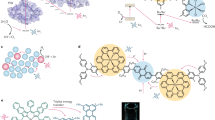
Abstract
Artificial photosynthesis is an attractive strategy for converting solar energy into fuels, largely because the Earth receives enough solar energy in one hour to meet humanity’s energy needs for an entire year. However, developing devices for artificial photosynthesis remains difficult and requires computational approaches to guide and assist the interpretation of experiments. In this Perspective, we discuss current and future computational approaches, as well as the challenges of designing and characterizing molecular assemblies that absorb solar light, transfer electrons between interfaces, and catalyze water-splitting and fuel-forming reactions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cramer, C. J. & Truhlar, D. G. Density functional theory for transition metals and transition metal chemistry. Phys. Chem. Chem. Phys. 11, 10757–10816 (2009).
Pacchioni, G. Modeling doped and defective oxides in catalysis with density functional theory methods: room for improvements. J. Chem. Phys. 128, 182505 (2008).
Scanlon, D. O., Morgan, B. J. & Watson, G. W. Modeling the polaronic nature of p-type defects in Cu2O: The failure of GGA and GGA+U. J. Chem. Phys. 131, 124703 (2009).
Casida, M. E. & Huix-Rotllant, M. in Density-Functional Methods for Excited States (eds Ferré, N. et al.) 1–60 (Springer, 2016).
Young, K. J. et al. Light-driven water oxidation for solar fuels. Coord. Chem. Rev. 256, 2503–2520 (2012).
Negre, C. F. A. et al. Efficiency of interfacial electron transfer from Zn-porphyrin dyes into TiO2 correlated to the linker single molecule conductance. J. Phys. Chem. C 117, 24462–24470 (2013).
Sivula, K. & van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 1, 15010 (2016).
Rego, L. G. C. & Batista, V. S. Quantum dynamics simulations of interfacial electron transfer in sensitized TiO2 semiconductors. J. Am. Chem. Soc. 125, 7989–7997 (2003).
Yang, K. R. et al. Solution structures of highly active molecular Ir water-oxidation catalysts from density functional theory combined with high-energy X-ray scattering and EXAFS spectroscopy. J. Am. Chem. Soc. 138, 5511–5514 (2016).
Doan, H. A. et al. Quantum chemistry-informed active learning to accelerate the design and discovery of sustainable energy storage materials. Chem. Mater. 32, 6338–6346 (2020).
Yao, Z. et al. Machine learning for a sustainable energy future. Nat. Rev. Mater. 8, 202–215 (2022).
Liu, J., Wan, L., Li, Z. & Yang, J. Simulating periodic systems on a quantum computer using molecular orbitals. J. Chem. Theory Comput. 16, 6904–6914 (2020).
Mizuta, K. et al. Deep variational quantum eigensolver for excited states and its application to quantum chemistry calculation of periodic materials. Phys. Rev. Res. 3, 043121 (2021).
Cao, Y. et al. Quantum chemistry in the age of quantum computing. Chem. Rev. 119, 10856–10915 (2019).
López, I. et al. A self‐improved water‐oxidation catalyst: is one site really enough? Angew. Chem. Int. Ed. 53, 205–209 (2014).
Heyd, J., Peralta, J. E., Scuseria, G. E. & Martin, R. L. Energy band gaps and lattice parameters evaluated with the Heyd-Scuseria-Ernzerhof screened hybrid functional. J. Chem. Phys. 123, 174101 (2005).
Wang, L., Long, R. & Prezhdo, O. V. Time-domain ab initio modeling of photoinduced dynamics at nanoscale interfaces. Annu. Rev. Phys. Chem. 66, 549–579 (2015).
González, L. & Lindh, R. Quantum Chemistry and Dynamics of Excited States: Methods and Applications (John Wiley and Sons, 2020).
Man, I. C. et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3, 1159–1165 (2011).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Stowasser, R. & Hoffmann, R. What do the Kohn−Sham orbitals and eigenvalues mean? J. Am. Chem. Soc. 121, 3414–3420 (1999).
Cohen, A. J., Mori-Sánchez, P. & Yang, W. Insights into current limitations of density functional theory. Science 321, 792–794 (2008).
Anisimov, V. I., Zaanen, J. & Andersen, O. K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 44, 943–954 (1991).
Liechtenstein, A. I., Anisimov, V. I. & Zaanen, J. Density-functional theory and strong interactions: orbital ordering in Mott-Hubbard insulators. Phys. Rev. B 52, R5467–R5470 (1995).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Liao, P. & Carter, E. A. New concepts and modeling strategies to design and evaluate photo-electro-catalysts based on transition metal oxides. Chem. Soc. Rev. 42, 2401–2422 (2013).
Meng, X. Y. et al. Enhanced photoelectrochemical activity for Cu and Ti doped hematite: the first principles calculations. Appl. Phys. Lett. 98, 112104 (2011).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Hedin, L. New method for calculating the one-particle Green’s function with application to the electron-gas problem. Phys. Rev. 139, A796–A823 (1965).
Kulik, H. et al. Roadmap on machine learning in electronic structure. Electron. Struct. 4, 023004 (2022).
Runge, E. & Gross, E. K. U. Density-functional theory for time-dependent systems. Phys. Rev. Lett. 52, 997–1000 (1984).
Carter-Fenk, K., Cunha, L. A., Arias-Martinez, J. E. & Head-Gordon, M. Electron-affinity time-dependent density functional theory: formalism and applications to core-excited states. J. Phys. Chem. Lett. 13, 9664–9672 (2022).
Hait, D. & Head-Gordon, M. Orbital optimized density functional theory for electronic excited states. J. Phys. Chem. Lett. 12, 4517–4529 (2021).
Kümmel, S. Charge-transfer excitations: a challenge for time-dependent density functional theory that has been met. Adv. Energy Mater. 7, 1700440 (2017).
Salpeter, E. E. & Bethe, H. A. A relativistic equation for bound-state problems. Phys. Rev. 84, 1232–1242 (1951).
Joung, J. F. et al. Deep learning optical spectroscopy based on experimental database: potential applications to molecular design. JACS Au 1, 427–438 (2021).
Abuabara, S. G., Rego, L. G. C. & Batista, V. S. Influence of thermal fluctuations on interfacial electron transfer in functionalized TiO2 semiconductors. J. Am. Chem. Soc. 127, 18234–18242 (2005).
Li, C. et al. Facet-dependent photoelectrochemical performance of TiO2 nanostructures: an experimental and computational study. J. Am. Chem. Soc. 137, 1520–1529 (2015).
Menzel, J. P. et al. Photoinduced electron injection in a fully solvated dye-sensitized photoanode: a dynamical semiempirical study. J. Phys. Chem. C 124, 27965–27976 (2020).
Jiang, J. et al. Molecular design of light-harvesting photosensitizers: effect of varied linker conjugation on interfacial electron transfer. Phys. Chem. Chem. Phys. 18, 18678–18682 (2016).
Liu, C. & Jakubikova, E. Two-step model for ultrafast interfacial electron transfer: limitations of Fermi’s golden rule revealed by quantum dynamics simulations. Chem. Sci. 8, 5979–5991 (2017).
Oliboni, R. S. et al. Vibronic effects in the ultrafast interfacial electron transfer of perylene-sensitized TiO2 surfaces. J. Phys. Chem. C 123, 12599–12607 (2019).
Komsa, H.-P., Broqvist, P. & Pasquarello, A. Alignment of defect levels and band edges through hybrid functionals: effect of screening in the exchange term. Phys. Rev. B 81, 205118 (2010).
Toroker, M. C. et al. First principles scheme to evaluate band edge positions in potential transition metal oxide photocatalysts and photoelectrodes. Phys. Chem. Chem. Phys. 13, 16644–16654 (2011).
Snyder, J. C., Rupp, M., Hansen, K., Müller, K.-R. & Burke, K. Finding density functionals with machine learning. Phys. Rev. Lett. 108, 253002 (2012).
Letchworth-Weaver, K. & Arias, T. A. Joint density functional theory of the electrode-electrolyte interface: application to fixed electrode potentials, interfacial capacitances, and potentials of zero charge. Phys. Rev. B 86, 075140 (2012).
Cheng, J. & Sprik, M. Aligning electronic energy levels at the TiO2/H2O interface. Phys. Rev. B 82, 081406 (2010).
Cramer, C. J. Essentials of Computational Chemistry: Theories and Models (Wiley, 2004).
Tomasi, J., Mennucci, B. & Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 105, 2999–3094 (2005).
Basdogan, Y., Maldonado, A. M. & Keith, J. A. Advances and challenges in modeling solvated reaction mechanisms for renewable fuels and chemicals. WIREs Comput. Mol. Sci. 10, e1446 (2020).
Pliego, J. R. & Riveros, J. M. The cluster−continuum model for the calculation of the solvation free energy of ionic species. J. Phys. Chem. A 105, 7241–7247 (2001).
Jinich, A., Sanchez-Lengeling, B., Ren, H., Harman, R. & Aspuru-Guzik, A. A mixed quantum chemistry/machine learning approach for the fast and accurate prediction of biochemical redox potentials and its large-scale application to 315 000 redox reactions. ACS Cent. Sci. 5, 1199–1210 (2019).
Toyao, T. et al. Machine learning for catalysis informatics: recent applications and prospects. ACS Catal. 10, 2260–2297 (2020).
Freeze, J. G., Kelly, H. R. & Batista, V. S. Search for catalysts by inverse design: artificial intelligence, mountain climbers, and alchemists. Chem. Rev. 119, 6595–6612 (2019).
Xu, Y., Yamazaki, M. & Villars, P. Inorganic materials database for exploring the nature of material. Jpn. J. Appl. Phys. 50, 11RH02 (2011).
Curtarolo, S. et al. AFLOWLIB.ORG: a distributed materials properties repository from high-throughput ab initio calculations. Comput. Mater. Sci. 58, 227–235 (2012).
Belsky, A., Hellenbrandt, M., Karen, V. L. & Luksch, P. New developments in the Inorganic Crystal Structure Database (ICSD): accessibility in support of materials research and design. Acta Crystallogr. Sect. B 58, 364–369 (2002).
Jain, A. et al. The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Saal, J. E., Kirklin, S., Aykol, M., Meredig, B. & Wolverton, C. Materials design and discovery with high-throughput density functional theory: the open quantum materials database (OQMD). JOM 65, 1501–1509 (2013).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015).
Palkovits, R. & Palkovits, S. Using artificial intelligence to forecast water oxidation catalysts. ACS Catal. 9, 8383–8387 (2019).
Guo, Y. et al. Machine-learning-guided discovery and optimization of additives in preparing Cu catalysts for CO2 reduction. J. Am. Chem. Soc. 143, 5755–5762 (2021).
Zheng, J. et al. Symbolic transformer accelerating machine learning screening of hydrogen and deuterium evolution reaction catalysts in MA2Z4 materials. ACS Appl. Mater. Interfaces 13, 50878–50891 (2021).
Takigawa, I., Shimizu, K.-i, Tsuda, K. & Takakusagi, S. Machine-learning prediction of the d-band center for metals and bimetals. RSC Adv. 6, 52587–52595 (2016).
Zhang, Y. & Xu, X. Machine learning band gaps of doped-TiO2 photocatalysts from structural and morphological parameters. ACS Omega 5, 15344–15352 (2020).
Spiegelman, F. et al. Density-functional tight-binding: basic concepts and applications to molecules and clusters. Adv. Phys. X 5, 1710252 (2020).
Senftle, T. P. et al. The ReaxFF reactive force-field: development, applications and future directions. NPJ Comput. Mater. 2, 15011 (2016).
Kmiecik, S. et al. Coarse-grained protein models and their applications. Chem. Rev. 116, 7898–7936 (2016).
Samanta, B. et al. Challenges of modeling nanostructured materials for photocatalytic water splitting. Chem. Soc. Rev. 51, 3794–3818 (2022).
Blase, X., Duchemin, I. & Jacquemin, D. The Bethe–Salpeter equation in chemistry: relations with TD-DFT, applications and challenges. Chem. Soc. Rev. 47, 1022–1043 (2018).
Wiktor, J., Reshetnyak, I., Ambrosio, F. & Pasquarello, A. Comprehensive modeling of the band gap and absorption spectrum of BiVO4. Phys. Rev. Mater. 1, 022401 (2017).
da Silva Oliboni, R., Bortolini, G., Torres, A. & Rego, L. G. C. A nonadiabatic excited state molecular Mechanics/Extended Hückel Ehrenfest method. J. Phys. Chem. C 120, 27688–27698 (2016).
Greene, S. M. & Batista, V. S. Tensor-train split-operator fourier transform (TT-SOFT) method: multidimensional nonadiabatic quantum dynamics. J. Chem. Theory Comput. 13, 4034–4042 (2017).
Westermayr, J. & Marquetand, P. Machine learning for electronically excited states of molecules. Chem. Rev. 121, 9873–9926 (2021).
Rudshteyn, B. et al. Calculation of metallocene ionization potentials via auxiliary field quantum Monte Carlo: toward benchmark quantum chemistry for transition metals. J. Chem. Theory Comput. 18, 2845–2862 (2022).
Gaggioli, C. A., Stoneburner, S. J., Cramer, C. J. & Gagliardi, L. Beyond density functional theory: the multiconfigurational approach to model heterogeneous catalysis. ACS Catal. 9, 8481–8502 (2019).
Szalay, S. et al. Tensor product methods and entanglement optimization for ab initio quantum chemistry. Int. J. Quantum Chem. 115, 1342–1391 (2015).
Kirkpatrick, J. et al. Pushing the frontiers of density functionals by solving the fractional electron problem. Science 374, 1385–1389 (2021).
Guda, A. A. et al. Understanding X-ray absorption spectra by means of descriptors and machine learning algorithms. NPJ Comput. Mater. 7, 203 (2021).
Behler, J. Machine learning potentials for atomistic simulations. J. Chem. Phys. 145, 170901 (2016).
Masood, H., Toe, C. Y., Teoh, W. Y., Sethu, V. & Amal, R. Machine learning for accelerated discovery of solar photocatalysts. ACS Catal. 9, 11774–11787 (2019).
Chen, Z., Bononi, F. C., Sievers, C. A., Kong, W.-Y. & Donadio, D. UV–visible absorption spectra of solvated molecules by quantum chemical machine learning. J. Chem. Theory Comput. 18, 4891–4902 (2022).
Hruska, E., Gale, A. & Liu, F. Bridging the experiment-calculation divide: machine learning corrections to redox potential calculations in implicit and explicit solvent models. J. Chem. Theory Comput. 18, 1096–1108 (2022).
Seritan, S. & Martinez, T. OMSC 2019 nonadiabatic dynamics study of the B850-B800 complex in LH2 using TeraChem Cloud. VIRT&L-COMM 19, VIRT&L-COMM.19.2019.21 (2019).
Acknowledgements
The authors acknowledge support from the Photosynthetic Systems Program, Office of Basic Energy Sciences, United States Department of Energy, under contract no. DESC0001423 (V.S.B.). We thank the National Energy Research Scientific Computing Center and the Yale Center for Research Computing for computing time. We also acknowledge support from the National Science Foundation under grant 2124511 (Centers for Chemical Innovation Phase I: National Science Foundation Center for Quantum Dynamics on Modular Quantum Devices) for the development of quantum computing algorithms applied to photosynthetic systems.
Author information
Authors and Affiliations
Contributions
K.R.Y., G.W.K. and V.S.B. reviewed the relevant material, brainstormed solutions and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Peer review
Peer review information
Nature Computational Science thanks Dimitrios Pantazis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Kaitlin McCardle, in collaboration with the Nature Computational Science team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, K.R., Kyro, G.W. & Batista, V.S. The landscape of computational approaches for artificial photosynthesis. Nat Comput Sci 3, 504–513 (2023). https://doi.org/10.1038/s43588-023-00450-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43588-023-00450-1