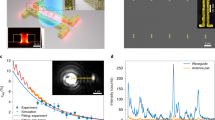
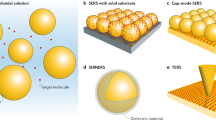
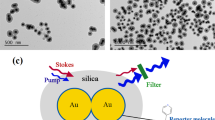
Abstract
Waveguide-enhanced Raman spectroscopy (WERS) exploits the electromagnetic enhancement that can be achieved at the surface of suitably designed waveguides to enhance the intensity of the Raman spectra of molecules close to the waveguide surface. This Primer describes practical aspects of WERS implementation including the choice of laser, choice of waveguide material, design and fabrication of the waveguides, coupling of light into and collection of light from the waveguide, and choice of spectrometer and filters. The methods for data collection and quantitative analysis of waveguide-enhanced Raman spectra are also described, together with the applications of WERS to problems in chemistry, materials science and bioscience. Issues of spectral reproducibility and key optimization factors are discussed together with a summary of technical limitations, current challenges and perspectives for future research. In many cases the material presented is supported by further, more detailed, discussion in the accompanying Supplementary Information.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fleischmann, M., Hendra, P. J. & McQuillan, A. J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 26, 163–166 (1974).
Jeanmaire, D. L. & Van Duyne, R. P. Surface Raman spectroelectrochemistry: part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. 84, 1–20 (1977).
Langer, J. et al. Present and future of surface-enhanced Raman scattering. ACS Nano 14, 28–117 (2019).
Smith, W. Practical understanding and use of surface enhanced Raman scattering/surface enhanced resonance Raman scattering in chemical and biological analysis. Chem. Soc. Rev. 37, 955–964 (2008).
Han, X. X., Rodriguez, R. S., Haynes, C. L., Ozaki, Y. & Zhao, B. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Primers 1, 87 (2022).
Lee, K. S. et al. Raman microspectroscopy for microbiology. Nat. Rev. Methods Primers 1, 80 (2021).
Walrafen, G. E. & Stone, J. Intensification of spontaneous Raman spectra by use of liquid core optical fibers. Appl. Spectrosc. 26, 585–589 (1972).
Levy, Y., Imbert, C., Cipriani, J., Racine, S. & Dupeyrat, R. Raman-scattering of thin-films as a waveguide. Opt. Commun. 11, 66–69 (1974).
Kanger, J. S., Otto, C., Slotboom, M. & Greve, J. Waveguide Raman spectroscopy of thin polymer layers and monolayers of biomolecules using high refractive index waveguides. J. Phys. Chem. 100, 3288–3292 (1996). This work is an early description of the optimization of waveguides for WERS and of WERS of biomolecular monolayers.
Dhakal, A. et al. Evanescent excitation and collection of spontaneous Raman spectra using silicon nitride nanophotonic waveguides. Opt. Lett. 39, 4025–4028 (2014).
Praveen, B. B., Steuwe, C., Mazilu, M., Dholakia, K. & Mahajan, S. Wavelength modulated surface enhanced (resonance) Raman scattering for background-free detection. Analyst 138, 2816–2820 (2013).
Alberti, S., Datta, A. & Jagerska, J. Integrated nanophotonic waveguide-based devices for IR and Raman gas spectroscopy. Sensors 21, 7224 (2021).
Raza, A. et al. High index contrast photonic platforms for on-chip Raman spectroscopy. Opt. Express 27, 23067–23079 (2019). This work describes WERS conversion efficiency and the WERS background from various waveguide materials to provide a FOM for optimized waveguides in terms of the SNR.
Evans, C. C., Liu, C. & Suntivich, J. TiO2 nanophotonic sensors for efficient integrated evanescent Raman spectroscopy. ACS Photonics 3, 1662–1669 (2016).
Jung, H. et al. Tantala Kerr nonlinear integrated photonics. Optica 8, 811–817 (2021).
Tyndall, N. F. et al. A low-loss, broadband, nitride-only photonic integrated circuit platform. in Quantum 2.0 Conference and Exhibition QTu4B.5 (Optica Publishing Group, 2022).
Le Thomas, N., Liu, Z., Lin, C., Zhao, H. & Baets, R. in Proc. SPIE Vol. 11689 (SPIE, 2021).
Michon, J., Kita, D. & Hu, J. J. Sensitivity comparison of free-space and waveguide Raman for bulk sensing. J. Opt. Soc. Am. B 37, 2012–2020 (2020). This work compares conventional free-space Raman, SERS and WERS in terms of the SNR, and therefore the LoD, for bulk analytes in contrast to monolayer and thin-film analytes.
Le Thomas, N., Dhakal, A., Raza, A., Peyskens, F. & Baets, R. Impact of fundamental thermodynamic fluctuations on light propagating in photonic waveguides made of amorphous materials. Optica 5, 328–336 (2018). This work proposes and characterizes thermodynamic fluctuations in amorphous waveguides as producing the WERS background which poses a fundamental limit to the WERS LoD.
Makela, M. et al. Benzene derivatives analysis using aluminum nitride waveguide raman sensors. Anal. Chem. 92, 8917–8922 (2020).
Dhakal, A. et al. Efficiency of evanescent excitation and collection of spontaneous Raman scattering near high index contrast channel waveguides. Opt. Express 23, 27391–27404 (2015). This work advances a theoretical model for the conversion efficiency in spontaneous WERS and applies it to various waveguide structures and materials with good experimental agreement.
Osgood, R. & Meng, X. Principles of Photonic Integrated Circuits: Materials, Device Physics, Guided Wave Design (Springer International, 2021).
Dhakal, A. et al. Single mode waveguide platform for spontaneous and surface-enhanced on-chip Raman spectroscopy. Interface Focus. 6, 20160015 (2016).
Fourkas, J. T. et al. Grand challenges in nanofabrication: there remains plenty of room at the bottom. Front. Nanotechnol. 3, 700849 (2021).
Wang, Z., Zervas, M. N., Bartlett, P. N. & Wilkinson, J. S. Surface and waveguide collection of Raman emission in waveguide-enhanced Raman spectroscopy. Opt. Lett. 41, 4146–4149 (2016).
Ettabib, M. A., Liu, Z., Zervas, M. N. & Wilkinson, J. S. Optimized design for grating-coupled waveguide-enhanced Raman spectroscopy. Opt. Express 28, 37226–37235 (2020).
Jones, R. R., Hooper, D. C., Zhang, L., Wolverson, D. & Valev, V. K. Raman techniques: fundamentals and frontiers. Nanoscale Res. Lett. 14, 231 (2019).
Pelletier, M. J. & Pelletier, C. C. in Encyclopedia of Analytical Science 2nd edn (eds Worsfold, P., Townshend, A. & Poole, C.) 94–104 (Elsevier, 2005).
IDEX Health & Science. What optical filters should you consider for Raman spectroscopy applications? IDEX Health & Science https://www.idex-hs.com/news-events/stories-and-features/detail/raman-spectroscopy-optical-filters (2023).
Bishop, E. Raman Spectroscopy Identifies Disease Characteristics and In Vitro Structure. Photonics https://www.photonics.com/Articles/Raman_Spectroscopy_Identifies_Disease/a68649 (2023).
Bērziņš, K., Fraser-Miller, S. J. & Gordon, K. C. Recent advances in low-frequency Raman spectroscopy for pharmaceutical applications. Int. J. Pharm. 592, 120034 (2021).
Dhakal, A. Nanophotonic Waveguide Enhanced Raman Spectroscopy. Doctoral dissertation, Ghent Univ. (2016).
Tuschel, D. Selecting an excitation wavelength for Raman spectroscopy. Spectroscopy 31, 14–23 (2016).
Mahajan, S. et al. Understanding the surface-enhanced Raman spectroscopy “background”. J. Phys. Chem. C. 114, 7242–7250 (2010).
Kita, D. M., Michon, J. & Hu, J. A packaged, fiber-coupled waveguide-enhanced Raman spectroscopic sensor. Opt. Express 28, 14963–14972 (2020). This work reports a fibre-pigtailed WERS chip with on-chip optical filters to minimize background emission from fibres and waveguides reaching the detector.
Reynkens, K. et al. Mitigation of photon background in nanoplasmonic all-on-chip Raman sensors. Opt. Express 28, 33564–33572 (2020).
Tyndall, N. F., Stievater, T. H., Kozak, D. A., Pruessner, M. W. & Rabinovich, W. S. Passive photonic integration of lattice filters for waveguide-enhanced Raman spectroscopy. Opt. Express 28, 34927–34934 (2020).
Dhakal, A. et al. Nanophotonic waveguide enhanced Raman spectroscopy of biological submonolayers. ACS Photonics 3, 2141–2149 (2016). This work demonstrates real-time measurements of the hybridization of DNA strands and the density of sub-monolayers of biotin−streptavidin complex using near-infrared WERS.
Tyndall, N. F. et al. Figure-of-merit characterization of hydrogen-bond acidic sorbents for waveguide-enhanced Raman spectroscopy. ACS Sens. 5, 831–836 (2020). This work demonstrates the application of WERS for trace-level gas phase analyte detection using sorbent coatings.
Lee, W. et al. Study on multiple waveguide platforms for waveguide integrated Raman spectroscopy. OSA Contin. 3, 1322–1333 (2020).
Midwinter, J. E. On the use of optical waveguide techniques for internal reflection spectroscopy. IEEE J. Quantum Electron. 7, 339–344 (1971).
Kanger, J. S. & Otto, C. Orientation effects in waveguide resonance Raman spectroscopy of monolayers. Appl. Spectrosc. 57, 1487–1493 (2003). This work is a theoretical and experimental study of polarization-resolved WERS using Raman depolarization ratios to determine molecular orientation in a monolayer at a waveguide surface.
Tyndall, N. F. et al. Waveguide-enhanced Raman spectroscopy of trace chemical warfare agent simulants. Opt. Lett. 43, 4803–4806 (2018).
Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 57, 783–826 (1985).
Long, D. A. The Raman Effect: A Unified Treatment of the Theory of Raman Scattering by Molecules 85–152 (Wiley, 2002).
Le Ru, E. C. & Etchegoin, P. G. Principles of Surface-Enhanced Raman Spectroscopy (Elsevier, 2009).
Zhao, H. L. et al. Multiplex volatile organic compound Raman sensing with nanophotonic slot waveguides functionalized with a mesoporous enrichment layer. Opt. Lett. 45, 447–450 (2020).
Lasch, P. Spectral pre-processing for biomedical vibrational spectroscopy and microspectroscopic imaging. Chemom. Intel. Lab. Syst. 117, 100–114 (2012).
Li, S. & Dai, L. An improved algorithm to remove cosmic spikes in Raman spectra for online monitoring. Appl. Spectrosc. 65, 1300–1306 (2011).
Barton, S. J. & Hennelly, B. M. Signal to noise ratio of Raman spectra of biological samples. in Biophotonics: Photonic Solutions for Better Health Care VI (eds Popp, J., Tuchin, V.V. & Pavone, F.S.) 106854F-1–106854F-11 (SPIE, 2018).
Ehrentreich, F. & Sümmchen, L. Spike removal and denoising of Raman spectra by wavelet transform methods. Anal. Chem. 73, 4364–4373 (2001).
Katsumoto, Y. & Ozaki, Y. Practical algorithm for reducing convex spike noises on a spectrum. Appl. Spectrosc. 57, 317–322 (2003).
Takeuchi, H., Hashimoto, S. & Harada, I. Simple and efficient method to eliminate spike noise from spectra recorded on charge-coupled device detectors. Appl. Spectrosc. 47, 129–131 (1993).
Gautam, R., Vanga, S., Ariese, F. & Umapathy, S. Review of multidimensional data processing approaches for Raman and infrared spectroscopy. EPJ Tech. Instrum. 2, 1–38 (2015). This work presents an extensive overview of diverse methods employed in the processing of multidimensional data acquired through Raman and infrared spectroscopy, valuable for WERS data processing.
Zhao, X., Liu, G., Sui, Y., Xu, M. & Tong, L. Denoising method for Raman spectra with low signal-to-noise ratio based on feature extraction. Spectrochim. Acta A 250, 119374 (2021).
Člupek, M., Matějka, P. & Volka, K. Noise reduction in Raman spectra: finite impulse response filtration versus Savitzky–Golay smoothing. J. Raman Spectrosc. 38, 1174–1179 (2007).
Chen, H., Xu, W., Broderick, N. & Han, J. An adaptive denoising method for Raman spectroscopy based on lifting wavelet transform. J. Raman Spectrosc. 49, 1529–1539 (2018).
Liu, Z. et al. Iterative non-negative constrained deconvolution for waveguide enhanced Raman spectroscopy signal recovery. in SMS/EGF/Sensors/ NanoMed 2021 209 (Setcor, 2021).
Xu, J. et al. High-speed diagnosis of bacterial pathogens at the single cell level by Raman microspectroscopy with machine learning filters and denoising autoencoders. ACS Chem. Biol. 17, 376–385 (2022).
Shreve, A. P., Cherepy, N. J. & Mathies, R. A. Effective rejection of fluorescence interference in Raman spectroscopy using a shifted excitation difference technique. Appl. Spectrosc. 46, 707–711 (1992).
Zhao, J., Carrabba, M. M. & Allen, F. S. Automated fluorescence rejection using shifted excitation Raman difference spectroscopy. App. Spectrosc. 56, 834–845 (2002).
Guo, S., Bocklitz, T. & Popp, J. Optimization of Raman-spectrum baseline correction in biological application. Analyst 141, 2396–2404 (2016).
Peng, J. et al. Asymmetric least squares for multiple spectra baseline correction. Anal. Chim. Acta 683, 63–68 (2010).
Cai, Y., Yang, C., Xu, D. & Gui, W. Baseline correction for Raman spectra using penalized spline smoothing based on vector transformation. Anal. Methods 10, 3525–3533 (2018).
Atakan, A. K., Blass, W. & Jennings, D. Elimination of baseline variations from a recorded spectrum by ultra-low frequency filtering. Appl. Spectrosc. 34, 369–372 (1980).
Hu, Y. et al. A background elimination method based on wavelet transform for Raman spectra. Chemom. Intel. Lab. Syst. 85, 94–101 (2007).
Schulze, G. et al. Investigation of selected baseline removal techniques as candidates for automated implementation. Appl. Spectrosc. 59, 545–574 (2005).
Jirasek, A., Schulze, G., Yu, M., Blades, M. & Turner, R. Accuracy and precision of manual baseline determination. Appl. Spectrosc. 58, 1488–1499 (2004).
Lórenz-Fonfría, V. A. & Padrós, E. Maximum entropy deconvolution of infrared spectra: use of a novel entropy expression without sign restriction. Appl. Spectrosc. 59, 474–486 (2005).
Chan, T. F. & Wong, C.-K. Total variation blind deconvolution. IEEE Trans. Image Process. 7, 370–375 (1998).
Davey, B., Lane, R. & Bates, R. Blind deconvolution of noisy complex-valued image. Opt. Commun. 69, 353–356 (1989).
Liu, T., Liu, H., Zhang, Z. & Liu, S. Nonlocal low-rank-based blind deconvolution of Raman spectroscopy for automatic target recognition. Appl. Opt. 57, 6461–6469 (2018).
Liu, H., Zhang, Z., Sun, J. & Liu, S. Blind spectral deconvolution algorithm for Raman spectrum with Poisson noise. Photonics Res. 2, 168–171 (2014).
Liu, H., Yan, L., Chang, Y., Fang, H. & Zhang, T. Spectral deconvolution and feature extraction with robust adaptive Tikhonov regularization. IEEE Trans. Instrum. Meas. 62, 315–327 (2012).
Rabolt, J. F., Santo, R., Schlotter, N. E. & Swalen, J. D. Integrated-optics and Raman-scattering - molecular-orientation in thin polymer-films and Langmuir–Blodgett monolayers. IBM J. Res. Dev. 26, 209–216 (1982).
Schlotter, N. E. & Rabolt, J. F. Raman-spectroscopy in polymeric thin-film optical-waveguides. 1. Polarized measurements and orientational effects in two-dimensional films. J. Phys. Chem. 88, 2062–2067 (1984).
Hu, D. B. & Qi, Z. M. Refractive-index-enhanced Raman apectroscopy and absorptiometry of ultrathin film overlaid on an optical waveguide. J. Phys. Chem. C. 117, 16175–16181 (2013).
Ellahi, S. & Hester, R. E. Enhanced wave-guide Raman-spectroscopy with thin-films. Analyst 119, 491–495 (1994).
Walker, D. S., Hellinga, H. W., Saavedra, S. S. & Reichert, W. M. Integrated optical wave-guide attenuated total-reflection spectrometry and resonance Raman-spectroscopy adsorbed cytochrome-c. J. Phys. Chem. 97, 10217–10222 (1993).
Zhao, H. L., Clemmen, S., Raza, A. & Baets, R. Stimulated Raman spectroscopy of analytes evanescently probed by a silicon nitride photonic integrated waveguide. Opt. Lett. 43, 1403–1406 (2018).
Qu, J. Y. & Shao, L. Near-infrared Raman instrument for rapid and quantitative measurements of clinically important analytes. Rev. Sci. Instrum. 72, 2717–2723 (2001).
Ashok, P. C., De Luca, A. C., Mazilu, M. & Dholakia, K. Enhanced bioanalyte detection in waveguide confined Raman spectroscopy using wavelength modulation. J. Biophotonics 4, 514–518 (2011).
Qi, D. H. & Berger, A. J. Quantitative concentration measurements of creatinine dissolved in water and urine using Raman spectroscopy and a liquid core optical fiber. J. Biomed. Opt. 10, 031115 (2005).
Yan, D., Popp, J., Pletz, M. W. & Frosch, T. Fiber enhanced Raman sensing of levofloxacin by PCF bandgap-shifting into the visible range. Anal. Methods 10, 586–592 (2018).
Holmstrom, S. A. et al. Trace gas Raman spectroscopy using functionalized waveguides. Optica 3, 891–896 (2016).
Liu, Z. Y. et al. Ultra-sensitive slot-waveguide-enhanced Raman spectroscopy for aqueous solutions of non-polar compounds using a functionalized silicon nitride photonic integrated circuit. Opt. Lett. 46, 1153–1156 (2021).
Ma, Y. M., Liu, H. L., Qian, K., Yang, L. B. & Liu, J. H. A displacement principle for mercury detection by optical waveguide and surface enhanced Raman spectroscopy. J. Colloid Interface Sci. 386, 451–455 (2012).
Choi, J., Lee, K. S., Jung, J. H., Sung, H. J. & Kim, S. S. Integrated real-time optofluidic SERS via a liquid-core/liquid-cladding waveguide. RSC Adv. 5, 922–927 (2015).
Zong, C. et al. Surface-enhanced Raman spectroscopy for bioanalysis: reliability and challenges. Chem. Rev. 118, 4946–4980 (2018).
Losada, J. et al. SERS detection via individual bowtie nanoantennas integrated in Si3N4 waveguides. IEEE J. Quantum Electron. 25, 1–6 (2019).
Chandler, L., Huang, B. & Mu, T. T. A smart handheld Raman spectrometer with cloud and AI deep learning algorithm for mixture analysis. in Proc. SPIE 10983, Next-Generation Spectroscopic Technologies XII (eds Crocombe, R. A., Profeta, L. T. M. & Azad A. K.) 1098308-1–1098308-9 (SPIE, 2019).
Cooman, T., Trejos, T., Romero, A. H. & Arroyo, L. E. Implementing machine learning for the identification and classification of compound and mixtures in portable Raman instruments. Chem. Phys. Lett. 787, 139283 (2022).
Wang, Z., Zong, S., Wu, L., Zhu, D. & Cui, Y. SERS-activated platforms for immunoassay: probes, encoding methods, and applications. Chem. Rev. 117, 7910–7963 (2017).
Ettabib, M. A. et al. Grating-incoupled waveguide-enhanced Raman sensor. PLOS ONE 18, e0284058 (2023).
Diez, M. et al. Direct patterning of polymer optical periodic nanostructures on CYTOP for visible light waveguiding. Opt. Mater. 82, 21–29 (2018).
Kasyutich, V. L. & Markus, W. S. Optimisation of laser linewidth and cavity alignment in off-axis cavity-enhanced absorption spectroscopy. Infrared Phys. Technol. 71, 179–186 (2015).
Maciel, M. P. et al. A tunable wavelength erbium doped fiber ring laser based on mechanically induced long-period fiber gratings. Photonic Fiber Cryst. Devices: Adv. Mater. Innov. Device Appl. IX 9586, 958618 (2015).
Principles of Raman spectroscopy (3) Raman spectroscopy measurements. JASCO https://www.jasco-global.com/principle/principles-of-raman-spectroscopy-3-raman-spectroscopy-measurements/ (2023).
Zhang, J. X. J. & Kazunori H. Molecular Sensors and Nanodevices 2nd edn Ch. 5 231–309 (Academic Press 2019).
Karlsson, H. & Illy, E. How to choose a laser: how to choose a laser for Raman spectroscopy. LaserFocusWorld https://www.laserfocusworld.com/lasers-sources/article/16555207/how-to-choose-a-laser-how-to-choose-a-laser-for-raman-spectroscopy (2018).
Flack, A. Grating selection for Raman spectroscopy. Edinburgh Instruments https://www.edinst.com/technical-note-grating-selection-for-raman-spectroscopy/ (2021).
Blumenthal, D. J. Photonic integration for UV to IR applications. APL. Photonics 5, 020903 (2020).
Acknowledgements
The authors acknowledge support from the UK Engineering and Physical Sciences Research Council (EPSRC) (EP/R011230/1). J.S.W. also acknowledges support from the UK EPSRC (EP/S03109X/1 and EP/V047663/1 (MISSION)) and from the European Research Council (ERC) (WIPFAB 291216).
Author information
Authors and Affiliations
Contributions
Introduction (P.N.B.); Experimentation (M.A.E. and J.S.W.); Results (J.S.W., Z.L., M.A.E. and M.N.Z.); Applications (P.N.B. and M.N.Z.); Reproducibility and data deposition (J.S.W.); Limitations and optimizations (J.S.W. and M.N.Z.); Outlook (J.S.W. and P.N.B.); Overview of the Primer (J.S.W. and P.N.B.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Bing Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ettabib, M.A., Liu, Z., Zervas, M.N. et al. Waveguide-enhanced Raman spectroscopy. Nat Rev Methods Primers 4, 5 (2024). https://doi.org/10.1038/s43586-023-00281-4
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-023-00281-4