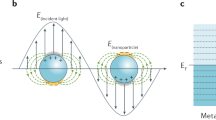
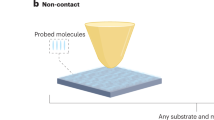
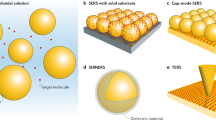
Abstract
Electrochemical surface-enhanced Raman spectroscopy (EC-SERS) is a spectroelectrochemical method that has been in existence since the first SERS measurements were collected, yet it is a technique that has only recently become mainstream. EC-SERS measurements involve the collection of greatly enhanced Raman spectra at the electrified interface of nanostructured metal surfaces, most often coinage metals such as gold or silver. Owing to the surface sensitivity and selectivity of EC-SERS measurements, this technique can provide insight into various interfacial processes. Recent applications of EC-SERS investigations have included biosensing, catalysis and environmental analysis. This Primer seeks to introduce researchers with interdisciplinary backgrounds to key concepts of EC-SERS, including signal enhancement mechanisms, practical experimentation, electrode preparation and data collection and interpretation. Finally, a selection of recent applications and novel directions for future research are presented.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Fleischmann, M., Hendra, P. J. & McQuillan, A. J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 26, 163–166 (1974). To the best of our knowledge, this article reports on the first SERS observation and the first EC-SERS study.
Jeanmaire, D. L. & Van Duyne, R. P. Surface Raman spectroelectrochemistry: part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 84, 1–20 (1977). To the best of our knowledge, this article is one of two which represents the first discovery of SERS.
Albrecht, M. G. & Creighton, J. A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 99, 5215–5217 (1977). To the best of our knowledge, this article is one of two which represents the first discovery of SERS.
Sharma, B., Frontiera, R. R., Henry, A.-I., Ringe, E. & Van Duyne, R. P. SERS: materials, applications, and the future. Mater. Today 15, 16–25 (2012).
Wu, D.-Y., Li, J.-F., Ren, B. & Tian, Z.-Q. Electrochemical surface-enhanced Raman spectroscopy of nanostructures. Chem. Soc. Rev. 37, 1025–1041 (2008). This article is a useful review of EC-SERS.
Willets, K. A. Probing nanoscale interfaces with electrochemical surface-enhanced Raman scattering. Curr. Opin. Electrochem. 13, 18–24 (2019).
Markin, A. V., Arzhanukhina, A. I., Markina, N. E. & Goryacheva, I. Y. Analytical performance of electrochemical surface-enhanced Raman spectroscopy: a critical review. Trends Anal. Chem. 157, 116776 (2022). This article provides a critical review of the analytical performance of EC-SERS.
Han, X. X., Rodriguez, R. S., Haynes, C. L., Ozaki, Y. & Zhao, B. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Primers 1, 87 (2022). This paper is a useful Primer for SERS.
Campion, A. & Kambhampati, P. Surface-enhanced Raman scattering. Chem. Soc. Rev. 27, 241 (1998).
Greene, B. H. C., Alhatab, D. S., Pye, C. C. & Brosseau, C. L. Electrochemical-surface enhanced Raman spectroscopic (EC-SERS) study of 6-thiouric acid: a metabolite of the chemotherapy drug azathioprine. J. Phys. Chem. C 121, 8084–8090 (2017).
Zaleski, S. et al. Investigating nanoscale electrochemistry with surface- and tip-enhanced Raman spectroscopy. Acc. Chem. Res. 49, 2023–2030 (2016).
Haes, A. J. et al. Plasmonic materials for surface-enhanced sensing and spectroscopy. MRS Bull. 30, 368–375 (2005).
Zhao, J., Dieringer, J. A., Zhang, X., Schatz, G. C. & Van Duyne, R. P. Wavelength-scanned surface-enhanced resonance Raman excitation spectroscopy. J. Phys. Chem. C 112, 19302–19310 (2008).
Zhao, Y. et al. A facile method for the synthesis of large-size Ag nanoparticles as efficient SERS substrates. J. Raman Spectrosc. 47, 662–667 (2016).
Tian, Z. Q. & Zhang, X. M. in Developments in Electrochemistry: Science Inspired by Martin Fleischmann 113–135 (John Wiley & Sons, Ltd, 2014).
Denson, S. C., Pommier, C. J. S. & Denton, M. B. The impact of array detectors on Raman spectroscopy. J. Chem. Educ. 84, 67–74 (2007).
Hollricher, O. in Springer Series in Surface Sciences Vol. 66, 69–87 (Springer International Publishing, 2018).
McCreery, R. L. Raman Spectroscopy for Chemical Analysis. Measurement Science and Technology Vol. 12 (John Wiley & Sons, Inc., 2000).
Butler, H. J. et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 11, 664–687 (2016).
Wang, X. et al. Tip-enhanced Raman spectroscopy for surfaces and interfaces. Chem. Soc. Rev. 46, 4020–4041 (2017).
Sartin, M. M., Su, H.-S., Wang, X. & Ren, B. Tip-enhanced Raman spectroscopy for nanoscale probing of dynamic chemical systems. J. Chem. Phys. 153, 170901 (2020).
Tian, Z.-Q. & Ren, B. Adsorption and reaction at electrochemical interfaces as probed by surface-enhanced raman spectroscopy. Annu. Rev. Phys. Chem. 55, 197–229 (2004).
Cai, W. B. et al. Investigation of surface-enhanced Raman scattering from platinum electrodes using a confocal Raman microscope: dependence of surface roughening pretreatment. Surf. Sci. 406, 9–22 (1998).
Robinson, A. M., Harroun, S. G., Bergman, J. & Brosseau, C. L. Portable electrochemical surface-enhanced Raman spectroscopy system for routine spectroelectrochemical analysis. Anal. Chem. 84, 1760–1764 (2012). This article is an early demonstration of the use of screen-printed electrodes for in situ EC-SERS.
Ibañez, D., Romero, E. C., Heras, A. & Colina, A. Dynamic Raman spectroelectrochemistry of single walled carbon nanotubes modified electrodes using a Langmuir–Schaefer method. Electrochim. Acta 129, 171–176 (2014).
Ibáñez, D. et al. Development of a novel Raman cell for the easy handling of spectroelectrochemical measurements. Microchem. J. 180, 107614 (2022).
Zeng, Z.-C. et al. Novel electrochemical Raman spectroscopy enabled by water immersion objective. Anal. Chem. 88, 9381–9385 (2016).
González-Diéguez, N., Colina, A., López-Palacios, J. & Heras, A. Spectroelectrochemistry at screen-printed electrodes: determination of dopamine. Anal. Chem. 84, 9146–9153 (2012).
Shou-Zhong, Z. et al. Extending SERS activity at silver electrodes to a wide potential region. Acta Phys. Chim. Sin. 11, 1020–1025 (1995).
Zhao, L., Blackburn, J. & Brosseau, C. L. Quantitative detection of uric acid by electrochemical-surface enhanced Raman spectroscopy using a multilayered Au/Ag substrate. Anal. Chem. 87, 441–447 (2015).
Farling, C. G., Stackaruk, M. C., Pye, C. C. & Brosseau, C. L. Fabrication of high quality electrochemical SERS (EC-SERS) substrates using physical vapour deposition. Phys. Chem. Chem. Phys. 23, 20065–20072 (2021).
Fan, M., Andrade, G. F. S. & Brolo, A. G. A review on the fabrication of substrates for surface enhanced Raman spectroscopy and their applications in analytical chemistry. Anal. Chim. Acta 693, 7–25 (2011).
Le Thi Ngoc, L. et al. Suppression of surface-enhanced Raman scattering on gold nanostructures by metal adhesion layers. J. Phys. Chem. C 120, 18756–18762 (2016).
Zhu, Q., Murphy, C. J. & Baker, L. R. Opportunities for electrocatalytic CO2 reduction enabled by surface ligands. J. Am. Chem. Soc. 144, 2829–2840 (2022).
Xu, L.-J. et al. Label-free detection of native proteins by surface-enhanced Raman spectroscopy using iodide-modified nanoparticles. Anal. Chem. 86, 2238–2245 (2014).
Zong, C., Chen, C. J., Zhang, M., Wu, D. Y. & Ren, B. Transient electrochemical surface-enhanced Raman spectroscopy: a millisecond time-resolved study of an electrochemical redox process. J. Am. Chem. Soc. 137, 11768–11774 (2015).
Bindesri, S. D., Alhatab, D. S. & Brosseau, C. L. Development of an electrochemical surface-enhanced Raman spectroscopy (EC-SERS) fabric-based plasmonic sensor for point-of-care diagnostics. Analyst 143, 4128–4135 (2018).
Karaballi, R. A., Merchant, S., Power, S. R. & Brosseau, C. L. Electrochemical surface-enhanced Raman spectroscopy (EC-SERS) study of the interaction between protein aggregates and biomimetic membranes. Phys. Chem. Chem. Phys. 20, 4513–4526 (2018).
Ibanez, D., Perez-Junquera, A., Begona Gonzalez-Garcia, M., Hernandez-Santos, D. & Fanjul-Bolado, P. Spectroelectrochemical elucidation of B vitamins present in multivitamin complexes by EC-SERS. Talanta 206, 120190 (2020).
Huang, C.-Y. & Hsiao, H.-C. Integrated EC-SERS chip with uniform nanostructured EC-SERS active working electrode for rapid detection of uric acid. Sensors 20, 7066 (2020).
Hutchinson, K., McQuillan, A. J. & Hester, R. E. Non-aqueous SERS: pyridine in N,N’-dimethylformamide solution at a silver electrode. Chem. Phys. Lett. 98, 27–31 (1983).
Wang, J. et al. In situ SERS and SHINERS study of electrochemical hydrogenation of p-ethynylaniline in nonaqueous solvents. Electrochem. Commun. 78, 16–20 (2017).
Lipkowski, J. & Stolberg, L. in Adsorption of Molecules at Metal Electrodes (eds Lipkowski, J. & Ross, P. N.) (VCH, 1992).
Moskovits, M. Surface selection rules. J. Chem. Phys. 77, 4408–4416 (1982). This article provides a description of the surface selection rules active in SERS.
Larkin, D., Guyer, K. L., Hupp, J. T. & Weaver, M. J. Determination of specific adsorption of some simple anions at a polycrystalline silver–aqueous interface using differential capacitance and kinetic probe techniques. J. Electroanal. Chem. Interfacial Electrochem. 138, 401–423 (1982).
Ali, A. H. & Foss, C. A. Electrochemically induced shifts in the plasmon resonance bands of nanoscopic gold particles adsorbed on transparent electrodes. J. Electrochem. Soc. 146, 628–636 (1999).
Perales-Rondon, J. V. et al. Electrochemical surface oxidation enhanced Raman scattering. Electrochim. Acta 282, 377–383 (2018). To the best of our knowledge, this article is the first demonstration of EC-SOERS.
Perales-Rondon, J. V., Hernandez, S., Heras, A. & Colina, A. Effect of chloride and pH on the electrochemical surface oxidation enhanced Raman scattering. Appl. Surf. Sci. 473, 366–372 (2019).
Perez-Estebanez, M. et al. Chemical selectivity in electrochemical surface oxidation enhanced Raman scattering. Electrochim. Acta 353, 136560 (2020).
Hernandez, S., Perales-Rondon, J. V., Heras, A. & Colina, A. Electrochemical SERS and SOERS in a single experiment: a new methodology for quantitative analysis. Electrochim. Acta 334, 135561 (2020).
Perez-Estebanez, M. et al. Raman spectroelectrochemical determination of clopyralid in tap water. Microchem. J. 183, 108018 (2022).
Hernandez, S., Perales-Rondon, J. V., Heras, A. & Colina, A. Enhancement factors in electrochemical surface oxidation enhanced Raman scattering. Electrochim. Acta 380, 138223 (2021).
Alessandri, I. & Lombardi, J. R. Enhanced Raman scattering with dielectrics. Chem. Rev. 116, 14921–14981 (2016).
Hernandez, S., Perales-Rondon, J. V., Heras, A. & Colina, A. Determination of uric acid in synthetic urine by using electrochemical surface oxidation enhanced Raman scattering. Anal. Chim. Acta 1085, 61–67 (2019).
Hu, J.-W. et al. Synthesis of Au@Pd core–shell nanoparticles with controllable size and their application in surface-enhanced Raman spectroscopy. Chem. Phys. Lett. 408, 354–359 (2005).
Li, J.-F. et al. Surface-enhanced Raman spectroscopy using gold-core platinum-shell nanoparticle film electrodes: toward a versatile vibrational strategy for electrochemical interfaces. Langmuir 22, 10372–10379 (2006).
Lu, L. et al. Fabrication of core–shell Au-Pt nanoparticle film and its potential application as catalysis and SERS substrate. J. Mater. Chem. 14, 1005–1009 (2004).
Tian, Z.-Q., Ren, B., Li, J.-F. & Yang, Z.-L. Expanding generality of surface-enhanced Raman spectroscopy with borrowing SERS activity strategy. Chem. Commun. 2007, 3514–3534 (2007).
Van Duyne, R. P. & Haushalter, J. P. Surface-enhanced Raman spectroscopy of adsorbates on semiconductor electrode surfaces. 2. In situ studies of transition metal (iron and ruthenium) complexes on silver/gallium arsenide and silver/silicon. J. Phys. Chem. 89, 4055–4061 (1985).
Van Duyne, R. P. & Haushalter, J. P. Surface-enhanced Raman spectroscopy of adsorbates on semiconductor electrode surfaces: tris(bipyridine)ruthenium(II) adsorbed on silver-modified n-gallium arsenide(100). J. Phys. Chem. 87, 2999–3003 (1983).
Leung, L. W. H. & Weaver, M. J. Extending surface-enhanced Raman spectroscopy to transition-metal surfaces: carbon monoxide adsorption and electrooxidation on platinum- and palladium-coated gold electrodes. J. Am. Chem. Soc. 109, 5113–5119 (1987).
Leung, L.-W. H. & Weaver, M. J. Extending the metal interface generality of surface-enhanced Raman spectroscopy: underpotential deposited layers of mercury, thallium, and lead on gold electrodes. J. Electroanal. Chem. 217, 367–384 (1987).
Leung, L. W. H. & Weaver, M. J. Adsorption and electrooxidation of carbon monoxide on rhodium- and ruthenium-coated gold electrodes as probed by surface-enhanced Raman spectroscopy. Langmuir 4, 1076–1083 (1988).
Zou, S., Williams, C. T., Chen, E. K. Y. & Weaver, M. J. Probing molecular vibrations at catalytically significant interfaces: a new ubiquity of surface-enhanced Raman scattering. J. Am. Chem. Soc. 120, 3811–3812 (1998).
Zou, S., Weaver, M. J., Li, X. Q., Ren, B. & Tian, Z. Q. New strategies for surface-enhanced Raman scattering at transition–metal interfaces: thickness-dependent characteristics of electrodeposited Pt-group films on gold and carbon. J. Phys. Chem. B 103, 4218–4222 (1999).
Li, J.-F., Zhang, Y.-J., Ding, S.-Y., Panneerselvam, R. & Tian, Z.-Q. Core–shell nanoparticle-enhanced Raman spectroscopy. Chem. Rev. 117, 5002–5069 (2017).
Li, J. F. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464, 392–395 (2010). To the best of our knowledge, this article is the first demonstration of SHINERS.
Li, J. F. et al. Surface analysis using shell-isolated nanoparticle-enhanced Raman spectroscopy. Nat. Protoc. 8, 52–65 (2013).
Li, J.-F., Anema, J. R., Wandlowski, T. & Tian, Z.-Q. Dielectric shell isolated and graphene shell isolated nanoparticle enhanced Raman spectroscopies and their applications. Chem. Soc. Rev. 44, 8399–8409 (2015).
Wang, Y.-H., Wei, J., Radjenovic, P., Tian, Z.-Q. & Li, J.-F. In situ analysis of surface catalytic reactions using shell-isolated nanoparticle-enhanced Raman spectroscopy. Anal. Chem. 91, 1675–1685 (2019).
Lin, X.-D. et al. Synthesis of ultrathin and compact Au@MnO2 nanoparticles for shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS). J. Raman Spectrosc. 43, 40–45 (2012).
Qiu, Z. et al. Raman spectroscopic investigation on TiO2–N719 dye interfaces using Ag@TiO2 nanoparticles and potential correlation strategies. ChemPhysChem 14, 2217–2224 (2013).
Li, J.-F., Rudnev, A., Fu, Y., Bodappa, N. & Wandlowski, T. In situ SHINERS at electrochemical single-crystal electrode/electrolyte interfaces: tuning preparation strategies and selected applications. ACS Nano 7, 8940–8952 (2013).
Li, J.-F. et al. Extraordinary enhancement of Raman scattering from pyridine on single crystal Au and Pt electrodes by shell-isolated Au nanoparticles. J. Am. Chem. Soc. 133, 15922–15925 (2011).
Anderson, M. S. Locally enhanced Raman spectroscopy with an atomic force microscope. Appl. Phys. Lett. 76, 3130–3132 (2000).
Hayazawa, N., Inouye, Y., Sekkat, Z. & Kawata, S. Metallized tip amplification of near-field Raman scattering. Opt. Commun. 183, 333–336 (2000).
Pettinger, B., Picardi, G., Schuster, R. & Ertl, G. Surface enhanced Raman spectroscopy: towards single molecule spectroscopy. Electrochemistry 68, 942–949 (2000).
Stöckle, R. M., Suh, Y. D., Deckert, V. & Zenobi, R. Nanoscale chemical analysis by tip-enhanced Raman spectroscopy. Chem. Phys. Lett. 318, 131–136 (2000). To the best of our knowledge, this article demonstrates the first conceptual demonstration of TERS.
Zeng, Z.-C. et al. Electrochemical tip-enhanced Raman spectroscopy. J. Am. Chem. Soc. 137, 11928–11931 (2015).
Kurouski, D., Mattei, M. & Van Duyne, R. P. Probing redox reactions at the nanoscale with electrochemical tip-enhanced Raman spectroscopy. Nano Lett. 15, 7956–7962 (2015).
Yang, B., Kazuma, E., Yokota, Y. & Kim, Y. Fabrication of sharp gold tips by three-electrode electrochemical etching with high controllability and reproducibility. J. Phys. Chem. C 122, 16950–16955 (2018).
Zhang, W., Yeo, B. S., Schmid, T. & Zenobi, R. Single molecule tip-enhanced Raman spectroscopy with silver tips. J. Phys. Chem. C 111, 1733–1738 (2007).
Ren, B., Picardi, G. & Pettinger, B. Preparation of gold tips suitable for tip-enhanced Raman spectroscopy and light emission by electrochemical etching. Rev. Sci. Instrum. 75, 837–841 (2004).
Yokota, Y. et al. Systematic assessment of benzenethiol self-assembled monolayers on Au(111) as a standard sample for electrochemical tip-enhanced Raman spectroscopy. J. Phys. Chem. C 123, 2953–2963 (2019).
Yeo, B.-S., Zhang, W., Vannier, C. & Zenobi, R. Enhancement of Raman signals with silver-coated tips. Appl. Spectrosc. 60, 1142–1147 (2006).
Taguchi, A. et al. Controlling the plasmon resonance wavelength in metal-coated probe using refractive index modification. Opt. Express 17, 6509–6518 (2009).
Deckert, V. et al. Spatial resolution in Raman spectroscopy. Faraday Discuss. 177, 9–20 (2015).
Saito, Y., Murakami, T., Inouye, Y. & Kawata, S. Fabrication of silver probes for localized plasmon excitation in near-field Raman spectroscopy. Chem. Lett. 34, 920–921 (2005).
Brejna, P. R. & Griffiths, P. R. Electroless deposition of silver onto silicon as a method of preparation of reproducible surface-enhanced Raman spectroscopy substrates and tip-enhanced Raman spectroscopy tips. Appl. Spectrosc. 64, 493–499 (2010).
Yang, L.-K. et al. Rational fabrication of a gold-coated AFM TERS tip by pulsed electrodeposition. Nanoscale 7, 18225–18231 (2015).
Huang, T.-X. et al. Rational fabrication of silver-coated AFM TERS tips with a high enhancement and long lifetime. Nanoscale 10, 4398–4405 (2018).
Kumar, N. et al. Nanoscale chemical imaging of solid–liquid interfaces using tip-enhanced Raman spectroscopy. Nanoscale 10, 1815–1824 (2018).
Schmid, T., Yeo, B.-S., Leong, G., Stadler, J. & Zenobi, R. Performing tip-enhanced Raman spectroscopy in liquids. J. Raman Spectrosc. 40, 1392–1399 (2009).
Bao, Y.-F. et al. Atomic force microscopy based top-illumination electrochemical tip-enhanced Raman spectroscopy. Anal. Chem. 92, 12548–12555 (2020).
Huang, S.-C. et al. Electrochemical tip-enhanced Raman spectroscopy with improved sensitivity enabled by a water immersion objective. Anal. Chem. 91, 11092–11097 (2019).
Touzalin, T., Joiret, S., Lucas, I. T. & Maisonhaute, E. Electrochemical tip-enhanced Raman spectroscopy imaging with 8 nm lateral resolution. Electrochem. Commun. 108, 106557 (2019).
Huang, S.-C. et al. Electrochemical tip-enhanced Raman spectroscopy: an in situ nanospectroscopy for electrochemistry. Annu. Rev. Phys. Chem. 72, 213–234 (2021).
Amemiya, S., Bard, A. J., Fan, F.-R. F., Mirkin, M. V. & Unwin, P. R. Scanning electrochemical microscopy. Annu. Rev. Anal. Chem. 1, 95–131 (2008).
Polcari, D., Dauphin-Ducharme, P. & Mauzeroll, J. Scanning electrochemical microscopy: a comprehensive review of experimental parameters from 1989 to 2015. Chem. Rev. 116, 13234–13278 (2016).
Danis, L., Polcari, D., Kwan, A., Gateman, S. M. & Mauzeroll, J. Fabrication of carbon, gold, platinum, silver, and mercury ultramicroelectrodes with controlled geometry. Anal. Chem. 87, 2565–2569 (2015).
Clausmeyer, J., Nebel, M., Grützke, S., Kayran, Y. U. & Schuhmann, W. Local surface modifications investigated by combining scanning electrochemical microscopy and surface-enhanced raman scattering. ChemPlusChem 83, 414–417 (2018).
Hatfield, K. O., Gole, M. T., Schorr, N. B., Murphy, C. J. & Rodríguez-López, J. Surface-enhanced Raman spectroscopy-scanning electrochemical microscopy: observation of real-time surface pH perturbations. Anal. Chem. 93, 7792–7796 (2021).
Gossage, Z. T. et al. Interrogating charge storage on redox active colloids via combined Raman spectroscopy and scanning electrochemical microscopy. Langmuir 33, 9455–9463 (2017).
Bentley, C. L., Kang, M. & Unwin, P. R. Nanoscale structure dynamics within electrocatalytic materials. J. Am. Chem. Soc. 139, 16813–16821 (2017).
Zhang, S., Li, M., Su, B. & Shao, Y. Fabrication and use of nanopipettes in chemical analysis. Annu. Rev. Anal. Chem. 11, 265–286 (2018).
Morris, C. A., Friedman, A. K. & Baker, L. A. Applications of nanopipettes in the analytical sciences. Analyst 135, 2190–2202 (2010).
Wang, Y., Wang, D. & Mirkin, M. V. Resistive-pulse and rectification sensing with glass and carbon nanopipettes. Proc. R. Soc. Math. Phys. Eng. Sci. 473, 20160931 (2017).
Brasiliense, V., Park, J. E., Chen, Z., Van Duyne, R. P. & Schatz, G. C. Nanopipette-based electrochemical SERS platforms: using electrodeposition to produce versatile and adaptable plasmonic substrates. J. Raman Spectrosc. 52, 339–347 (2021).
Vitol, E. A. et al. In situ intracellular spectroscopy with surface enhanced Raman spectroscopy (SERS)-enabled nanopipettes. ACS Nano 3, 3529–3536 (2009).
Masson, J.-F. et al. Plasmonic nanopipette biosensor. Anal. Chem. 86, 8998–9005 (2014).
Fang, W. et al. Portable SERS-enabled micropipettes for microarea sampling and reliably quantitative detection of surface organic residues. Anal. Chem. 87, 9217–9224 (2015).
Yang, J.-M. et al. Surface-enhanced Raman scattering probing the translocation of DNA and amino acid through plasmonic nanopores. Anal. Chem. 91, 6275–6280 (2019).
Li, P., Ge, M., Lin, D. & Yang, L. Functionalized acupuncture needle as a SERS-active platform for rapid and sensitive determination of adenosine triphosphate. Anal. Bioanal. Chem. 411, 5669–5679 (2019).
Lussier, F., Missirlis, D., Spatz, J. P. & Masson, J.-F. Machine-learning-driven surface-enhanced Raman scattering optophysiology reveals multiplexed metabolite gradients near cells. ACS Nano 13, 1403–1411 (2019).
Vitol, E. A., Orynbayeva, Z., Friedman, G. & Gogotsi, Y. Nanoprobes for intracellular and single cell surface-enhanced Raman spectroscopy (SERS). J. Raman Spectrosc. 43, 817–827 (2012).
Nie, X.-L. et al. Recognition of plastic nanoparticles using a single gold nanopore fabricated at the tip of a glass nanopipette. Chem. Commun. 55, 6397–6400 (2019).
LI, H.-N. et al. Facile fabrication of gold functionalized nanopipette for nanoscale electrochemistry and surface enhanced Raman spectroscopy. Chin. J. Anal. Chem. 47, e19104–e19112 (2019).
Shi, C., Zhang, W., Birke, R. L. & Lombardi, J. R. Detection of short-lived intermediates in electrochemical reactions using time-resolved surface-enhanced Raman spectroscopy. J. Phys. Chem. 94, 4766–4769 (1990).
Wilson, A. J., Molina, N. Y. & Willets, K. A. Modification of the electrochemical properties of Nile Blue through covalent attachment to gold as revealed by electrochemistry and SERS. J. Phys. Chem. C 120, 21091–21098 (2016).
Willets, K. A. Super-resolution imaging of SERS hot spots. Chem. Soc. Rev. 43, 3854–3864 (2014).
Wilson, A. J. & Willets, K. A. Visualizing site-specific redox potentials on the surface of plasmonic nanoparticle aggregates with superlocalization SERS microscopy. Nano Lett. 14, 939–945 (2014).
Weber, M. L., Wilson, A. J. & Willets, K. A. Characterizing the spatial dependence of redox chemistry on plasmonic nanoparticle electrodes using correlated super-resolution surface-enhanced Raman scattering imaging and electron microscopy. J. Phys. Chem. C 119, 18591–18601 (2015).
Huang, S.-C. et al. Probing nanoscale spatial distribution of plasmonically excited hot carriers. Nat. Commun. 11, 4211 (2020).
Schulze, G. et al. Investigation of selected baseline removal techniques as candidates for automated implementation. Appl. Spectrosc. 59, 545–574 (2005).
Wei, D., Chen, S. & Liu, Q. Review of fluorescence suppression techniques in Raman spectroscopy. Appl. Spectrosc. Rev. 50, 387–406 (2015).
Galloway, C. M., Ru, E. C. L. & Etchegoin, P. G. An iterative algorithm for background removal in spectroscopy by wavelet transforms. Appl. Spectrosc. 63, 1370–1376 (2009).
Lieber, C. A. & Mahadevan-Jansen, A. Automated method for subtraction of fluorescence from biological Raman spectra. Appl. Spectrosc. 57, 1363–1367 (2003).
Guo, S., Popp, J. & Bocklitz, T. Chemometric analysis in Raman spectroscopy from experimental design to machine learning-based modeling. Nat. Protoc. 16, 5426–5459 (2021).
Lin, K.-Q. et al. Plasmonic photoluminescence for recovering native chemical information from surface-enhanced Raman scattering. Nat. Commun. 8, 14891 (2017).
Plieth, W., Wilson, G. S. & Gutiérrez De La Fe, C. Spectroelectrochemistry: a survey of in situ spectroscopic techniques. Pure Appl. Chem. 70, 1395–1414 (1998).
Luo, S. et al. Developing a peak extraction and retention (PEER) algorithm for improving the temporal resolution of Raman spectroscopy. Anal. Chem. 93, 8408–8413 (2021).
Savitzky, A. & Golay, M. J. E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 36, 1627–1639 (1964).
Wang, X., Liu, G., Xu, M., Ren, B. & Tian, Z. Development of weak signal recognition and an extraction algorithm for Raman imaging. Anal. Chem. 91, 12909–12916 (2019).
Barclay, V. J., Bonner, R. F. & Hamilton, I. P. Application of wavelet transforms to experimental spectra: smoothing, denoising, and data set compression. Anal. Chem. 69, 78–90 (1997).
Wang, A. et al. In situ identification of intermediates of benzyl chloride reduction at a silver electrode by SERS coupled with DFT calculations. J. Am. Chem. Soc. 132, 9534–9536 (2010).
Huang, Y.-F., Kooyman, P. J. & Koper, M. T. M. Intermediate stages of electrochemical oxidation of single-crystalline platinum revealed by in situ Raman spectroscopy. Nat. Commun. 7, 12440 (2016).
Dong, J.-C. et al. In situ Raman spectroscopic evidence for oxygen reduction reaction intermediates at platinum single-crystal surfaces. Nat. Energy 4, 60–67 (2019).
Hernandez, S. et al. Multiamperometric-SERS detection of melamine on gold screen-printed electrodes. J. Electroanal. Chem. 918, 116478 (2022).
Wang, Y.-H. et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 600, 81–85 (2021).
Bro, R. PARAFAC. Tutorial and applications. Chemom. Intell. Lab. Syst. 38, 149–171 (1997).
Andersson, C. A. & Bro, R. The N-way toolbox for MATLAB. Chemom. Intell. Lab. Syst. 52, 1–4 (2000).
Marro, M., Nieva, C., De Juan, A. & Sierra, A. Unravelling the metabolic progression of breast cancer cells to bone metastasis by coupling Raman spectroscopy and a novel use of MCR-ALS algorithm. Anal. Chem. 90, 5594–5602 (2018).
Grys, D.-B., de Nijs, B., Huang, J., Scherman, O. A. & Baumberg, J. J. SERSbot: revealing the details of SERS multianalyte sensing using full automation. ACS Sens. 6, 4507–4514 (2021).
Morais, C. L. M., Lima, K. M. G., Singh, M. & Martin, F. L. Tutorial: multivariate classification for vibrational spectroscopy in biological samples. Nat. Protoc. 15, 2143–2162 (2020).
Bursch, M., Mewes, J.-M., Hansen, A. & Grimme, S. Best-practice DFT protocols for basic molecular computational chemistry**. Angew. Chem. Int. Ed. 61, e202205735 (2022).
Su, M. et al. In situ Raman study of CO electrooxidation on Pt(hkl) single-crystal surfaces in acidic solution. Angew. Chem. Int. Ed. 59, 23554–23558 (2020).
Dai, K. et al. Adsorption and reduction reactions of anthraquinone derivatives on gold electrodes studied with electrochemical surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 43, 1367–1373 (2012).
Perez-Estebanez, M. et al. Double fingerprint characterization of uracil and 5-uracil. Electrochim. Acta 388, 138615 (2021).
Quinet, O., Champagne, B. & Kirtman, B. Analytical TDHF second derivatives of dynamic electronic polarizability with respect to nuclear coordinates. Application to the dynamic ZPVA correction. J. Comput. Chem. 22, 1920–1932 (2001).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Ozaki, Y. et al. Advances, challenges and perspectives of quantum chemical approaches in molecular spectroscopy of the condensed phase. Chem. Soc. Rev. 50, 10917–10954 (2021).
Li, C.-Y. et al. In situ probing electrified interfacial water structures at atomically flat surfaces. Nat. Mater. 18, 697–701 (2019).
Li, P. et al. Hydrogen bond network connectivity in the electric double layer dominates the kinetic pH effect in hydrogen electrocatalysis on Pt. Nat. Catal. 5, 900–911 (2022).
Le, J.-B., Yang, X.-H., Zhuang, Y.-B., Jia, M. & Cheng, J. Recent progress toward ab initio modeling of electrocatalysis. J. Phys. Chem. Lett. 12, 8924–8931 (2021).
Wang, H., Zhang, L., Han, J. & Weinan, E. DeePMD-kit: a deep learning package for many-body potential energy representation and molecular dynamics. Comput. Phys. Commun. 228, 178–184 (2018).
Aranda, D. et al. Computational model for electrochemical surface-enhanced Raman scattering: key role of the surface charges and synergy between electromagnetic and charge-transfer enhancement mechanisms. J. Chem. Theory Comput. 18, 6802–6815 (2022).
Tian, Z.-Q. & Ren, B. in Encyclopedia of Electrochemistry Vol. 3 (eds Bard, A. J., Stratmann, M. & Unwin, P. R.) 576–659 (Wiley-VCH, 2007).
Okejas, V., Sjostrom, C. & Harris, J. M. SERS detection of the vibrational stark effect from nitrile-terminated SAMs to probe electric fields in the diffuse double-layer. J. Am. Chem. Soc. 124, 2408–2409 (2002).
Zhang, N. et al. Probing double layer structure at Au/[BMIm]BF4 interface by molecular length-dependent SERS Stark effect. J. Electroanal. Chem. 751, 137–143 (2015).
Marr, J. M. & Schultz, Z. Imaging electric fields in SERS and TERS using the vibrational stark effect. J. Phys. Chem. Lett. 4, 3268–3272 (2013).
Huang, Y.-F. et al. When the signal is not from the original molecule to be detected: chemical transformation of para-aminothiophenol on Ag during the SERS measurement. J. Am. Chem. Soc. 132, 9244–9246 (2010).
Zhan, C. et al. From plasmon-enhanced molecular spectroscopy to plasmon-mediated chemical reactions. Nat. Rev. Chem. 2, 216–213 (2018).
Zhan, C., Moskovits, M. & Tian, Z.-Q. Recent progress and prospects in plasmon-mediated chemical reaction. Matter 3, 42056 (2020).
Moldovan, R. et al. Review on combining surface-enhanced Raman spectroscopy and electrochemistry for analytical applications. Anal. Chim. Acta 1209, 339250 (2022).
Hendricks-Leukes, N. R., Jonas, M. R., Mlamla, Z. C., Smith, M. & Blackburn, J. M. Dual-approach electrochemical surface-enhanced Raman scattering detection of Mycobacterium tuberculosis in patient-derived biological specimens: proof of concept for a generalizable method to detect and identify bacterial pathogens. ACS Sens. 7, 1403–1418 (2022).
Albarghouthi, N., Eisnor, M. M., Pye, C. C. & Brosseau, C. L. Electrochemical surface-enhanced Raman spectroscopy (EC-SERS) and computational study of atrazine: toward point-of-need detection of prevalent herbicides. J. Phys. Chem. C 126, 9836–9842 (2022).
Velička, M., Zacharovas, E., Adomavičiūtė, S. & Šablinskas, V. Detection of caffeine intake by means of EC-SERS spectroscopy of human saliva. Spectrochim. Acta A Mol. Biomol. Spectrosc. 246, 118956 (2021).
Lin, Y.-K., Tai, R.-J., Wei, S.-C. & Luo, S.-C. Electrochemical SERS on 2D mapping for metabolites detection. Langmuir 36, 5990–5996 (2020).
Velicka, M., Adomaviciute, S., Zacharovas, E. & Sablinskas, V. in Plasmonics in Biology and Medicine XVII Vol. 11257 (eds VoDinh, T., Ho, H. & Ray, K.) (SPIE, 2020).
Karaballi, R. A., Nel, A., Krishnan, S., Blackburn, J. & Brosseau, C. L. Development of an electrochemical surface-enhanced Raman spectroscopy (EC-SERS) aptasensor for direct detection of DNA hybridization. Phys. Chem. Chem. Phys. 17, 21356–21363 (2015).
Lynk, T. P., Sit, C. S. & Brosseau, C. L. Electrochemical surface-enhanced Raman spectroscopy as a platform for bacterial detection and identification. Anal. Chem. 90, 12639–12646 (2018).
McLeod, K. E. R., Lynk, T. P., Sit, C. S. & Brosseau, C. L. On the origin of electrochemical surface-enhanced Raman spectroscopy (EC-SERS) signals for bacterial samples: the importance of filtered control studies in the development of new bacterial screening platforms. Anal. Methods 11, 924–929 (2019).
Moldovan, R. et al. EC-SERS detection of thiobendazole in apple juice using activated screen-printed electrodes. Food Chem. 405, 134713 (2023).
Santos, V. O., Leite, I. R., Brolo, A. G. & Rubim, J. C. The electrochemical reduction of CO2 on a copper electrode in 1-N-butyl-3-methyl imidazolium tetrafluoroborate (BMI.BF4) monitored by surface-enhanced Raman scattering (SERS). J. Raman Spectrosc. 47, 674–680 (2016).
Henckel, D. A. et al. Potential dependence of the local pH in a CO2 reduction electrolyzer. ACS Catal. 11, 255–263 (2021).
Shan, W. et al. In situ surface-enhanced Raman spectroscopic evidence on the origin of selectivity in CO2 electrocatalytic reduction. ACS Nano 14, 11363–11372 (2020).
An, H. et al. Sub-second time-resolved surface-enhanced Raman spectroscopy reveals dynamic CO intermediates during electrochemical CO2 reduction on copper. Angew. Chem. Int. Ed. 60, 16576–16584 (2021).
Tian, Z.-Q., Ren, B., Chen, Y.-X., Zou, S.-Z. & Mao, B.-W. Probing electrode/electrolyte interfacial structure in the potential region of hydrogen evolution by Raman spectroscopy. J. Chem. Soc. Faraday Trans. 92, 3829 (1996).
Ren, B. et al. Surface Raman spectra of pyridine and hydrogen on bare platinum and nickel electrodes. J. Electroanal. Chem. 415, 175–178 (1996).
Chen, Y. X. & Tian, Z. Q. Dependence of surface enhanced Raman scattering of water on the hydrogen evolution reaction. Chem. Phys. Lett. 281, 379–383 (1997).
Tian, Z. Q., Ren, B. & Mao, B. W. Extending surface Raman spectroscopy to transition metal surfaces for practical applications. 1. Vibrational properties of thiocyanate and carbon monoxide adsorbed on electrochemically activated platinum surfaces. J. Phys. Chem. B 101, 1338–1346 (1997).
Ren, B., Xu, X., Li, X. Q., Cai, W. B. & Tian, Z. Q. Extending surface Raman spectroscopic studies to transition metals for practical applications: II. Hydrogen adsorption at platinum electrodes. Surf. Sci. 427–428, 157–161 (1999).
Ren, B., Cui, L., Lin, X.-F. & Tian, Z.-Q. Probing different adsorption behavior of CO on Pt at solid/liquid and solid/gas interfaces by Raman spectroscopy with a three-phase Raman cell. Chem. Phys. Lett. 376, 130–135 (2003).
Zhang, P. et al. Potential-dependent chemisorption of carbon monoxide at a gold core−platinum shell nanoparticle electrode: a combined study by electrochemical in situ surface-enhanced Raman spectroscopy and density functional theory. J. Phys. Chem. C 114, 403–411 (2010).
Zhang, B. et al. Electrochemical and surfaced-enhanced Raman spectroscopic investigation of CO and SCN- adsorbed on Au core−Pt shell nanoparticles supported on GC electrodes. Langmuir 21, 7449–7455 (2005).
Hu, J.-W. et al. Palladium-coated gold nanoparticles with a controlled shell thickness used as surface-enhanced Raman scattering substrate. J. Phys. Chem. C 111, 1105–1112 (2007).
Li, J.-F. et al. Core–shell nanoparticle based SERS from hydrogen adsorbed on a rhodium(111) electrode. Chem. Commun. 47, 2023–2025 (2011).
Li, J.-F. et al. SERS and DFT study of water on metal cathodes of silver, gold and platinum nanoparticles. Phys. Chem. Chem. Phys. 12, 2493–2502 (2010).
Li, C.-Y. et al. In situ monitoring of electrooxidation processes at gold single crystal surfaces using shell-isolated nanoparticle-enhanced Raman spectroscopy. J. Am. Chem. Soc. 137, 7648–7651 (2015).
Li, J. et al. Electrokinetic and in situ spectroscopic investigations of CO electrochemical reduction on copper. Nat. Commun. 12, 3264 (2021).
Kang, G., Yang, M., Mattei, M. S., Schatz, G. C. & Van Duyne, R. P. In situ nanoscale redox mapping using tip-enhanced Raman spectroscopy. Nano Lett. 19, 2106–2113 (2019).
Pfisterer, J. H. K., Baghernejad, M., Giuzio, G. & Domke, K. F. Reactivity mapping of nanoscale defect chemistry under electrochemical reaction conditions. Nat. Commun. 10, 5702 (2019).
Ding, S.-Y. et al. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 1, 16021 (2016).
Chen, J.-J. et al. Conductive Lewis base matrix to recover the missing link of Li2S8 during the sulfur redox cycle in Li–S battery. Chem. Mater. 27, 2048–2055 (2015).
Huang, J. X. et al. Structural evolution of NM (Ni and Mn) lithium-rich layered material revealed by in-situ electrochemical Raman spectroscopic study. J. Power Sources 310, 85–90 (2016).
Peng, Z. et al. Oxygen reactions in a non-aqueous Li+ electrolyte. Angew. Chem. Int. Ed. 50, 6351–6355 (2011).
Johnson, L. et al. The role of LiO2 solubility in O2 reduction in aprotic solvents and its consequences for Li-O2 batteries. Nat. Chem. 6, 1091–1099 (2014).
Peng, Z., Chen, Y., Bruce, P. G. & Xu, Y. Direct detection of the superoxide anion as a stable intermediate in the electroreduction of oxygen in a non-aqueous electrolyte containing phenol as a proton source. Angew. Chem. Int. Ed. 54, 8165–8168 (2015).
Peng, Z., Freunberger, S. A., Chen, Y. & Bruce, P. G. A reversible and higher-rate Li-O2 battery. Science 337, 563–566 (2012).
Gittleson, F. S., Ryu, W. H. & Taylor, A. D. Operando observation of the gold–electrolyte interface in Li-O2 batteries. ACS Appl. Mater. Inter. 6, 19017–19025 (2014).
Hy, S. et al. In situ surface enhanced Raman spectroscopic studies of solid–electrolyte interphase formation in lithium ion battery electrodes. J. Power Sources 256, 324–328 (2014).
Gajan, A. et al. Solid electrolyte interphase instability in operating lithium-ion batteries unraveled by enhanced-Raman spectroscopy. ACS Energy Lett. 6, 1757–1763 (2021).
Hu, R. et al. Dramatically enhanced reversibility of Li2O in SnO2-based electrodes: the effect of nanostructure on high initial reversible capacity. Energy Environ. Sci. 9, 595–603 (2016).
Chen, D. et al. Operando investigation into dynamic evolution of cathode–electrolyte interfaces in a Li-ion battery. Nano Lett. 19, 2037–2043 (2019).
Hy, S., Felix, F., Rick, J., Su, W. N. & Hwang, B. J. Direct in situ observation of Li2O evolution on Li-rich high-capacity cathode material, Li[Ni(x)Li((1−2x)/3)Mn((2−x)/3)]O2 (0 ≤ x ≤ 0.5). J. Am. Chem. Soc. 136, 999–1007 (2014).
Martin-Yerga, D. et al. Dynamics of solid–electrolyte interphase formation on silicon electrodes revealed by combinatorial electrochemical screening. Angew. Chem. Int. Ed. 61, e202207184 (2022).
Nanda, J. et al. Unraveling the nanoscale heterogeneity of solid electrolyte interphase using tip-enhanced Raman spectroscopy. Joule 3, 2001–2019 (2019).
Zhong, J. H. et al. Quantitative correlation between defect density and heterogeneous electron transfer rate of single layer graphene. J. Am. Chem. Soc. 136, 16609–16617 (2014).
Chen, J. et al. Ag@MoS2 core–shell heterostructure as SERS platform to reveal the hydrogen evolution active sites of single-layer MoS2. J. Am. Chem. Soc. 142, 7161–7167 (2020).
Zhang, S. P. et al. In situ Raman study of the photoinduced behavior of dye molecules on TiO2(hkl) single crystal surfaces. Chem. Sci. 11, 6431–6435 (2020).
Yang, L. et al. Engineering large-scaled electrochromic semiconductor films as reproductive SERS substrates for operando investigation at the solid/liquid interfaces. Chin. Chem. Lett. 33, 5169–5173 (2022).
Smith, G. & Dickinson, E. J. F. Error, reproducibility and uncertainty in experiments for electrochemical energy technologies. Nat. Commun. 13, 6832 (2022).
Eisnor, M. M. et al. Electrochemical surface-enhanced Raman spectroscopy (EC-SERS): a tool for the identification of polyphenolic components in natural lake pigments. Phys. Chem. Chem. Phys. 24, 347–356 (2022).
Chotoye, S. A. B., Eisnor, M. M., Ball, R. B. E. & Brosseau, C. L. Development of an electrochemical surface-enhanced Raman spectroscopic biosensor for the direct detection of glutathione. J. Raman Spectrosc. 54, 587–595 (2023).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Chakrabarti, A. et al. A decade+ of operando spectroscopy studies. Catal. Today 283, 27–53 (2017).
Chang Xie, W. et al. In-situ electrochemical surface-enhanced Raman spectroscopy study of formic acid electrooxidation at variable temperatures by high-frequency heating technology. Electrochim. Acta 281, 323–328 (2018).
Wang, X. & Guo, L. SERS activity of semiconductors: crystalline and amorphous nanomaterials. Angew. Chem. Int. Ed. 59, 4231–4239 (2020).
Qu, S. et al. An electrochromic Ag-decorated WO3−x film with adjustable defect states for electrochemical surface-enhanced Raman spectroscopy. Nanomaterials 12, 1637 (2022).
Hao, R. & Zhang, B. Nanopipette-based electroplated nanoelectrodes. Anal. Chem. 88, 614–620 (2016).
Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council of Canada (C.L.B.) and the Canada Research Chairs Program (C.L.B.). A.J.W. and P.B.J. acknowledge support from the Oak Ridge Associated Universities through a Ralph E. Powe Junior Faculty Enhancement Award and start-up funds from the University of Louisville. A.C. acknowledges the Ministerio de Ciencia e Innovación and Agencia Estatal de Investigación (MCIN/AEI/10.13039/501100011033, PID2020-113154RB-C21). J.V.P.-R. acknowledges Ministerio de Universidades and NextGenerationEU for his Maria Zambrano fellowship. B.R. and X.W. acknowledge the financial support of the National Natural Science Foundation of China (NSFC) (Grant Nos 22021001, 22227802 and 92061118).
Author information
Authors and Affiliations
Contributions
Introduction (C.L.B.); Experimentation (A.C., J.V.P.-R., C.L.B., B.R., X.W., A.J.W. and P.B.J.); Results (A.C., J.V.P.-R., B.R. and X.W.); Applications (C.L.B., B.R. and X.W.); Reproducibility and data deposition (C.L.B.); Limitations and optimizations (C.L.B.); Outlook (C.L.B.); Overview of the Primer (C.L.B.).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Zhu Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Gaussian: https://gaussian.com
IRUG: http://www.irug.org/
ORCA: https:// orcaforum.kofo.mpg.de
RRUFF: https://rruff.info/
Glossary
- Chemical enhancement mechanism
-
Charge-transfer processes between the adsorbate and the metal surface result in an enhancement of the Raman signal, typically in the order of 101–102.
- Electrochemical Stark tuning
-
Changes in vibrational frequencies of adsorbed species as a function of applied potential that originates from potential-dependent metal–adsorbate bonding and from the influence of electric field on the adsorbed layer.
- Electromagnetic enhancement
-
Excitation of localized surface plasmons on the nanoparticle surface leads to a generation of an enhanced electric field at the surface, which results in an E4 enhancement of the Raman-scattered photons.
- Epi-illumination
-
An arrangement of optical components in microscopy that allows for illumination of the specimen from above.
- Glancing angle geometry
-
In optics, the angle between the incident beam and the surface.
- Localized surface plasmon resonance
-
Collective oscillation of conduction electrons present in metal nanoparticles on irradiation of light.
- Potential of zero charge
-
(PZC). Potential at which the surface free charge of a particular metal is zero.
- Savitzky–Golay smoothing
-
A digital data filter commonly used to smooth data.
- Signal filtering
-
Process whereby unwanted components or features of a signal are removed.
- VoltaRamangram
-
A plot of the spectral peak evolution in Raman spectroscopy as a function of the applied potential.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brosseau, C.L., Colina, A., Perales-Rondon, J.V. et al. Electrochemical surface-enhanced Raman spectroscopy. Nat Rev Methods Primers 3, 79 (2023). https://doi.org/10.1038/s43586-023-00263-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-023-00263-6