Abstract
Background
Sex and gender are believed to influence vaccine response. Yet, the relationship between sex and gender and COVID-19 vaccine efficacy is poorly understood and remains under-investigated.
Methods
We conducted a systematic review to determine whether and to what extent post-approval COVID-19 vaccine effectiveness (VE) studies report sex-disaggregated VE data.
We searched four publication and pre-publication databases and additional grey literature sources for relevant published/preprint studies released between 1 January 2020 and 1 October 2021 (i.e., pre-Omicron era). We included observational studies providing VE estimates for one or more licensed/approved COVID-19 vaccines and including both males and females. Two reviewers independently assessed study eligibility, extracted data, and assessed risk-of-bias through a modified version of Cochrane’s ROBINS-I tool. A qualitative data synthesis was performed.
Results
Here we show that, among 240 eligible publications, 68 (28.3%) do not report the sex distribution among participants. Only 21/240 (8.8%) studies provide sex-disaggregated VE estimates, and high between-study heterogeneity regarding design, target population, outcomes, and vaccine type/timing prevent the assessment of sex in determining COVID-19 VE across studies.
Conclusions
Our findings indicate that few COVID-19 vaccine research publications account for sex. Improved adherence to recommended reporting guidelines will ensure that the evidence generated can be used to better understand the relationship between sex and gender and VE.
Plain Language Summary
The level of protection that vaccines provide against COVID-19 might depend on a person’s sex or gender. However, sex and gender are not always reported in studies on the effectiveness of COVID-19 vaccines. Here, we systematically reviewed the literature on COVID-19 vaccine effectiveness and looked at whether the studies we found separated out their data on vaccine effectiveness by participants’ sex. Out of the 240 publications we identified, 68 (28.3%) did not report the sex of the participants in their study, and only 21 studies (8.8%) reported vaccine effectiveness data separated by sex. These results show that a substantial proportion of COVID-19 vaccine research publications do not account for sex. Efforts should be made by researchers to study and report the relationship between sex and vaccine effectiveness, to help to optimise vaccination strategies so that all people are adequately protected.
Similar content being viewed by others
Introduction
The importance of considering sex as a biological variable (SABV) and gender as a social construct in statistical analyses is receiving increasing recognition1,2,3,4. In recent decades, funding agencies and regulatory authorities have introduced requirements to include both male and female participants and to report sex-disaggregated results in biological research, focusing primarily on preclinical studies and clinical trials5. In 2016, the Gender Policy Committee of European Association of Science Editors developed the Sex and Gender Equity in Research (SAGER) guidelines6, which have been widely adopted by scientific publishers to encourage authors to report sex- and gender-specific results more systematically and transparently across study types.
A growing body of evidence indicates that sex and gender are associated with and are among key factors in shaping immunogenicity, pharmacokinetics, and vaccine response7,8,9. In particular, sex differences in reported adverse events following vaccination is one of the clearest examples that we have of the role of sex in vaccine response10,11. Furthermore, multiple factors, ranging from the recipient and pathogen-related characteristics to context-specific aspects, can affect a vaccine’s ability to induce an immune response and confer protection against a range of key outcomes, but these are often underexplored in preclinical and clinical trials12. The scope and scale of COVID-19 vaccine research published since 2020 provides an important opportunity to assess the degree to which sex is being considered in vaccine effectiveness (VE) studies. While reduced VE for many vaccines among older age groups due to immunosenescence is well-recognized13, the degree to which sex and age-sex interaction impact VE is less clear and less frequently investigated and reported. In fact, females typically present stronger immune responses, which might lead to greater VE compared to males14,15. The relationship between sex and immune response to vaccination may therefore have important implications for COVID-19 prevention, especially given that male sex has been identified as a risk factor for severe COVID-19 and COVID-19-related death16.
Hundreds of vaccine clinical trials have been conducted since the start of the COVID-19 pandemic, and an estimated 50 COVID-19 vaccines have been approved in at least one country as of 2 December 202217. Yet, a systematic review of COVID-19 vaccine clinical trials that were published by 22 April 2021 found that only 24% of the included trials reported sex-disaggregated estimates for their primary outcome18. Considering the limited availability of sex-disaggregated efficacy data from COVID-19 vaccine clinical trials18, post-approval observational studies investigating VE offers an opportunity to provide additional sex-specific estimates relevant to vaccine evaluation and can help advance our understanding of the relationship between sex and vaccine response. In this study, we examine the literature for observational COVID-19 VE studies to determine the proportion of studies that reported vaccine effectiveness disaggregated by sex and compare the characteristics of these studies. After collating the evidence from eligible COVID-19 VE studies identified through systematic searches, we show that a substantial proportion of studies did not report participants’ sex and/or failed to account for sex in their analyses. Furthermore, we find that a very small number of studies reported sex-disaggregated VE data, thus missing an important opportunity to shed light on whether and how sex affects a person’s response to COVID-19 vaccines.
Methods
We conducted a systematic review of the published literature based on a prespecified protocol that was registered in the International Prospective Register of Systematic Reviews (PROSPERO; identifier: CRD42021289263). We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines 2020, as indicated in the PRISMA checklists19,20.
Search strategy
Using combinations of terms related to the concepts of “COVID-19 vaccine” and “effectiveness”, we searched Ovid MEDLINE, Europe PMC Preprints, Embase (Ovid) and the WHO COVID-19 Research Database for relevant published or preprint studies released between 1 January 2020 and 1 October 2021 (Supplementary Table 1). We retrieved additional records through hand-searching websites of relevant research initiatives and public health agencies such as the COVID-19 Evidence Network to support Decision-Making (COVID-END), the International Vaccine Access Center of the Johns Hopkins University, the UK Health Security Agency, and the Public Health Agency of Canada (PHAC) as of 27 October 2021. Where available, we applied filters to exclude studies based on animal models; no restrictions were placed with respect to the language of publication. For all eligible preprints based on inclusion and exclusion criteria as detailed below, we searched for any corresponding published version of the study up to 15 September 2022 and used the published version for data extraction. Fully reproducible search strategies are available in Supplementary Note 1.
Review process
Two reviewers among GS, VR, JK, AP, MH, and AU independently screened each record first by title and abstract, and then in full text based on predefined eligibility criteria. Data extraction and risk of bias assessment of eligible studies were also carried out independently by two reviewers among those listed above. At all steps, disagreements were solved by discussion or arbitration of a third author (GS).
Study eligibility criteria
All observational studies that reported on the effectiveness of one or more COVID-19 vaccines and their combinations for those COVID-19 vaccines that had been approved/authorized for use were eligible. The list of eligible vaccines was obtained from the list of approved/authorized vaccines reported in the COVID-19 Vaccine Development and Approvals Tracker (covid19.trackvaccines.org) at the time of the search17. Studies were included if they met the eligibility criteria regardless of the type of measure being reported and the outcome event(s) of interest (e.g., documented test positivity, symptomatic infection, severe disease, hospitalization, infection transmission etc.). No restrictions were placed on the geographic area where the studies were conducted.
We excluded non-primary studies (e.g. reviews, commentaries, opinion pieces), study protocols without reporting of results, studies without a control group (e.g. case series, studies including only vaccinated individuals), clinical trials (as these studies estimate vaccine efficacy but not effectiveness), economic analyses, modelling studies, qualitative studies, and studies that did not specify the type of vaccine(s) under investigation.
Risk of bias assessment
We assessed the risk of bias in all studies that reported sex-specific VE data using Cochrane’s ROBINS-I (Risk Of Bias In Non-randomized Studies of Interventions) tool21, adapted to our research question as detailed in Supplementary Note 2.
Data extraction and synthesis
For each COVID-19 VE study that met the eligibility criteria described above, we recorded whether it reported sex-disaggregated VE data. For studies not reporting such data, we included only the following details: bibliographic information, type of publication (published refereed article, preprint, report from a public health agency), study country, vaccine(s) under evaluation, type of study population, whether the sex distribution of the study population was reported, and percentage of female participants included in the study. For the purpose of this review, study participants were considered exposed if they had received at least one dose of any COVID-19 vaccine that had been approved, authorized, licensed, granted emergency use authorization, or made available for use outside of clinical trials via any pathway by a regulatory agency, a national authority, or another entity.
For studies that reported sex-disaggregated VE data, we developed a standardized data extraction form that was pilot tested by all members of the review team on five randomly selected studies and refined as needed. Through this form, we collected the same basic details as indicated above for studies not reporting sex-disaggregated VE data, along with additional study-level data including (but not limited to): study period, study methodology, setting, primary variant circulating, population characteristics (e.g. demographics, prior infection status), vaccine type and schedule, the timing of outcome assessment relative to dose administration, number of vaccinated and unvaccinated individuals with and without the events of interest, VE estimates and other relevant outcomes.
For all studies, country groupings were based on the geographic region classification of the United Nations Department of Economic and Social Affairs and the income classification of the World Bank22,23.
Meta-analyses of sex-specific VE were planned but could not be undertaken owing to the high between-study heterogeneity in terms of populations, vaccine types, outcomes, and approaches. Therefore, we only performed a qualitative synthesis of the evidence.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
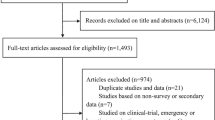
After screening 8639 unique records, we identified 376 studies eligible for full-text review and 78 additional records retrieved through other sources (Fig. 1). We determined that 248 studies of COVID-19 VE met the eligibility criteria. Of these, eight studies included participants of one sex only—five were studies on pregnant women, two were carried out among incarcerated males, and one involved an all-male population of workers within the Indian Armed Forces. The list of studies excluded after full-text review and their reasons for exclusion are provided in Supplementary Data 1.
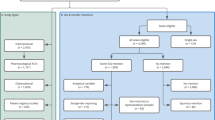
The main features of the 240 studies that included both males and females in the study population are summarized in Table 1 and full references are provided in Supplementary Data 2. While most studies accounted for sex in their analyses (e.g., by matching on sex or adjusting for sex in regression models), 68/240 (28.3%) studies did not explicitly report the sex distribution of their study population. Of these, the vast majority (54/68, 77.9%) were published in peer-reviewed journals, 12 (17.7%) were preprints, and 3 (4.4%) were reports from public health agencies. Across all other studies, the percentage of female individuals ranged from 2.9 to 95.4% (median, IQR: 54.2%, 49.0–63.9) (Table 1), reflecting the demographic characteristics of the population under assessment (e.g., fewer male participants are typically enrolled in studies of healthcare workers because most individuals in this occupational group are female) and/or selection bias issues (e.g., females less likely than males to be recruited into the study in some contexts).
Only 21/240 (8.8%) studies provided sex-specific VE estimates or provided sufficient aggregated data allowing to calculate VE for one or both sexes. Across the 21 studies that reported COVID-19 VE data disaggregated by sex, we found substantial heterogeneity with respect to the design and methods of data collection, the study populations, the vaccine(s) under investigation, the types of outcome events against which effectiveness was assessed, and the timing of VE evaluation relative to the last vaccine dose (Supplementary Data 3). For all studies reporting sex-specific VE data, the risk of bias was judged to be moderate or serious mostly due to the high potential for confounding along with moderate concerns regarding selection bias and outcome misclassification (Supplementary Data 4).
We did not observe meaningful differences with respect to the country income level, the geographic areas where the study was carried out, or the vaccine(s) under investigation between studies that reported sex-disaggregated measures of COVID-19 VE versus those that did not (Table 1). Most studies reporting VE by sex were conducted in the general population (15/21, 71.4%). Among the 219 studies not reporting sex-specific VE data, almost half (101, 46.1%) involved the general population, 51 (23.3%) were restricted to healthcare workers, 18 (8.2%) focused on older adults, and 40 (18.3%) on other special populations (e.g., children/adolescents, residents of long-term care facilities, employees of a particular sector/firm, etc.).
Discussion
The importance of evaluating the role of sex and gender across a range of biological processes has become more widely recognized by the scientific community and research funding agencies in recent years, and many publishing and reporting guidelines have devoted efforts to standardize reporting. Nonetheless, opportunities for improvement remain in evaluating the role of sex and gender in vaccine research. In this systematic review, we found that less than 10% of eligible post-vaccine authorization/approval observational studies investigating the effectiveness of one or more COVID-19 vaccines reported sex-disaggregated VE estimates. Therefore, we are missing an opportunity to investigate thoroughly the role of sex in determining an individual’s response to COVID-19 vaccines.
Almost all studies identified in our review included male and female participants in variable proportions, but—among those that considered sex in their analyses—sex was mainly handled as a potential confounder. As such, it was often used for matching and/or included in multivariable regression models aimed at estimating adjusted VE. However, to elucidate whether males and females respond differently to vaccination, sex should be investigated as a potential effect modifier. To this end, sex-specific VE estimates are needed24. Given that only a highly heterogenous group of 21 studies did provide such sex-specific estimates, whether sex plays a clinically relevant role in determining COVID-19 VE remains unclear.
Our work was focused on the variable sex, which must be clearly differentiated from gender, though both these variables need to be investigated further. The complex set of socially constructed roles, behaviours, and identities that define an individual’s gender is worth noting, as this is also widely neglected as a potential factor shaping effectiveness estimates. Without systematic and transparent reporting of sex- and gender-stratified analyses, as well as adjusted estimates, we simply lack the necessary information to understand whether and how these important factors contribute to determining the effectiveness of any COVID-19 vaccine. Gender may be just as likely, or more likely, than biological sex to confound the relationship between vaccination and vaccine effectiveness, by affecting both the exposure (whether someone gets vaccinated) and the outcome (whether the vaccine succeeds at preventing a given event, e.g., severe COVID-19). Notably, research has shown that both sex and gender are associated with factors that directly influence the outcome such as immunological responses to immunization and the likelihood of acquiring an infection25,26, and can play a role in determining an individual’s access to vaccination and their willingness to get vaccinated27,28.
Of note, over 85% of the studies included in our review were articles published in peer-reviewed journals (as opposed to preprints or reports from public health agencies), suggesting that the principles outlined in the SAGER guidelines6, which have already been endorsed by several journals, are not systematically applied, if incorporated at all, within a journal’s publication policies. Therefore, greater efforts should be made to increase researchers’ awareness of, and adherence to, recommended and/or required reporting practices, especially given that these practices are beneficial to not only the scientific community, but society at large, in addition to being relatively easy to implement. In fact, understanding whether vaccine effectiveness varies by sex and gender has important implications for vaccination policies and practices as this could lead to sex- and/or gender-specific recommendations regarding dosages, intervals between doses, or preferred vaccines, in order to maximize the benefits of vaccination. Failing to report sex- and gender-disaggregated VE estimates hampers the ability to draw conclusions on whether relevant differences exist between sexes and genders with respect to COVID-19 vaccine effectiveness. Instead, systematic reporting of sex- and gender-disaggregated estimates, regardless of statistical significance, would not only clarify the role (or lack thereof) of sex and gender in vaccine research but also avoid publication bias.
The biggest limitation of this systematic review is that, given the timing of the search, we only included studies conducted and released during the pre-Omicron era. It is to be noted that as our search strategy was limited to research conducted and released up to October 2021, our systematic review also does not capture all relevant studies carried out right before the emergence of Omicron as some were released later. While it is possible that a more substantial proportion of studies published after October 2021 reported sex-disaggregated VE data compared to previous studies, major changes in reporting practices occurring abruptly over the past year are unlikely. As such, we believe that our findings apply more broadly and highlight key concerns about reporting practices.
Our findings call for renewed attention to the widespread lack of reporting of results relevant to the role of sex and gender in COVID-19 vaccine effectiveness observational studies and the need for a substantial paradigm change. Simple, yet highly impactful, steps can be taken to standardize and normalize this practice in the research community. For example, accounting for sex and gender can be made mandatory for any manuscript submitted for publication, similar to ethics statements or funding disclosure requirements. Our review provides solid evidence that additional efforts are needed urgently to improve reporting of sex-related differences in responses to vaccines, including but not limited to those aimed at preventing COVID-19 and its complications.
Data availability
Our review protocol is publicly available in the International Prospective Register of Systematic Reviews (PROSPERO; identifier: CRD42021289263), and our fully reproducible search strategies are provided in the supplementary material. Template data collection forms can be obtained from the corresponding author upon reasonably motivated request. Data extracted from included studies are reported in the article and its supplementary material along with references of all studies included and excluded after full-text review.
References
Arnegard, M. E., Whitten, L. A., Hunter, C. & Clayton, J. A. Sex as a biological variable: a 5-year progress report and call to action. J. Womens Health (Larchmt) 29, 858–864 (2020).
Lee, H. et al. It is time to integrate sex as a variable in preclinical and clinical studies. Exp. Mol. Med. 50, 1–2 (2018).
Canadian Institutes of Health Research - Institute of Gender and Health. Online Training Modules. Integrating Sex and Gender in Health Research. Available at: http://www.cihr-irsc.gc.ca/e/49347.html. [Accessed: December 20, 2022].
National Institutes of Health (NIH). NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research (2001). Available at: https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm. [Accessed: December 20, 2022].
Gendered Innovations. Policy timeline: Key Governmental, Agency, and Institutional Policies related to Sex and Gender Research (2020). Available at: http://genderedinnovations.stanford.edu/policy/timeline.html. [Accessed: December 20, 2022].
Heidari, S., Babor, T. F., De Castro, P., Tort, S. & Curno, M. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res. Integr. Peer Rev. 1, 2 (2016).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Zucker, I. & Prendergast, B. J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 11, 32 (2020).
Flanagan, K. L., Fink, A. L., Plebanski, M. & Klein, S. L. Sex and gender differences in the outcomes of vaccination over the life course. Annu. Rev. Cell Dev. Biol. 33, 577–599 (2017).
Harris, T., Nair, J., Fediurek, J. & Deeks, S. L. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012-15. Vaccine 35, 2600–2604 (2017).
Tadount, F. et al. Is there a difference in the immune response, efficacy, effectiveness and safety of seasonal influenza vaccine in males and females? A systematic review. Vaccine 38, 444–459 (2020).
Falahi, S. & Kenarkoohi, A. Host factors and vaccine efficacy: Implications for COVID-19 vaccines. J. Med. Virol. 94, 1330–1335 (2022).
Wang, J., Tong, Y., Li, D., Li, J. & Li, Y. The impact of age difference on the efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis. Front Immunol. 12, 758294 (2021).
McCartney, P. R. Sex-based vaccine response in the context of COVID-19. J. Obstet. Gynecol. Neonatal Nurs. 49, 405–408 (2020).
Vijayasingham, L., Bischof, E. & Wolfe, J. Sex-disaggregated data in COVID-19 vaccine trials. Lancet 397, 966–967 (2021).
Peckham, H. et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 11, 6317 (2020).
Basta, N. E., Moodie, E. M. M. & the VIPER (Vaccines, Infectious disease Prevention, and Epidemiology Research) Group COVID-19 Vaccine Development and Approvals Tracker Team. COVID-19 Vaccine Development and Approvals Tracker. (2020). Last updated: December 2, 2022. Available at: https://covid19.trackvaccines.org. [Accessed: December 2, 2022].
Heidari, S., Palmer-Ross, A. & Goodman, T. A Systematic review of the sex and gender reporting in COVID-19 Clinical Trials. Vaccines (Basel) 9 https://doi.org/10.3390/vaccines9111322 (2021).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71 (2021).
Haddaway, N. R., Page, M. J., Pritchard, C. C. & McGuinness, L. A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Systematic Reviews 18, e1230 (2022).
Sterne, J. A. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj 355, i4919 (2016).
UN Department of Economic and Social Affairs. Definition of Regions. (United Nations Department of Economic and Social Affairs, Population Division. Available at: https://population.un.org/wpp/DefinitionOfRegions/, New York, NY, USA). [Accessed: December 20, 2022].
The World Bank. World Bank Country and Lending Groups. (2020). Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. [Accessed: December 20, 2022].
Vander Weele, T. J. Confounding and effect modification: distribution and measure. Epidemiol. Methods 1, 55–82 (2012).
Takahashi, T. et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 588, 315–320 (2020).
Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 27, 28–33 (2021).
Zintel, S. et al. Gender differences in the intention to get vaccinated against COVID-19: a systematic review and meta-analysis. Z Gesundh Wiss, 1–25 https://doi.org/10.1007/s10389-021-01677-w (2022).
Wendt, A. et al. Children of more empowered women are less likely to be left without vaccination in low- and middle-income countries: a global analysis of 50 DHS surveys. J. Glob. Health 12, 04022 (2022).
Acknowledgements
This work was supported by research funds from the Canada Research Chair (CRC) Program [PI: Nicole E. Basta, CRC in Infectious Disease Prevention (Tier 2)]. Research reported in this publication was also supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health (https://www.niaid.nih.gov) under Award Number R01AI132496 (PI: Dr. Nicole E. Basta). The content is solely the responsibility of the authors and does not necessarily represent the official views of any funding agency. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
G.S. designed the study with critical feedback from V.R., J.K., A.P., M.H., A.U., and N.E.B. G.G. developed the search strategy in consultation with G.S. G.S., V.R., J.K., A.P., M.H., and A.U. screened the studies and extracted the data. G.S., V.R., J.K., A.P., and M.H. conducted the risk of bias assessment of eligible studies. G.S. synthesized and interpreted the data, with input from V.R., J.K., A.P., and N.E.B. G.S. wrote the initial draft. G.S., V.R., J.K., A.P., G.G., M.H., A.U., and N.E.B. revised the manuscript until finalization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sulis, G., Kim, J.Y., Rodrigue, V. et al. Sex-disaggregated effectiveness data reporting in COVID-19 vaccine research: a systematic review. Commun Med 3, 69 (2023). https://doi.org/10.1038/s43856-023-00297-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-023-00297-7