with type 2 diabetes mellitus in the DECLARE-TIMI 58 trial. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehab530 (2021).
Rådholm, K. et al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation 138, 458–468 (2018).
Vaduganathan, M. et al. Stress cardiac biomarkers, cardiovascular and renal outcomes, and response to canagliflozin. J. Am. Coll. Cardiol. 79, 432–444 (2022).
Wang, Q., Liu, L., Gao, L. & Li, Q. Cardiovascular safety of GLP-1 receptor agonists for diabetes patients with high cardiovascular risk: a meta-analysis of cardiovascular outcomes trials. Diab. Res. Clin. Pract. 143, 34–42 (2018).
Marsico, F. et al. Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with Type 2 diabetes mellitus with or without established cardiovascular disease: a meta-analysis of randomized controlled trials. Eur. Heart J. 41, 3346–3358 (2020).
He, L. et al. Subpopulation differences in the cardiovascular efficacy of long-acting glucagon-like peptide 1 receptor agonists in type 2 diabetes mellitus: a systematic review and meta-analysis. Diab. Therapy 11, 2121–2143 (2020).
Giugliano, D. et al. GLP-1 receptor agonists for prevention of cardiorenal outcomes in type 2 diabetes: An updated meta-analysis including the REWIND and PIONEER 6 trials. Diab. Obes. Metab. 21, 2576–2580 (2019).
Mannucci, E., Dicembrini, I., Nreu, B. & Monami, M. Glucagon-like peptide-1 receptor agonists and cardiovascular outcomes in patients with and without prior cardiovascular events: an updated meta-analysis and subgroup analysis of randomized controlled trials. Diab. Obes. Metab. 22, 203–211 (2020).
Zelniker, T. A. et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation 139, 2022–2031 (2019).
Singh, A. K. & Singh, R. Gender difference in cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in type 2 diabetes: a systematic review and meta-analysis of cardio-vascular outcome trials. Diab. Metab. Synd. 14, 181–187 (2020).
Uneda, K. et al. Systematic review and meta-analysis for prevention of cardiovascular complications using GLP-1 receptor agonists and SGLT-2 inhibitors in obese diabetic patients. Sci. Rep. 11, 10166 (2021).
Husain, M. et al. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diab. Obes. Metab. 22, 442–451 (2020).
Qiu, M. et al. The effects of the baseline characteristics on the efficacy of glp-1 ras in reducing cardiovascular events in type 2 diabetes: a meta-analysis. Int. J. Clin. Exp. Med. 13, 7437–7445 (2020).
Husain, M. et al. Effects of semaglutide on risk of cardiovascular events across a continuum of cardiovascular risk: combined post hoc analysis of the SUSTAIN and PIONEER trials. Cardiovas. Diabetol. 19, 156 (2020).
Kim, Y. G., Han, S. J., Kim, D. J., Lee, K. W. & Kim, H. J. Association between sodium glucose co-transporter 2 inhibitors and a reduced risk of heart failure in patients with type 2 diabetes mellitus: a real-world nationwide population-based cohort study. Cardiovas. Diab. 17, 91 (2018).
Raparelli, V. et al. Sex differences in cardiovascular effectiveness of newer glucose-lowering drugs added to metformin in type 2 diabetes mellitus. J. Am. Heart Assoc. 9, e012940 (2020).
Becher, P. M. et al. Use of sodium-glucose co-transporter 2 inhibitors in patients with heart failure and type 2 diabetes mellitus: data from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 23, 1012–1022 (2021).
Idris, I. et al. Lower risk of hospitalization for heart failure, kidney disease and death with sodium-glucose co-transporter-2 inhibitors compared with dipeptidyl peptidase-4 inhibitors in type 2 diabetes regardless of prior cardiovascular or kidney disease: a retrospective cohort study in UK primary care. Diab. Obes. Metab. 23, 2207–2214 (2021).
Patorno, E. et al. Sodium-glucose cotransporter-2 inhibitors versus glucagon-like peptide-1 receptor agonists and the risk for cardiovascular outcomes in routine care patients with diabetes across categories of cardiovascular disease. Ann. Int. Med. 174, 1528–1541 (2021).
Htoo, P. T. et al. Cardiovascular effectiveness of sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists in older patients in routine clinical care with or without history of atherosclerotic cardiovascular diseases or heart failure. J. Am. Heart Assoc. 11, e022376 (2022).
Yang, C. T., Yang, C. Y., Ou, H. T. & Kuo, S. Comparative cardiovascular safety of GLP-1 receptor agonists versus other glucose-lowering agents in real-world patients with type 2 diabetes: a nationwide population-based cohort study. Cardiovas. Diabetol. 19, 83 (2020).
Chen, J. J. et al. Association of glucagon-like peptide-1 receptor agonist vs dipeptidyl peptidase-4 inhibitor use with mortality among patients with type 2 diabetes and advanced chronic kidney disease. JAMA Netw. Open 5, e221169 (2022).
Birkeland, K. I. et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diab. Endocrinol. 5, 709–717 (2017).
Chan, Y. H. et al. Impact of the initial decline in estimated glomerular filtration rate on the risk of new-onset atrial fibrillation and adverse cardiovascular and renal events in patients with type 2 diabetes treated with sodium-glucose co-transporter-2 inhibitors. Diab. Obes. Metab. 23, 2077–2089 (2021).
Neuen, B. L. et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diab. Endocrinol. 7, 845–854 (2019).
Zelniker, T. A. et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393, 31–39 (2019).
Bae, J. H. et al. Effects of sodium-glucose cotransporter 2 inhibitors on renal outcomes in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 9, 13009 (2019).
Bakris, G. et al. Effects of canagliflozin in patients with baseline eGFR <30 ml/min per 1.73 m(2): subgroup analysis of the randomized CREDENCE trial. Clin. J. Am. Soc. Nephrol. 15, 1705–1714 (2020).
Jardine, M. et al. Kidney, cardiovascular, and safety outcomes of canagliflozin according to baseline albuminuria: a CREDENCE secondary analysis. Clin. J. Am. Soc. Nephrol. 16, 384–395 (2021).
Mosenzon, O. et al. The effect of dapagliflozin on albuminuria in DECLARE-TIMI 58. Diab. Care 44, 1805–1815 (2021).
Neuen, B. L. et al. Relative and absolute risk reductions in cardiovascular and kidney outcomes with canagliflozin across KDIGO risk categories: findings from the CANVAS program. Am. J. Kidney Dis. 77, 23–34.e21 (2021).
Wanner, C. et al. Consistent effects of empagliflozin on cardiovascular and kidney outcomes irrespective of diabetic kidney disease categories: Insights from the EMPA-REG OUTCOME trial. Diab. Obes. Metab. 22, 2335–2347 (2020).
Neuen, B. L. et al. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS program. J. Am. Soc. Nephrol. 30, 2229–2242 (2019).
Januzzi, J. L. Jr. et al. Insulin-like growth factor binding protein 7 predicts renal and cardiovascular outcomes in the canagliflozin cardiovascular assessment study. Diab. Care 44, 210–216 (2021).
Riddle, M. et al. Efficacy and safety of dulaglutide in older patients: a post hoc Analysis of the REWIND trial. J. Clin. Endocrinol. Metab. 106, https://doi.org/10.1210/clinem/dgab065 (2021).
van der Aart-van der Beek, A. B. et al. Effect of once-weekly exenatide on estimated glomerular filtration rate slope depends on baseline renal risk: a post hoc analysis of the EXSCEL trial. Diab. Obes. Metab. 22, 2493–2498 (2020).
Leiter, L. A. et al. The effect of glucagon-like peptide-1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across baseline blood pressure categories: analysis of the LEADER and SUSTAIN 6 trials. Diab. Obes. Metab. 22, 1690–1695 (2020).
Verma, S. et al. Duration of diabetes and cardiorenal efficacy of liraglutide and semaglutide: a post hoc analysis of the LEADER and SUSTAIN 6 clinical trials. Diab. Obes. Metab. 21, 1745–1751 (2019).
Marso, S. et al. Effects of liraglutide on cardiovascular outcomes in patients with diabetes with or without heart failure. J. Am. Coll. Cardiol. 75, https://doi.org/10.1016/j.jacc.2019.12.063 (2020).
Verma, S. et al. Effects of glucagon-like peptide-1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across body mass index categories in type 2 diabetes: results of the LEADER and SUSTAIN 6 trials. Diab. Obes. Metab. 22, 2487–2492 (2020).
Mosenzon, O. et al. Cardiovascular and renal outcomes by baseline albuminuria status and renal function: results from the LEADER randomized trial. Diab. Obes. Metab. 22, 2077–2088 (2020).
Muskiet, M. H. A. et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diab. Endocrinol. 6, 859–869 (2018).
Koh, E. S. et al. Renal outcomes and all-cause death associated with sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL 3 Korea). Diab. Obes. Metab. 23, 455–466 (2021).
Nagasu, H. et al. Kidney outcomes associated with SGLT2 inhibitors versus other glucose-lowering drugs in real-world clinical practice: the Japan chronic kidney disease database. Diab. Care 44, 2542–2551 (2021).
Kent, D. M., Steyerberg, E. & van Klaveren, D. Personalized evidence based medicine: predictive approaches to heterogeneous treatment effects. BMJ 363, k4245 (2018).
Kent, D. et al. The predictive approaches to treatment effect heterogeneity (PATH) statement. Ann. Int. Med. 172, https://doi.org/10.7326/M18-3667 (2020).
Shields, B. M. et al. Patient stratification for determining optimal second-line and third-line therapy for type 2 diabetes: the TriMaster study. Nat. Med. 29, 376–383 (2022).
Brandon, R. et al. Stratified glucose-lowering response to vildagliptin and pioglitazone by obesity and hypertriglyceridemia in a randomized crossover trial. Front. Endocrinol. 13, https://doi.org/10.3389/fendo.2022.1091421 (2023).
Dennis, J. M. et al. Derivation and validation of a type 2 diabetes treatment selection algorithm for SGLT2-inhibitor and DPP4-inhibitor therapies based on glucose-lowering efficacy: cohort study using trial and routine clinical data. medRxiv, 2021.2011.2011.21265959, https://doi.org/10.1101/2021.11.11.21265959 (2021).
Dawed, A. Y. et al. Pharmacogenomics of GLP-1 receptor agonists: a genome-wide analysis of observational data and large randomised controlled trials. Lancet Diab. Endocrinol. 11, 33–41 (2023).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Solomon, S. D. et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 387, 1089–1098 (2022).
Herrington, W. G. et al. Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 388, 117–127 (2023).
Kyriakidou, A. et al. Clinical and genetic predictors of glycemic control and weight loss response to liraglutide in patients with type 2 diabetes. J. Pers. Med. 12, https://doi.org/10.3390/jpm12030424 (2022).
McAdam-Marx, C. et al. Glycemic control and weight outcomes for exenatide once weekly versus liraglutide in patients with type 2 diabetes: a 1-year retrospective cohort analysis. Clin. Therap. 38, 2642–2651 (2016).
Gorgojo-Martínez, J. J., Gargallo-Fernández, M. A., Brito-Sanfiel, M. & Lisbona-Catalán, A. Real-world clinical outcomes and predictors of glycaemic and weight response to exenatide once weekly in patients with type 2 diabetes: the CIBELES project. Int. J. Clin. Pract. 72, e13055 (2018).
Brekke, L. et al. The use of decomposition methods in real-world treatment benefits evaluation for patients with type 2 diabetes initiating different injectable therapies: findings from the INITIATOR study. Value Health 20, 1252–1259 (2017).
Carrington, M. J. et al. Long-term tolerance and efficacy of adjunctive exenatide therapy on glycaemic control and bodyweight in type 2 diabetes: a retrospective study from a specialist diabetes outpatient clinic. Int. Med. J. 44, 345–353 (2014).
Mirabelli, M. et al. Clinical effectiveness and safety of once-weekly glp-1 receptor agonist dulaglutide as add-on to metformin or metformin plus insulin secretagogues in obesity and type 2 diabetes. J. Clin. Med. 10, 1–13 (2021).
Yu, M., Yuan, G. Y., Zhang, B., Wu, H. Y. & Lv, X. F. Efficacy and safety of dulaglutide by baseline HbA1c in Chinese patients with type 2 diabetes: a post hoc analysis. Diab. Therapy 11, 1147–1159 (2020).
Yabe, D. et al. Efficacy and safety of oral semaglutide in Japanese patients with type 2 diabetes: a subgroup analysis by baseline variables in the PIONEER 9 and PIONEER 10 trials. J. Diabetes Investig., https://doi.org/10.1111/jdi.13764 (2022).
Terauchi, Y. et al. Benefits of the fixed-ratio combination of insulin glargine 100 units/mL and lixisenatide (iGlarLixi) in Japanese people with type 2 diabetes: a subgroup and time-to-control analysis of the LixiLan JP phase 3 trials. Diab. Obes. Metab. 22, 35–47 (2020).
Gentilella, R. et al. Change in HbA(1c) across the baseline HbA(1c) Range in type 2 diabetes patients receiving once-weekly dulaglutide versus other incretin agents. Diabetes Therapy 10, 1113–1125 (2019).
Cho, Y. K. et al. Clinical parameters affecting the therapeutic efficacy of empagliflozin in patients with type 2 diabetes. PLoS ONE 14, e0220667 (2019).
Brown, R. E., Gupta, N. & Aronson, R. Effect of dapagliflozin on glycemic control, weight, and blood pressure in patients with type 2 diabetes attending a specialist endocrinology practice in canada: a retrospective cohort analysis. Diabetes Technology & Therapeutics 19, 685–691 (2017).
Zhou, F. L. et al. Identification of subgroups of patients with type 2 diabetes with differences in renal function preservation, comparing patients receiving sodium-glucose co-transporter-2 inhibitors with those receiving dipeptidyl peptidase-4 inhibitors, using a supervised machine-learning algorithm (PROFILE study): A retrospective analysis of a Japanese commercial medical database. Diabetes, Obesity & Metabolism 21, 1925–1934 (2019).
Montanya, E. et al. Improvement in glycated haemoglobin evaluated by baseline body mass index: a meta-analysis of the liraglutide phase III clinical trial programme. Diabetes, Obesity & Metabolism 18, 707–710 (2016).
Eto, K., Naito, Y. & Seino, Y. Evaluation of the efficacy and safety of lixisenatide add-on treatment to basal insulin therapy among T2DM patients with different body mass indices from GetGoal trials. Diabetology and Metabolic Syndrome 7, https://doi.org/10.1186/s13098-015-0104-6 (2015).
Boustani, M. A. et al. Similar efficacy and safety of once-weekly dulaglutide in patients with type 2 diabetes aged ≥65 and <65 years. Diabetes, Obesity & Metabolism 18, 820–828 (2016).
Blonde, L. et al. Achievement of treatment goals with canagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of randomized controlled trials. Curr. Med. Res. Opin. 31, 1993–2000 (2015).
Gimeno-Orna, J. A. et al. Baseline ALT levels as a marker of glycemic response to treatment with GLP-1 receptor agonists. Endocrinol. Nutr. 63, 164–170 (2016).
Yang, K. et al. High baseline FGF21 levels are associated with poor glucose-lowering efficacy of exenatide in patients with type 2 diabetes. Acta Diabetolog. 58, 595–602 (2021).
Neeland, I. J. et al. The impact of empagliflozin on obstructive sleep apnea and cardiovascular and renal outcomes: an exploratory analysis of the EMPA-REG OUTCOME trial. Diab. Care 43, 3007–3015 (2020).
Kaku, K. et al. Empagliflozin and cardiovascular outcomes in asian patients with type 2 diabetes and established cardiovascular disease - results from EMPA-REG OUTCOME(®). Circ. J. 81, 227–234 (2017).
Kaku, K. et al. The effect of empagliflozin on the total burden of cardiovascular and hospitalization events in the Asian and non-Asian populations of the EMPA-REG OUTCOME trial of patients with type 2 diabetes and cardiovascular disease. Diab. Obes. Metab. 24, 662–674 (2022).
Verma, S. et al. Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus with or without history of myocardial infarction or stroke. Circulation 138, 2884–2894 (2018).
Verma, S. et al. Empagliflozin reduces the risk of mortality and hospitalization for heart failure across thrombolysis in myocardial infarction risk score for heart failure in diabetes categories: post hoc analysis of the EMPA-REG OUTCOME trial. Diab. Obes. Metab. 22, 1141–1150 (2020).
Mentz, R. J. et al. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL trial. J. Am. Heart Assoc. 7, e009304 (2018).
Leiter, L. A. et al. Cardiovascular risk reduction with once-weekly semaglutide in subjects with type 2 diabetes: a post hoc analysis of gender, age, and baseline CV risk profile in the SUSTAIN 6 trial. Cardiovas. Diabetol. 18, 73 (2019).
Fudim, M. et al. Effect of once-weekly exenatide in patients with type 2 diabetes mellitus with and without heart failure and heart failure-related outcomes: insights from the EXSCEL trial. Circulation 140, 1613–1622 (2019).
Badjatiya, A. et al. Clinical outcomes in patients with type 2 diabetes mellitus and peripheral artery disease: results from the EXSCEL trial. Circ.: Cardiovas. Interventions 12, e008018 (2019).
Verma, S. et al. Empagliflozin reduces cardiovascular events, mortality and renal events in participants with type 2 diabetes after coronary artery bypass graft surgery: subanalysis of the EMPA-REG OUTCOME® randomised trial. Diabetologia 61, 1712–1723 (2018).
Bonaca, M. P. et al. Dapagliflozin and cardiac, kidney, and limb outcomes in patients with and without peripheral artery disease in DECLARE-TIMI 58. Circulation 142, 734–747 (2020).
Gilbert, M. P. et al. Effect of liraglutide on cardiovascular outcomes in elderly patients: a post Hoc analysis of a randomized controlled trial. Ann. Int. Med. 170, 423–426 (2019).
Inzucchi, S. E. et al. Cardiovascular benefit of empagliflozin across the spectrum of cardiovascular risk factor control in the EMPA-REG OUTCOME trial. J. Clin. Endocrinol. Metab. 105, 3025–3035 (2020).
Zinman, B. et al. Empagliflozin in women with type 2 diabetes and cardiovascular disease - an analysis of EMPA-REG OUTCOME®. Diabetologia 61, 1522–1527 (2018).
O’Donoghue, M. L. et al. The efficacy and safety of dapagliflozin in women and men with type 2 diabetes mellitus. Diabetologia 64, 1226–1234 (2021).
Mahmoud, A. N. et al. Does Gender Influence the Cardiovascular Benefits Observed with Sodium Glucose Co-Transporter-2 (SGLT-2) Inhibitors? A Meta-Regression Analysis. Cardiol Ther 6, 129–132 (2017).
Rådholm, K., Zhou, Z., Clemens, K., Neal, B. & Woodward, M. Effects of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes in women versus men. Diabetes, Obesity & Metabolism 22, 263–266 (2020).
Mann, J. F. E. et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation 138, 2908–2918 (2018).
Zelniker, T. A. et al. Effect of dapagliflozin on cardiovascular outcomes according to baseline kidney function and albuminuria status in patients with type 2 diabetes: a prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol 6, 801–810 (2021).
Verma, S. et al. Association between uric acid levels and cardio-renal outcomes and death in patients with type 2 diabetes: a subanalysis of EMPA-REG OUTCOME. Diab. Obes. Metab. 22, 1207–1214 (2020).
Sen, T. et al. Association between circulating GDF-15 and cardio-renal outcomes and effect of canagliflozin: results from the CANVAS trial. J. Am. Heart Assoc. 10, e021661 (2021).
Sen, T. et al. Effects of the SGLT2 inhibitor canagliflozin on plasma biomarkers TNFR-1, TNFR-2 and KIM-1 in the CANVAS trial. Diabetologia 64, 2147–2158 (2021).
Oikonomou, E. K., Suchard, M. A., McGuire, D. K. & Khera, R. Phenomapping-derived tool to individualize the effect of canagliflozin on cardiovascular risk in type 2 diabetes. Diabetes Care 45, 965–974 (2022).
Barbery, C. et al. Effect of once-weekly exenatide on hospitalization for acute coronary syndrome or coronary revascularization in patients with type 2 diabetes mellitus. Am. Heart J. 239, https://doi.org/10.1016/j.ahj.2021.03.013 (2021).
Fitchett, D. et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. European Heart Journal 37, 1526–1534 (2016).
Ji, Q. et al. Effect of empagliflozin on cardiorenal outcomes and mortality according to body mass index: a subgroup analysis of the EMPA-REG OUTCOME trial with a focus on Asia. Diabetes, Obesity & Metabolism 23, 1886–1891 (2021).
Fitchett, D. et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation 139, 1384–1395 (2019).
Berg, D. D. et al. Heart failure risk stratification and efficacy of sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes mellitus. Circulation 140, 1569–1577 (2019).
Savarese, G. et al. Empagliflozin in heart failure with predicted preserved versus reduced ejection fraction: data from the EMPA-REG OUTCOME trial. J. Cardiac Fail. 27, 888–895 (2021).
Sharma, A. et al. Patient phenotypes and SGLT-2 inhibition in Type 2 diabetes: insights from the EMPA-REG OUTCOME trial. JACC Heart Fail. 9, 568–577 (2021).
Kadowaki, T. et al. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from the EMPA-REG OUTCOME(®) trial. J Diabetes Investig. 10, 760–770 (2019).
Böhm, M. et al. Efficacy of empagliflozin on heart failure and renal outcomes in patients with atrial fibrillation: data from the EMPA-REG OUTCOME trial. Eur. J. Heart Fail. 22, 126–135 (2020).
Acknowledgements
The ADA/EASD Precision Diabetes Medicine Initiative, within which this work was conducted, has received the following support: The Covidence license was funded by Lund University (Sweden) for which technical support was provided by Maria Björklund and Krister Aronsson (Faculty of Medicine Library, Lund University, Sweden). Administrative support was provided by Lund University (Malmö, Sweden), University of Chicago (IL, USA), and the American Diabetes Association (Washington D.C., USA). The Novo Nordisk Foundation (Hellerup, Denmark) provided grant support for in-person writing group meetings (PI: L Phillipson, University of Chicago, IL, USA). This study was supported by the National Institute for Health and Care Research Exeter Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. K.G.Y. and J.M.D. are supported by Research England’s Expanding Excellence in England (E3) fund. A.R.K. is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR002490. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. S.R. is funded by a US Department of Veterans Affairs Award IK2-CX001907, and a Webb-Waring Biomedical Research Award from the Boettcher Foundation. J.M.D. is funded by the EFSD Rising Star Fellowship Programme, the UK Medical Research Council (MR/N00633X/1), and a BHF-Turing Cardiovascular Data Science Award (SP/19/6/34809). M.S. is funded by the National Institutes of Health, K01HL157658. The funders had no role in the study design, data extraction or interpretation, writing of the article, or the decision to submit for publication.
Author information
Authors and Affiliations
Consortia
Contributions
K.G.Y., E.H.M., R.J.M., A.R.K., S.J.P., S.R., M.A.S., D.K.T., A.P.M., A.Y.D., A.G.J., E.R.P., and J.M.D. designed the study. K.G.Y., E.H.M., R.J.M., A.R.K., S.J.P., S.R., M.A.S., A.P.M., A.Y.D., A.G.J., E.R.P., and J.M.D. implemented the systematic review and contributed to full-text data extraction. K.G.Y., E.H.M., R.J.M., A.R.K., S.J.P., S.R., M.A.S., A.Y.D., A.G.J., E.R.P., and J.M.D. synthesized the data and drafted the article. All authors critically revised the article and approved the final article. E.R.P. and J.M.D. jointly supervised this work. E.R.P. and J.M.D. attest that all listed authors meet authorship criteria, and that no others meeting the criteria have been omitted. E.R.P. and J.M.D. were responsible for the decision to submit for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare the following competing interests: A.P.M. declares previous research funding from Eli Lilly and Company, Pfizer, and AstraZeneca. A.G.J. has received research funding from the Novo Nordisk foundation. E.R.P. has received honoraria for speaking from Lilly, Novo Nordisk, and Illumina. All other authors declare no competing interest.
Peer review
Peer review information
Communications Medicine thanks Sheyu Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Young, K.G., McInnes, E.H., Massey, R.J. et al. Treatment effect heterogeneity following type 2 diabetes treatment with GLP1-receptor agonists and SGLT2-inhibitors: a systematic review. Commun Med 3, 131 (2023). https://doi.org/10.1038/s43856-023-00359-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-023-00359-w