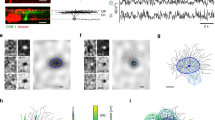
Abstract
The brain is an intricate assembly of intercommunicating neurons whose input–output function is only partially understood. The role of active dendrites in shaping spiking responses, in particular, is unclear. Although existing models account for active dendrites and spiking responses, they are too complex to analyze analytically and demand long stochastic simulations. Here we combine cable and renewal theory to describe how input fluctuations shape the response of neuronal ensembles with active dendrites. We found that dendritic input readily and potently controls interspike interval dispersion. This phenomenon can be understood by considering that neurons display three fundamental operating regimes: one mean-driven regime and two fluctuation-driven regimes. We show that these results are expected to appear for a wide range of dendritic properties and verify predictions of the model in experimental data. These findings have implications for the role of interspike interval dispersion in learning and for theories of attractor states.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The raw electrophysiology data can be obtained by writing to the corresponding author, who will seek the permission of the data owners (Matthew Larkum and Christine Grienberger). Source data are provided with this paper. Source data used in Fig. 1, Extended Data Fig. 1 and Supplementary Figs. 1, 3 and 4 can also be generated using the publicly available code.
Code availability
All code used in this manuscript is publicly available on Code Ocean (ref. 38) and on GitHub at https://github.com/ZachFriedenberger/dendritic-renewal-theory.
References
Brunel, N. Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. J. Comput. Neurosci. 8, 183–208 (2000).
van Vreeswijk, C. Stability of the asynchronous state in networks of non-linear oscillators. Phys. Rev. Lett. 84, 5110 (2000).
Renart, A., Moreno-Bote, R., Wang, X.-J. & Parga, N. Mean-driven and fluctuation-driven persistent activity in recurrent networks. Neural Comput. 19, 1–46 (2007).
Schwalger, T., Deger, M. & Gerstner, W. Towards a theory of cortical columns: from spiking neurons to interacting neural populations of finite size. PLoS Comput. Biol. 13, e1005507 (2017).
Tiberi, L. et al. Gell-Mann–Low criticality in neural networks. Phys. Rev. Lett. 128, 168301 (2022).
Ricciardi, L. M. Diffusion Processes and Related Topics in Biology (Springer, 1977).
Renart, A., Song, P. & Wang, X. J. Robust spatial working memory through homeostatic synaptic scaling in heterogeneous cortical networks. Neuron 38, 473–485 (2003).
Vilela, R. D. & Lindner, B. Comparative study of different integrate-and-fire neurons: spontaneous activity, dynamical response and stimulus-induced correlation. Phys. Rev. E 80, 031909 (2009).
Rauch, A., Camera, G. L., Luscher, H., Senn, W. & Fusi, S. Neocortical pyramidal cells respond as integrate-and-fire neurons to in vivo-like input currents. J. Neurophysiol. 90, 1598–1612 (2003).
Mensi, S., Naud, R., Avermann, M., Petersen, C. C. H. & Gerstner, W. Parameter extraction and classification of three neuron types reveals two different adaptation mechanisms. J. Neurophysiol. 107, 1756–1775 (2012).
Mel, B. W. A connectionist model may shed light on neural mechanisms for visually guided reaching. J. Cogn. Neurosci. 3, 273–292 (1991).
Poirazi, P. & Mel, B. W. Choice and value flexibility jointly contribute to the capacity of a subsampled quadratic classifier. Neural Comput. 12, 1189–1205 (2000).
Schiller, J., Major, G., Koester, H. J. & Schiller, Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature 404, 285–289 (2000).
Larkum, M., Zhu, J. & Sakmann, B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 398, 338–341 (1999).
Magee, J. C. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat. Neurosci. 2, 508–514 (1999).
Judák, L. et al. Sharp-wave ripple doublets induce complex dendritic spikes in parvalbumin interneurons in vivo. Nat. Commun. 13, 6715 (2022).
Larkum, M. E., Senn, W. & Luscher, H.-R. Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb. Cortex 14, 1059–1070 (2004).
Poirazi, P., Brannon, T. & Mel, B. W. Pyramidal neuron as two-layer neural network. Neuron 37, 989–999 (2003).
Ujfalussy, B. B., Makara, J. K., Lengyel, M. & Branco, T. Global and multiplexed dendritic computations under in vivo-like conditions. Neuron 100, 579–592 (2018).
Dembrow, N. C. & Spain, W. J. Input rate encoding and gain control in dendrites of neocortical pyramidal neurons. Cell Rep. 38, 110382 (2022).
Harkin, E. F., Shen, P. R., Goel, A., Richards, B. A. & Naud, R. Parallel and recurrent cascade models as a unifying force for understanding subcellular computation. Neuroscience 489, 200–215 (2022).
Magó, Á., Kis, N., Lüko, B. & Makara, J. K. Distinct dendritic Ca2+ spike forms produce opposing input-output transformations in rat CA3 pyramidal cells. eLife 10, e74493 (2021).
Polsky, A., Mel, B. & Schiller, J. Encoding and decoding bursts by NMDA spikes in basal dendrites of layer 5 pyramidal neurons. J. Neurosci. 29, 11891–11903 (2009).
Xu, N.-l. et al. Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature 492, 247–251 (2012).
Rall, W. & Rinzel, J. Branch input resistance and steady attenuation for input to one branch of a dendritic neuron model. Biophys. J. 13, 648–687 (1973).
Rall, W. in Methods in Neuronal Modeling (eds Koch, C. & Segev, I.) 9–62 (MIT Press, 1989).
Pillow, J. et al. Spatio-temporal correlations and visual signalling in a complete neuronal population. Nature 454, 995–999 (2008).
Pozzorini, C., Naud, R., Mensi, S. & Gerstner, W. Temporal whitening by power-law adaptation in neocortical neurons. Nat. Neurosci. 16, 942–948 (2013).
Gerstner, W. Time structure of the activity in neural network models. Phys. Rev. E 51, 738–758 (1995).
Naud, R. & Gerstner, W. Coding and decoding with adapting neurons: a population approach to the peri-stimulus time histogram. PLoS Comput. Biol. 8, e1002711 (2012).
Senzai, Y., Fernandez-Ruiz, A. & Buzsáki, G. Layer-specific physiological features and interlaminar interactions in the primary visual cortex of the mouse. Neuron 101, 500–513 (2019).
Holt, G. R., Softky, W. R., Koch, C. & Douglas, R. J. Comparison of discharge variability in vitro and in vivo in cat visual cortex neurons. J. Neurophysiol. 75, 1806–1814 (1996).
Grienberger, C., Chen, X. & Konnerth, A. NMDA receptor-dependent multidendrite Ca2+ spikes required for hippocampal burst firing in vivo. Neuron 81, 1274–1281 (2014).
Compte, A. et al. Temporally irregular mnemonic persistent activity in prefrontal neurons of monkeys during a delayed response task. J. Neurophysiol. 90, 3441–3454 (2003).
Barbieri, F. & Brunel, N. Can attractor network models account for the statistics of firing during persistent activity in prefrontal cortex?. Front. Neurosci. 2, 114–122 (2008).
Prescott, S. A. & De Koninck, Y. Gain control of firing rate by shunting inhibition: roles of synaptic noise and dendritic saturation. Proc. Natl Acad. Sci. USA 100, 2076–2081 (2003).
Jarsky, T., Roxin, A., Kath, W. L. & Spruston, N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat. Neurosci. 8, 1667–1676 (2005).
Friedenberger, Z. & Naud, R. Dendritic Excitability Controls Overdispersion [code] https://doi.org/10.24433/CO.9810370.v1 (2023).
Acknowledgements
We thank all members of the Neural Coding Lab for helpful discussions regarding the manuscript. This work was supported by an NSERC Discovery Grant (to R.N., RGPIN-2017-06872) and an NSERC PGS-D Scholarship (to Z.F.).
Author information
Authors and Affiliations
Contributions
Z.F. provided conceptualization, writing, mathematical derivations, simulations and data analysis. R.N. provided conceptualization, writing and supervision. All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Computational Science thanks Maria Psarrou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ananya Rastogi, in collaboration with the Nature Computational Science team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Dendritic control of overdispersion is observed for a wide range of dendritic spike amplitudes and durations.
Dendritic input controls frequency shift, gain, and overdispersion. a Schematic of the parametrization of dendritic input modulation in terms of the shift (a) and gain (g) of the f-I curve, as well as overdispersion (c). b-d Heatmaps of the effect on shift (a), gain (g) and overdispersion (c) for different amplitude and duration of the dendritic spikes. The color scale bar corresponds to the dimensionless values of (a), (g), and (c). The undefined region in c-d corresponds to a region where our definitions of gain and dispersion break down (see Methods section ‘Effect of the dendritic spike amplitude and duration’).
Supplementary information
Source data
Source Data Fig. 1
CSV files for the data in Fig. 1.
Source Data Fig. 2
CSV files for the data in Fig. 2.
Source Data Extended Data Fig. 1
CSV files for the data in Extended Data Fig. 1.
Rights and permissions
About this article
Cite this article
Friedenberger, Z., Naud, R. Dendritic excitability controls overdispersion. Nat Comput Sci 4, 19–28 (2024). https://doi.org/10.1038/s43588-023-00580-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43588-023-00580-6