Abstract
Expanded ocean anoxia and global cooling have been invoked as major causal mechanisms for the Late Ordovician mass extinction, but the factors underpinning the extinction remain unresolved. Here, we document two intervals of particularly intense phosphorus recycling in marine rocks deposited across a bathymetric transect in the Yangtze Shelf Sea. The first occurred during the initial phase of the extinction and, coincident with global cooling, drove the development of ocean euxinia on the shelf. The second re-established shelf euxinia after the peak of glaciation, leading to the second phase of extinction. Integration of these data into a global biogeochemical model indicates that phosphorus recycling would have doubled the long-term burial rate of organic carbon, driving ~4°C of global cooling. Thus, through its impact on both the spread of anoxia and global cooling, extensive redox-promoted phosphorus recycling was a critical factor in Earth’s first catastrophic loss of animal life.
Similar content being viewed by others
Introduction
An estimated 53% of marine species became extinct during the late Ordovician mass extinction (LOME) ~445 million years ago (Ma)1. This loss of marine fauna is considered to have occurred in two phases, with the initial, major extinction phase (LOMEI-1) occurring at the Katian/Hirnantian stage boundary, and the second interval (LOMEI-2) occurring in the late Hirnantian2. Both rapid global cooling leading to the Hirnantian glaciation, and expanded marine anoxia and/or euxinia (anoxic, sulfidic conditions), have been invoked as causal mechanisms for the LOME3,4,5,6,7,8.
The precise role of each of these mechanisms in driving the LOME is controversial. In part, this is due to uncertainty in stratigraphic correlations between sections used to evaluate the potential roles of glaciation and ocean anoxia, and even mismatches in the timing of the onset of the LOME4,5. However, geochemical evidence from the same stratigraphic sections in the Yangtze Shelf Sea, South China, suggests that expanded euxinia was likely directly responsible for the two pulses of the LOME, with global cooling playing a secondary role4. Nevertheless, while there is abundant evidence for water column anoxia at various stages of the LOME3,4,5,9,10, an integrated understanding of links between water column deoxygenation and coeval global cooling has remained elusive.
Cooling during the late Ordovician has commonly been considered to be due to drawdown of atmospheric CO2 through global silicate weathering and organic carbon burial. These processes are thought to have been enhanced through mountain building11,12 and by the expansion of early land plants13,14,15,16. The late Ordovician also saw periods of intense volcanism17, which may have supplied large amounts of phosphorus (P) through ash deposition and the weathering of tephra18. Phosphorus is the ultimate limiting nutrient for marine primary productivity on geological timescales19 and increased P bioavailability due to enhanced volcanism, weathering and/or land plant expansion during the Hirnantian stage may have stimulated primary productivity and contributed to the drawdown of CO218. This increased P influx would also have increased oxygen demand in the water column20, potentially leading to the development of water column anoxia. However, recent biogeochemical modelling has shown that reproducing the amount of cooling evidenced in the geologic record would require substantially more P than was available from the volcanic source18, and the P supply from weathering and plant expansion is not well known14.
Phosphorus bioavailability is also closely linked to redox conditions in the ocean and during early diagenesis21,22,23,24,25. During organic matter (Corg) remineralization under anoxic conditions in the water column and during early diagenesis, intense preferential recycling of P commonly occurs21,22. This process is particularly prevalent during the microbial generation of dissolved sulfide (which elevates Corg/Porg values above the Redfield ratio of 106:1), while the reductive dissolution of Fe (oxyhydr)oxide minerals also releases associated P (PFe) to solution21,26. Some of the released P may undergo ‘sink-switching’ to authigenic phases, such as carbonate fluorapatite (Paut)27 or vivianite (which is extracted as part of the PFe pool)28,29. However, dependent on the precise redox condition of the water column and underlying sediments, P may also be recycled back to the water column, where it has the potential to promote a positive productivity feedback20. Indeed, it has been speculated that such recycling may have played a critical, but unsubstantiated, role in both global cooling and the development of water column anoxia during the LOME18.
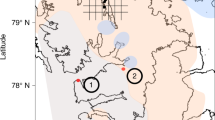
Here, we utilize a P phase partitioning approach30 to provide an empirical reconstruction of the behavior of the P cycle at two locations across a bathymetric transect in the Yangtze Shelf Sea, South China (Fig. 1; Supplementary Note 1; Supplementary Fig. 1). These locations comprise the proximal Shuanghe inner-shelf (SH) outcrop section and the distal outer-shelf Wuxi (WX) drill core section (Supplementary Notes 2, 3; Supplementary Figs. 2, 3), which were deposited during the Late Ordovician (Katian Stage) to Early Silurian (Rhudanian stage) (Fig. 1). We then integrate these new data into a biogeochemical model to provide detailed constraints on the role of P recycling in both the development of anoxia and global cooling during Earth’s first catastrophic loss of animal life.
Data for δ13Corg, Corg, Ptot, climate change and redox conditions for the SH section are modified from ref. 4. See Supplementary Information for the climate and redox characterization of the WX drill core. Six stratigraphic horizons (A to F) are distinguished based on these water column redox dynamics. The two extinction intervals of the LOME are denoted as LOMEI-1 and LOMEI-22, 4. Open circles represent samples with anomalous Pdet contents or Pdet/Al ratios, which likely represent post-depositional transfer of Paut to Pdet (see main text and Supplementary Information for details). While this affects Corg/Preac ratios (and hence the ratios for these samples are discluded from consideration), it does not affect Corg/Porg ratios. Dashed blue line represents the average Ptot concentration for Post-Archean Australian Shale (PAAS). A molar C/P ratio of 106:1 denotes the Redfield ratio (red dashed line). The ages of graptolite zones are from ref. 1. Graptolite zones: D. cn. Dicellograptus complanatus, D. cx. Dicellograptus complexus, P. pacificus Paraorthograptus pacificus, M. e. Metabolograptus extraordinarius, M. p. Metabolograptus persculptus, A. a. Akidograptus ascensus. Rhud. Rhuddanian, Lx Linxiang Formation, KB Kuangyinchiao Bed, Lm.Fm. Lungmachi Formation.
Results
Both sections have organic carbon concentrations (see Methods) that start with low values (0.17 ± 0.09 wt%) in the Linxiang and basal Wufeng formations, followed by an increase to significantly higher concentrations (4.29 ± 1.58 wt%), but with a transient decrease prior to the LOMEI-2 horizon (Fig. 1; see Supplementary Tables 1–4 for all data). Organic C isotope profiles (δ13Corg) are typical for this time period4, with values of ~−29‰ at the base of the succession, followed by an excursion to more negative values and then an increase that starts at the LOMEI-1 horizon and persists until a second decrease coincident with the LOMEI-2 horizon (Fig. 1; Supplementary Fig. 2).
Total phosphorus (Ptot) concentrations vary through each section (see Supplementary Note 4 and Supplementary Figs. 4–7 for detailed discussion of P systematics), and this variability is largely controlled by changes in the detrital P pool (Fig. 1). One concern with chemical extractions of P phases is the potential for transfer of authigenic P, particularly carbonate fluorapatite, to more crystalline apatite during diagenesis and metamorphism, which would result in an overestimation of Pdet at the expense of the Paut pool30,31. However, Pdet concentrations are also affected by the degree of chemical weathering, whereby more intense chemical weathering would result in enhanced dissolution of apatite and hence lower concentrations of detrital P in sediments delivered to the ocean. Indeed, while Pdet concentrations are generally low and within the range commonly found in modern continental shelf settings at the more distal site27,32, at the more proximal site, Pdet increases from the middle of Horizon B to a peak during the glacial interval in Horizon D, followed by a subsequent decrease during the recovery from glaciation (Fig. 1). This apparent paleo-weathering control on Pdet, which might be expected to be more pronounced in proximal settings, is supported by a strong negative correlation between Pdet and the chemical index of alteration weathering proxy (Supplementary Fig. 7). Thus, variability in Pdet (and hence Ptot) appears to be largely controlled by climate-induced changes in the intensity of chemical weathering, rather than significant post-depositional transfer of Paut to Pdet. Possible exceptions to this concern two samples with anomalously high Pdet (>800 ppm), and four samples from Horizon C in the proximal site, which have elevated Pdet/Al ratios (shown as open circles on Fig. 1). Some of these samples deviate from the weathering induced trend in Pdet concentrations (see Supplementary Fig. 7), suggesting potential formation of crystalline apatite during diagenesis, and thus we take a conservative approach whereby these anomalous samples are excluded from further consideration.
Discussion
Based on published redox characteristics for the SH section4, combined with new data for the WX drill core (see Supplementary Note 2 for water column redox interpretations), we identify six distinct horizons (Fig. 1). The lower part of Horizon A was deposited under oxic conditions, before anoxic ferruginous (Fe-containing) conditions developed in Horizon B, followed by a transition to weak or intermittent euxinia during the LOMEI-1 interval in Horizon C (Fig. 1). Persistent euxinia then developed in Horizon D, as well as in Horizon F, the base of which coincides with the LOMEI-2 interval, with Horizon E marking ferruginous (anoxic, Fe-containing) deposition in the interim. Importantly, this ocean redox history is broadly consistent with the reported evolution of ocean redox chemistry at other global sites (Supplementary Fig. 1; Supplementary Table 5).
All samples have molar Corg/Porg ratios above the Redfield ratio (Figs. 1, 2), consistent with preferential release of P from organic matter during microbial remineralization21,33. Most molar Corg/Preac ratios (Figs. 1, 2) also fall above the Redfield ratio, and thus document Porg and PFe recycling back to the water column21,26,29. However, Corg/Preac ratios are consistently below Corg/Porg ratios throughout the succession, due to a proportion of the dissolved P being incorporated into authigenic phases. These ratios do, however, show distinct differences between the six horizons (Fig. 2). From Horizon A to C during the Katian stage, Corg/Porg and Corg/Preac ratios increase gradually, suggesting a progressive increase in P recycling to the water column (Fig. 3), consistent with the proposed redox evolution from oxic, through ferruginous, to weakly euxinic conditions (Fig. 1). In particular, we note that the sediments deposited under ferruginous conditions document a relatively high degree of pyritization (see Supplementary Table 3 and ref. 4), which suggests that significant sulfide production during diagenesis promoted P recycling, even though the water column itself was not euxinic. These data imply a biogeochemical cascade, whereby enhanced bioavailability of P initially increased marine primary productivity and organic carbon production20,34, thus intensifying oxygen demand in the water column.
a Corg/Porg for the proximal section; b Corg/Porg for the distal section; c Corg/Preac for the proximal section; d Corg/Preac for the distal section. Horizons A–F are as identified in Fig. 1, and are broadly grouped into ranges (larger oval groupings). Samples that fall outside of the Horizon A–F groupings on the Corg/Preac plots reflect transitional redox conditions at the boundary between different horizons. These samples appear to have been affected by diagenetic over-printing as the redox state of the overlying water column evolved, and thus are not included in the modelling analysis. The red lines represent the molar Redfield ratio of 106:1.
The initial increase in bioavailable P may be related to a gradual increase in the weathering influx of P due to early plant evolution13, the weathering of volcanic arcs35,36, or deposition of volcanic ash17,18. In this latter regard, the presence of volcanic ash layers supports a pulse of volcanism at the base of the Wufeng Formation (Horizon A), which gradually decreased to Horizon C37, consistent with a mercury (Hg) anomaly taken to indicate enhanced volcanism across this interval38, as well as the presence of mass-independent sulfur isotope anomalies39. The close correspondence between the timing of enhanced volcanism and the initial development of water column anoxia suggests a potential link18, which may have been particularly pronounced in areas such as the Yangtze shelf Sea, where upwelling likely focused the supply of bioavailable P40, although we note that the initial driver of enhanced P inputs remains unresolved. Nevertheless, while enhanced P bioavailability would have initially promoted oxygen depletion in the water column and sulfide production in the sediments, the consequent enhanced recycling of P from the sediments then tipped the ocean into a weakly euxinic state in Horizon C, which progressed to more intense euxinia in Horizon D (Figs. 1, 3).
During deposition of Horizon D, which marks the onset and progression of the Hirnantian glaciation to its peak (Fig. 1), the intensity of chemical weathering significantly decreased4. This would have progressively decreased the weathering influx of sulfate, and sulfate would also have been extensively depleted from the euxinic water column through microbial reduction and removal as pyrite. Global sulfate limitation is supported by an increase in the δ34S composition of sedimentary pyrite at most sites during deposition of Horizon E (Supplementary Fig. 7; Supplementary Table 5), and this appears to have driven a global change in ocean redox state. However, while ferruginous conditions developed in the Yangtze Shelf Sea area during Horizon E (Figs. 1, 3), other locations appear to have been characterized by the development of oxic to dysoxic conditions. This suggests that the precise nature of the redox transition was controlled by local processes, specifically the depth of the redoxcline, which fluctuated as a function of glacio-eustasy.
Under ferruginous conditions in Horizon E, drawdown of phosphate from the water column in association with Fe minerals would be expected, which is supported by an increase in Paut (likely due to ‘sink switching’ from PFe), particularly in the more distal setting (Supplementary Fig. 6). Furthermore, the extent of recycling was significantly diminished, as indicated by Corg/Porg and Corg/Preac ratios closer to the Redfield ratio (Fig. 2). This would likely have led to decreased productivity, which is supported by lower organic carbon concentrations (Fig. 1). Less efficient recycling during deposition of Horizon E is also evident on a global scale, with a general increase in Ptot and lower Corg/Ptot ratios (Supplementary Note 5; Supplementary Fig. 8). This is consistent with the development of oxic or dysoxic conditions at other sites, and supports a global-scale sequestration of the high concentrations of dissolved P produced via recycling during deposition of Horizon D.
Water column sulfate concentrations would be expected to increase progressively after the cessation of euxinic conditions. Indeed, during the onset of deglaciation (Horizon F) there was a return to more widespread water column euxinia both in the Yangtze Shelf Sea (Fig. 3) and globally10, which may also be related to nutrient and sulfate upwelling during rapid deglaciation7,40,41. Under these conditions, Corg/Porg and Corg/Preac ratios again increased across the Yangtze Sea transect, suggesting a reintensification of P recycling back to the water column (Fig. 2). This helped to maintain persistent euxinic conditions4,10 and high organic carbon production and burial throughout this horizon (Fig. 1).
The Hirnantian glaciation was relatively short-lived, and its apex is thought to have involved ~5 °C of global cooling over a 1–2 Myr timeframe7. It is usually assumed that cooling resulted from CO2 drawdown13,35, and recent proxy estimates of late Ordovician CO2 point to levels below 1000 ppm42. These CO2 estimations appear consistent with a climate that was similar to the preindustrial (given the reduced solar flux) but substantial uncertainty remains, and previous model-based estimates were much higher43. Recent work has shown that a large volcanic P input event, which occurred coincident with the Hirnantian δ13C isotope excursion (HICE)18, appears to be capable of driving cooling synchronous with the Hirnantian glaciation. This is the only quantitative estimate of Hirnantian P inputs currently available, but biogeochemical modelling suggests that it was not sufficient to drive significant cooling unless there was a high degree of P recycling, estimated at around four times the P input flux18. Our data show that elevated P recycling during the later Katian and Hirnantian is indeed synchronous with late Ordovician cooling (Figs. 1, 3), implying that the enhanced organic carbon burial brought about by P recycling contributed significantly to the Hirnantian glaciation. If correct, this may explain why the glaciation was relatively short-lived, given that the recycling feedback is a transient phenomenon driven by oceanic redox state. Indeed, a substantial contribution from CO2 drawdown through enhanced burial of organic carbon, rather than via changes in silicate weathering, is supported by records of increased organic carbon burial44,45 during the Hirnantian.
To explore this hypothesis we utilize the COPSE biogeochemical model46 (see Methods). We begin with the simulations of ref. 18, and plot model runs under the assumed enhanced P input from volcanism (i.e., with no recycling) as the red line in Fig. 4. Here, we assume that the maximum estimate of the total P influx from ash deposition and the weathering of tephra was delivered between about 450 and 440 Ma (see Methods). For the next model runs, shown in purple, we alter the COPSE model so that sedimentary P burial is co-constrained by the model rate of organic carbon burial and our measured Corg:Preac ratio. In this way the model is forced to implement the degree of P recycling observed in the geological record, but is still free to calculate organic carbon burial rates and marine [PO4] based on its internal biosphere and continental inputs of P. We force the model with the mean Corg:Preac ratio of our combined proximal and distal datasets, and apply the forcing to 80% of the organic carbon burial flux, which is a conservative estimate for the proportion of organic carbon deposited on the shelf47. Here, we note that while there are no other datasets with which to compare our P phase association data, global trends in Ptot and Corg/Ptot across horizons A–F provide strong support for the globally representative nature of the relative changes we observe in our Corg/Preac data (Supplementary Fig. 8; Supplementary Table 5).
a Corg/Preac; b Corg burial; c Atmospheric CO2; d Global average surface temperature and ice cap latitude. Model background run from ref. 54 shown as a black dashed line. Red lines show the effects of a pulse of P weathering18, and purple lines show the combined effect of the same P weathering pulse with additional P recycling, as imposed using the sedimentary Corg:Preac ratios from our empirical data (shown as the average and lower limit in panel a). The combined model output is shown as a range representing the average and lower limit (where the purple shading represents the average and lower limits, and the purple line represents the mid-point of these two values), in order to provide a conservative estimate for the impact of P recycling. The model predicts substantial increases in P recycling and organic C burial across the interval sampled (b). This also draws down atmospheric CO2 (c), reducing surface temperature (d). Ice cap latitude is shown in panel d as a blue shaded area, following ref. 36. As discussed in the main text and Supplementary Information, the model results imply a more rapid increase in organic C burial and consequent decrease in CO2 and temperature across Horizons A and B (shown as a dashed purple line) than suggested by the geologic record. This is attributed to a global overestimation of phosphate bioavailability based on our data from shelf settings, due to subsequent drawdown of recycled phosphate in deeper ocean settings that were poised at a less reducing state than during horizons C–F.
Our full model results (Fig. 4) show that changes to the P recycling rate derived from our data result in substantial changes to the carbon cycle. As sedimentary Corg/Preac ratios begin to increase across Horizons A–C, the global rate of organic carbon burial more than doubles, and atmospheric CO2 concentration is reduced from around 900 ppm to around 500 ppm. This causes ~4 °C of global cooling between the earlier Katian and the main phase of Hirnantian glaciation, which is similar to the decrease predicted by isotopic temperature proxies7,48 (although oxygen isotope temperature proxies tend to predict higher temperatures overall49). The model also produces a simplified carbonate-carbon isotope (δ13Ccarb) record, and this records a positive excursion of around 7‰, similar to the observed Hirnantian δ13Ccarb excursion (Supplementary Fig. 9). As expected, organic C burial is reduced during Horizon E where euxinia abates and P recycling is curtailed, and then increases again during Horizon F when shelf euxinia returns (Fig. 4). In fact, on a global scale, the decrease in organic C burial during Horizon E was likely even greater, since our identification of a degree of P recycling under ferruginous conditions on the Yangtze Shelf (Fig. 2) appears to contrast with more extensive P drawdown under oxic-dysoxic conditions in other global localities (Supplementary Fig. 8; Supplementary Table 5).
The model temperature prediction is compared qualitatively to the expansion of ice caps from the geological record36 (Fig. 4d). The timing of ice cap advance and retreat, which occurs between ~447 and ~442 Ma shows a reasonable agreement with our model predictions, although the model has the onset of cooling earlier, between 448 and 447 Ma (Fig. 4d), and also predicts an earlier onset of the positive δ13Ccarb excursion than is observed in the δ13Ccarb record (Supplementary Note 6; Supplementary Fig. 9). This may be explained by the simplicity of the model and its input variables, which are not defined at sufficiently high resolution and lack spatial characteristics that are important for calculating continental surface processes. In particular, the moderate level of P recycling recorded under ferruginous conditions in Horizon B drives the beginning of the carbon burial event (Figs. 2, 4). However, during these early stages of anoxia, it is likely that the global deeper ocean would have been poised at an overall less reducing state, which would have promoted drawdown of recycled phosphate25,32, thus reducing the global bioavailability of P. This deeper ocean drawdown of P during the initiation of shallower water anoxia is not factored into our biogeochemical model, which thus results in an earlier onset of CO2 drawdown. Nevertheless, despite this discrepancy, the fact that our model reproduces both the observed ~7‰ δ13Ccarb excursion and a multi-million-year negative temperature shift by several degrees, supports the suggestion that P recycling played a critical role in driving the Hirnantian glaciation.
Conclusions
Our high-resolution reconstruction of redox and P recycling on the Yangtze Shelf Sea in South China clearly demonstrates two expansions of euxinia and associated rises in P recycling during the late Ordovician (Fig. 3). Once anoxia was established, the increased recycling of P promoted a major increase in marine primary productivity. This not only drove the two extinction phases of the LOME through the development of euxinic conditions, but also resulted in large-scale CO2 drawdown and transient deep glaciation during the Hirnantian, which played a secondary role in driving the extinction of certain low-latitude taxa4. We thus conclude that redox-promoted P recycling was a critical factor in driving Earth’s first major extinction of animal life.
Methods
Total organic carbon (Corg) and carbon isotope (δ13Corg) analyses
Samples for all analyses were collected by the authors and no permissions were required. Corg and δ13Corg values for the proximal Shuanghe inner-shelf (SH) outcrop section are from ref. 4. For samples from the distal outer-shelf Wuxi (WX) drill core section, sample powders were decarbonated with HCl (10% vol/vol) prior to Corg analysis on a LECO CS-230 analyzer. Analytical precision for Corg was generally better than 5%. Sample powders for δ13Corg measurements were also first decarbonated with HCl (10% vol/vol). The decalcified samples were combusted for isotopic analysis using cryogenically purified CO2 in a Finnigan MAT-253 mass spectrometer. All δ13Corg data are reported in standard δ-notation relative to the Vienna Pee Dee Belmnite (VPDB) standard, with a precision of ±0.1‰ (1σ level).
Elemental concentrations
Major (FeT and Ptot) and trace elements (Mo and U) in the distal outer-shelf Wuxi (WX) drill core section were measured by the methods in ref. 4. Sample powders for major elements were heated and mixed with flux (Li2B4O7) in a proportion of 1:5 before analysis. Sample powders for trace elements were dissolved with HF + HNO3 prior to analysis. Major elements were measured by X-ray fluorescence spectrometery (XRF-1500), and trace elements were measured on a VG PQ2 Turbo inductively coupled plasma source mass spectrometer (FINNIGAN MAT). Analytical precision was better than 3% for all elements of interest.
Fe-S systematics
For the operationally defined Fe extraction method50, Fecarb (targeting Fe in carbonate minerals, including siderite and ankerite) was extracted with a sodium acetate solution (pH = 4.5) for 48 h at 50 °C, Feox (targeting Fe (oxyhydr)oxide minerals) was extracted with a citrate-dithionite-acetate solution (pH = 4.8) for 2 h at room temperature, and Femag (targeting mixed ferrous-ferric phases, particularly magnetite) was extracted with an ammonium oxalate solution for 6 h at room temperature. These three steps were performed sequentially, with the extracted Fe measured by atomic adsorption spectroscopy. FeAVS (acid volatile sulfide) and FePy (pyrite) were extracted by the two step-acid Cr(II) method51, with concentrations determined gravimetrically. Replicate analysis gave RSDs of <5% for all phases, and accuracy was confirmed relative to an Fe speciation reference material (WHIT)52.
P phase partitioning
A recently revised sequential extraction method30 was used to determine the phase partitioning of P. PFe1 (which targets poorly crystalline Fe (oxyhydr)oxide-bound P) was extracted with a dithionite-citrate-bicarbonate solution (pH 7.6) for 8 h at room temperature. Authigenic P (Paut; which targets carbonate fluorapatite) was extracted with a sodium acetate solution at pH 4 for 6 h at room temperature. Detrital apatite (Pdet) was extracted with a 10% HCl solution for 16 h at room temperature. Pmag (which targets P associated with magnetite) was extracted with an ammonium oxalate solution for 6 h at room temperature. PFe2 (which targets more crystalline Fe (oxyhydr)oxide minerals) was extracted with a dithionite-citrate-acetate solution (pH 4.8) for 8 h at room temperature. Finally, the residue was ashed (550 °C for 2 h) and reacted with 10% HCl for 16 h at room temperature to liberate organic-bound P (Porg). The sum of PFe1 + PFe2 + Pmag gives PFe. The P content of the various extracts was determined either spectrophotometrically via the molybdate blue method53, or by ICP-OES for solutions where the matrix interferes with the molybdate blue method (PFe1, PFe2 and Pmag). Replicate extractions gave RSDs of <2.1% for PFe1, <2.2% for Pauth, <2.9% for Pdet, <1.0% for Pmag, <1.0% for PFe2, and <2.0% for Porg.
Biogeochemical modeling
We use the COPSE (Carbon Oxygen Phosphorus Sulfur Evolution) global biogeochemical model54. COPSE is an extension of the GEOCARB55 box model framework, which incorporates a dynamic biosphere. We use the latest model version54, which provides a ‘baseline’ run through the Paleozoic Era. A key uncertainty in this previous run is the degree to which the evolution of vascular land plants enhanced continental weathering rates56, which alters background conditions in the prevascular world, and which we set here at a factor of 0.5 relative to present day, resulting in background Ordovician CO2 concentrations of around 1000 ppm, close to proxy estimates42. To produce the red lines in Fig. 4 we use the model baseline and add an assumed enhancement of P weathering inputs in line with previously published work18. This is a gaussian increase in P input between 450 and 440 Ma, with a total flux of 4.59 × 1016 mol P, taken directly from the maximum estimates of ref. 18, assuming weathering continued for the entire 10 Myr input timeframe. We also alter the P burial functions to calculate total marine P burial based on the model organic C burial rate and the Corg:Preac ratio from our dataset (purple lines). When Corg:Preac rises substantially above the Redfield ratio, the P burial term becomes very small, and most potentially bioavailable P is recycled from the sediment. The model P burial is calculated as (1):
where fshelf is the fraction of organic carbon burial that occurs on the shelf, taken as 0.8. Fmocb is the marine organic carbon burial rate, CPreac is the measured Corg:Preac ratio, and CPreac0 is the first value in our dataset to which the model is initiated. This formulation therefore assumes that the deep ocean P burial flux is unaffected by the dynamics we observe in our sections. Note also that we do not assume that organic carbon burial is directly affected by anoxia. Increasing Fmocb in this way would bury more P for a given CPreac, which would reduce the P recycling flux. However, this would be countered by having buried more organic carbon in the first place, and would still produce a Corg burial event.
Data availability
All data generated during this study are included in the Supplementary Information and are available at EarthChem (https://doi.org/10.26022/IEDA/112232).
Code availability
The COPSE model can be downloaded for free at bjwmills.com.
References
Fan, J. et al. A high-resolution summary of Cambrian to Early Triassic marine invertebrate biodiversity. Science 367, 272–277 (2020).
Harper, D. A. T., Hammarlund, E. U. & Rasmussen, C. M. Ø. End Ordovician extinctions: a coincidence of causes. Gondwana Res. 25, 1294–1307 (2014).
Hammarlund, E. U. et al. A sulfidic driver for the end-Ordovician mass extinction. Earth Planet. Sci. Lett. 331–332, 128–139 (2012).
Zou, C. et al. Ocean euxinia and climate change ‘double whammy’ drove the Late Ordovician mass extinction. Geology 46, 535–538 (2018).
Bartlett, R. et al. Abrupt global-ocean anoxia during the Late Ordovician–Early Silurian detected using uranium isotopes of marine carbonates. Proc. Natl Acad. Sci. USA 115, 5896–5901 (2018).
Vandenbroucke, T. R. A. et al. Polar front shift and atmospheric CO2 during the glacial maximum of the Early Paleozoic Icehouse. Proc. Natl Acad. Sci. USA 107, 14983–14986 (2010).
Finnegan, S. et al. The magnitude and duration of Late Ordovician-Early Silurian glaciation. Science 331, 903–906 (2011).
Saupe, E. E. et al. Extinction intensity during Ordovician and Cenozoic glaciations explained by cooling and palaeogeography. Nat. Geosci. 13, 65–70 (2020).
Ahm, A. S. C., Bjerrum, C. J. & Hammarlund, E. U. Disentangling the record of diagenesis, local redox conditions, and global seawater chemistry during the latest Ordovician glaciation. Earth Planet. Sci. Lett. 459, 145–156 (2017).
Stockey, R. G. et al. Persistent global marine euxinia in the early Silurian. Nat. Commun. 11, 1804 (2020).
Kump, L. R. et al. A weathering hypothesis for glaciation at high atmospheric pCO2 during the Late Ordovician. Palaeogeogr. Palaeoclimatol. Palaeoecol. 152, 173–187 (1999).
Finlay, A. J., Selby, D. & Grocke, D. R. Tracking the Hirnantian glaciation using Os isotopes. Earth Planet. Sci. Lett. 293, 339–348 (2010).
Lenton, T., Crouch, M., Johnson, M., Pires, N. & Dolan, L. First plants cooled the Ordovician. Nat. Geosci. 5, 86–89 (2012).
Lenton, C. M. O. et al. Earliest land plants created modern levels of atmospheric oxygen. Proc. Natl Acad. Sci. USA 113, 9704–9709 (2016).
Kenrick, P., Wellman, C., Schneider, H. & Edgecombe, G. A timeline for terrestrialization: consequences for the carbon cycle in the Palaeozoic. Phil. Trans. R Soc. Lond. B 367, 519–536 (2012).
Porada, P. et al. High potential for weathering and climate effects of nonvascular vegetation in the Late Ordovician. Nat. Commun. 7, 12113 (2016).
Buggisch, W. et al. Did intense volcanism trigger the first Late Ordovician icehouse? Geology 38, 327–330 (2010).
Longman, J. et al. Volcanic nutrient supply initiated Late Ordovician climate change and extinctions. Nat. Geosci 14, 924–929 (2021).
Tyrrell, T. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 400, 525–531 (1999).
Van Cappellen, P. & Ingall, E. D. Redox stabilization of the atmosphere and oceans by phosphorus-limited marine productivity. Science 271, 493–496 (1996).
Ingall, E. D., Bustin, R. M. & Cappellen, P. V. Influence of water column anoxia on the burial of organic carbon and phosphorus in marine shales. Geochim. Cosmochim. Acta 57, 303–316 (1993).
Ingall, E. D. & Jahnke, R. Evidence for enhanced phosphorus regeneration from marine sediments overlain by oxygen depleted waters. Geochim. Cosmochim. Acta 58, 2571–2575 (1994).
Algeo, T. J. et al. Changes in productivity and redox conditions in the Panthalassic Ocean during the latest Permian. Geology 38, 187–190 (2010).
Bowyer, F. T. et al. Regional nutrient decrease drove redox stabilisation and metazoan diversification in the late Ediacaran Nama Group, Namibia. Sci. Rep. 10, 2240 (2020).
Schobben, M. et al. A nutrient control on marine anoxia during the end-Permian mass extinction. Nat. Geosci. 13, 640–946 (2020).
Dale, A. W., Boyle, R. A., Lenton, T. M., Ingall, E. D. & Wallmann, K. A model for microbial phosphorus cycling in bioturbated marine sediments: Significance for phosphorus burial in the early Paleozoic. Geochim. Cosmochim. Acta 189, 251–268 (2016).
Ruttenberg, K. C. Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnol. Oceanogr. 37, 1460–1482 (1992).
Egger, M., Jilbert, T., Behrends, T., Rivard, C. & Slomp, C. P. Vivianite is a major sink for phosphorus in methanogenic coastal surface sediments. Geochim. Cosmochim. Acta 169, 217–235 (2015).
Xiong, Y. et al. Phosphorus cycling in Lake Cadagno, Switzerland: a low sulfate euxinic ocean analogue. Geochim. Cosmochim. Acta 251, 116–135 (2019).
Thompson, J. et al. Development of a modified SEDEX phosphorus speciation method for ancient rocks and modern iron-rich sediments. Chem. Geol. 524, 383–393 (2019).
Creveling, J. R. et al. Phosphorus sources for phosphatic Cambrian carbonates. Geol. Soc. Am. Bull. 126, 145–163 (2014).
Guilbaud, R. et al. Phosphorus-limited conditions in the early Neoproterozoic ocean maintained low levels of atmospheric oxygen. Nat. Geosci. 13, 296–301 (2020).
Krom, M. D. & Berner, R. A. The diagenesis of phosphorus in a nearshore marine sediment. Geochim. Cosmochim. Acta 45, 207–216 (1981).
Bjerrum, C. J., Bendtsen, J. & Legarth, J. J. F. Modeling organic carbon burial during sea level rise with reference to the Cretaceous. Geochem. Geophys. Geosyst. 7, Q05008 (2006).
Young, S. A. et al. A major drop in seawater 87Sr/86Sr during the Middle Ordovician (Darriwilian): Links to volcanism and climate? Geology 37, 951–954 (2009).
Macdonald, F. A. et al. Arc-continent collisions in the tropics set Earth’s climate state. Science 364, 181–184 (2019).
Qiu, Z. & Zou, C. Unconventional petroleum sedimentology: connotation and prospect. Acta Sediment. Sin. 38, 1–29 (2020).
Hu, D. et al. Major volcanic eruptions linked to the Late Ordovician mass extinction: Evidence from mercury enrichment and Hg isotopes. Glob. Planet. Chang. 196, 103374 (2021).
Hu, D. et al. Large mass independent sulphur isotope anomalies link stratospheric volcanism to the Late Ordovician mass extinction. Nat. Commun. 11, 2297 (2020).
Yang, S., Hu, W. & Wang, X. Mechanism and implications of upwelling from the Late Ordovician to early Silurian in the Yangtze region, South China. Chem. Geol. 565, 120074 (2021).
Rong, J. Y. et al. The latest Ordovician Hirnantian brachiopod faunas: new global insights. Earth Sci. Rev. 208, 103280 (2020).
Witkowski, C. R. et al. Molecular fossils from phytoplankton reveal secular PCO2 trend over the Phanerozoic. Sci. Adv 4, eaat4556 (2018).
Berner, R. A. & Kothavala, Z. Geocarb III: a revised model of atmospheric CO2 over Phanerozoic time. Am. J. Sci 301, 182–204 (2001).
Brenchley, P. et al. High-resolution stable isotope stratigraphy of Upper Ordovician sequences: constraints on the timing of bioevents and environmental changes associated with mass extinction and glaciation. Geol. Soc. Am. Bull. 115, 89–104 (2003).
Shen, J. et al. Improved efficiency of the biological pump as a trigger for the Late Ordovician glaciation. Nat. Geosci. 11, 510–514 (2018).
Lenton, T. M. et al. COPSE reloaded: an improved model of biogeochemical cycling over Phanerozoic time. Earth Sci. Rev. 178, 1–28 (2018).
Slomp, C. P. & Van Cappellen, P. The global marine phosphorus cycle: sensitivity to oceanic circulation. Biogeosciences 4, 155–171 (2007).
Scotese, C. R. et al. Phanerozoic Paleotemperatures: The Earth’s Changing Climate during the Last 540 million years. Earth Sci. Rev. 215, 103503 (2021).
Grossman, E. L. & Joachimski, M. M. in Geologic Time Scale 2020, 279–307, (Elsevier, 2020).
Poulton, S. W. & Canfield, D. E. Development of a sequential extraction procedure for iron: implications for iron partitioning in continentally derived particulates. Chem. Geol. 214, 209–221 (2005).
Canfield, D. E. et al. The use of chromium reduction in the analysis of reduced inorganic sulfur in sediments and shales. Chem. Geol. 54, 149–155 (1986).
Alcott, L. et al. Development of iron speciation reference materials for palaeoredox analysis. Geostand. Geoanal. Res. 44, 581–591 (2020).
Strickland, J. D. H. & Parsons, T. R. in A Practical Handbook of Seawater Analysis, 2nd edn., 167, 45–64 (Fisheries Research Board of Canada, Ottawa, Bulletin, 1972).
Tostevin, R. & Mills, B. J. W. Reconciling proxy records and models of Earth’s oxygenation during the Neoproterozoic and Palaeozoic. Interface Focus 10, 20190137 (2020).
Berner, R. A. A model for atmospheric CO2 over Phanerozoic time. Am. J. Sci. 291, 339–376 (1991).
Lenton, T. The role of land plants, phosphorus weathering and fire in the rise and regulation of atmospheric oxygen. Global Chang. Biol. 7, 613–6297 (2001).
Acknowledgements
This work was supported by NSFC (grants 41888101 and 41602119) and the Scientific Research and Technological Development Program of CNPC (2021yjcq02). B.J.W.M. and S.W.P. are supported by the UK Natural Environment Research Council (grants NE/S009663/1 and NE/R010129/1), and S.W.P. acknowledges support from a Royal Society Wolfson Research Merit Award. We thank Christian Bjerrum for useful comments on earlier versions of the manuscript, Tianchen He for helpful discussion, and Hongyan Wang, Qun Zhao, and Dazhong Dong for assistance with sampling and geochemical analysis. We are also grateful to professors Rixing Zhu, Jiayu Rong, Xu Chen, Pingan Peng, Chengshan Wang, Shuzhong Shen, Zhijun Jin, and Fang Hao of the Chinese Academy of Sciences for helpful discussions.
Author information
Authors and Affiliations
Contributions
C.N.Z., Z.Q., and S.W.P. designed the research. Z.Q., H.F.T., and B.L. collected samples. Z.Q., Y.J.X., H.F.T., and H.L.L. performed analyses. Z.Q., S.W.P., B.J.W.M., C.N.Z., and W.J.X. interpreted the data. Z.Q., S.W.P., and B.J.W.M. wrote the manuscript, with contributions from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Kazumi Ozaki, Ellery Ingall and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Joe Aslin, Heike Langenberg.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qiu, Z., Zou, C., Mills, B.J.W. et al. A nutrient control on expanded anoxia and global cooling during the Late Ordovician mass extinction. Commun Earth Environ 3, 82 (2022). https://doi.org/10.1038/s43247-022-00412-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-022-00412-x
This article is cited by
-
In-situ δ18O and 87Sr/86Sr proxies in an unconformable clastic unit at the Ordovician–Silurian transition
Scientific Reports (2023)
-
The influence of Tethyan evolution on changes of the Earth’s past environment
Science China Earth Sciences (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.