Abstract
Within the breath lie numerous health indicators, encompassing respiratory patterns and biomarkers extending beyond respiratory conditions to cardiovascular health. Recently, the emergence of the SARS-CoV-2 pandemic has not only underscored the necessity of on-the-spot breath analysis but has also normalized the use of masks in everyday life. Simultaneously, the rapid evolution of wearable technology has given rise to innovative healthcare monitoring tools, with a specific emphasis on wearable breath sensors. This review explores current research trends in utilizing wearable breathing sensors to detect diverse respiratory biomarkers and monitor respiratory parameters, including airflow, temperature, and humidity. Additionally, it explores diverse applications, ranging from recognizing breathing patterns to swiftly detecting diseases. Integrating the Internet of Things and machine learning technologies into these applications highlights their potential to offer a personalized, accurate, and efficient healthcare solution.
Similar content being viewed by others
Introduction
Breath analysis has long been a cornerstone in clinical diagnostics, offering a window into an individual’s systemic health. This non-invasive method harnesses the diverse array of compounds within exhaled breath, including volatile organic compounds (VOCs), semi-volatile organic compounds (SVOCs), proteins, lipids, DNA, bacteria, and viruses, offering a diagnostic window into various physiological and pathological states1,2,3. Recent advancements in material science, nanotechnology, and soft electronics have propelled the development of intelligent wearable sensors4,5,6. These breakthroughs, Internet of Things (IoT) technologies, and machine learning algorithms have expanded sensor capabilities far beyond conventional monitoring7,8. Specifically, the latest respiratory sensing technologies allow wearable breathing sensors to seamlessly integrate into patches under the nose or within masks, ensuring the continuous collection of real-time breathing data9,10. This convergence, complemented by IoT systems and machine learning integration, promises real-time monitoring and analysis of sensor data, poised to revolutionize healthcare and clinical applications.
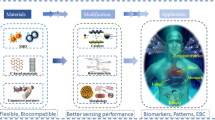
This review article delves into traditional breath sampling and analysis methods, transitioning to exploring the latest research trends in wearable breath sensors for real-time health monitoring. It elucidates the construction and mechanisms of these sensors, highlighting their real-time detection capabilities for various respiratory biomarkers and their ability to monitor crucial parameters such as airflow, temperature, and humidity. Beyond technological advancements, the article explores diverse applications, from breathing pattern recognition to real-time disease detection, illustrating how integrating IoT and machine learning technologies enhances the functionality of these devices, ensuring more personalized, accurate, and efficient health monitoring through comprehensive breath analysis (Fig. 1).
Breath sampling
Discussing effective breath-capturing methods is essential to analyze the nuanced composition within exhaled breath thoroughly. Conventionally, the breath sample could be sampled in two ways: Blow exhaled breath directly to the analysis equipment or in a container such as polymer bags11,12. Additional breath sample pre-concentration process for more precise analyzation would be conducted via several methods, utilizing thermal desorption tubes, solid phase microextraction (SPME), and needle trap devices12.
Several breath-collecting devices have been established for a more comfortable and portable collection of exhaled breath. For example, the ReCIVA® (Owlstone Medical, Cambridge, UK) device comprises a disposable face mask connected to a portable sampling device, which could collect and pre-concentration breath VOCs13. In addition to the VOC inside the exhaled breath, collection methods like exhaled breath condensate (EBC) and associated exhaled breath aerosol (EBA) sampling have gained attention for retrieving SVOCs, proteins, lipids, DNA, bacteria, and viruses from the lower airways1,14. Portable EBC collection devices include a mouthpiece, a one-way valve, and a tube with a cooling container or sleeve to condense exhaled aerosols into EBC15.
Recently, due to the COVID-19 pandemic, the adoption of face masks for public health has surged, prompting innovations in wearable breath collector systems for daily use. A simple example is the integration of a porous membrane as a breathing collector inside a face mask. For example, Soto et al. introduced a porous polycarbonate membrane integrated into various face masks, serving as a flexible exhaled breath collector16. Leveraging the porous membrane’s amplified surface area facilitated increased liquid collection compared to nonporous surfaces. Similarly, porous paper strips were affixed to the masks’ inner and outer surfaces, demonstrating an effective capture of exhaled VOCs and droplets and the adsorption of ambient air during inhalation17. Another notable strategy showed an EBA collection system utilizing SPME techniques, employing SPME fibers fixed within face masks to collect EBA18. Furthermore, a wearable EBC collector integrated within face masks was developed, utilizing a Teflon-coated cooling trap to gather EBC for SARS-CoV-2 detection efficiently19. Pre-cooling the mask at −20 °C enabled the condensation of exhaled breath on the polytetrafluoroethylene trap surface, facilitating easy sample retrieval without exposing the mask’s interior.
Breath analysis
Conventional breath analysis methods such as gas chromatography coupled with mass spectrometry (GC-MS), selected-ion flow-tube mass spectrometry (SIFT-MS), and proton-transfer-reaction mass spectrometry (PTR-MS) have been widely used to non-invasively diagnose related conditions by detecting various components in exhaled breath20. GC-MS, the most widely used tool, separates and analyzes breath samples through a two-step process involving gas chromatography and mass spectrometry21. SIFT-MS and PTR-MS, on the other hand, provide real-time analysis, with SIFT-MS utilizing soft chemical ionization for rapid identification and quantification of VOCs and PTR-MS employing proton transfer reactions for high-sensitivity analysis of metabolic processes and disease biomarkers in exhaled breath22,23.
An optical method has been developed as an alternative technique to analyze the exhaled breath components. Optical absorption spectroscopy in the near- to mid-infrared has proven its usefulness in biomarker detection. Nevertheless, challenges arise in detecting a diverse array of molecules due to limitations in controlling laser light sources24. To address this, researchers proposed using optical frequency combs-based techniques, spanning the ultraviolet to far-infrared region, for a more comprehensive approach24,25. In addition, cavity-enhanced absorption spectroscopies, which utilize multiple reflections of light within an optical cavity, have also been proposed to increase sensitivity26. For instance, Liang et al. utilized mid-infrared cavity-enhanced direct frequency comb spectroscopy to detect multiple biomarkers in real-time with parts-per-trillion level sensitivity27. Moreover, studies utilizing surface-enhanced Raman scattering (SERS) have also been reported that exploit scattering spectra beyond conventional light absorption. For example, Huang et al. demonstrated the feasibility of aldehyde and ketone biomarker detection and subsequent noninvasive gastric cancer diagnosis by performing high-performance SERS analysis by effectively trapping gases through a silver nanoparticle/metal-organic framework-coated glass capillary-like chamber28.
An alternative approach utilizes electrochemical techniques for gas analysis, incorporating a transistor-type structure and substances with selectivity for specific gases. This method facilitates the analysis of VOCs and other gases. For example, carbon-based materials like carbon nanotubes (CNTs) and two-dimensional MXene can be employed for detecting oxygen (O2) and ammonia (NH3)29,30,31. Additionally, catalytic nanoparticles such as Pt, Pd, and Au react with NH3, leading to changes in resistance and conductivity32,33,34,35. These modifications influence the channel conductance, resulting in changes in source-drain current. Detecting this current alteration allows for identifying the existence and concentration of each gas. In addition, recent efforts focus on integrating these gas sensors into a unified array system. Individual sensors in this array system are tailored with selectivity for specific gases, reacting chemically only to particular gases, thereby inducing changes in electrical characteristics. A prominent example of this approach is an electronic nose (e-nose), which mimics the olfactory system of mammals. The e-nose consists of multiple gas sensors into a single system, ensuring each sensor exhibits responsiveness to specific gases. The overall configuration involves capturing and adsorbing gases using materials that undergo electrochemical changes and measuring the difference in electrical signals due to the altered electrical characteristics of these materials. The variance in electrical signals is typically measured through changes in source-drain current, particularly in organic field-effect transistor configurations. Recently developed healthcare-oriented e-noses are designed at a portable level, enabling real-time data collection. Attempts to apply machine learning algorithms to the collected data are ongoing, aiming to enable assessments of diseases and health conditions based on the gathered information.
In addition to VOC analysis of the exhaled breath, there have been attempts to detect pathogens within EBC using various molecular diagnostic methods. For instance, Houspie et al. employed reverse transcription polymerase chain reaction to detect 14 respiratory viruses in EBC36. Furthermore, Loop-mediated isothermal amplification was used to screen for bacterial pathogens in EBC collected from subjects exhibiting respiratory infection symptoms37.
Wearable breath sensor for biomarker detection
Conventional techniques for sampling and analyzing breath biomarkers offer high sensitivity, specificity, and quantitative analysis, facilitating the diagnosis of various health conditions. Nevertheless, their drawbacks, such as high cost, complexity, and large instrument size, pose challenges to portability and accessibility, limiting their application primarily to clinical or experimental settings. In response to these challenges, there is a growing interest in continuous sampling with direct sensing, particularly through wearable devices like masks or patches. Integrating biomarker detection into these wearable technologies enables real-time monitoring with enhanced comfort and portability. This shift from conventional methods to wearable real-life monitoring promises to revolutionize disease detection and expand the scope of healthcare applications, making biomarker analysis a seamless and integral part of everyday life.
Oxygen
O2 is a vital element for sustaining life and is necessary for cellular metabolism. Monitoring the O2 levels in the breath allows for assessing an individual’s lung function, tissue oxygenation, and overall respiratory health. Deviations in O2 levels can signal conditions like hypoxia (O2 deficiency) or hyperoxia (excess O2), both holding clinical significance in healthcare38,39.
One common method for O2 detection involves employing an electrochemical sensing mechanism, which leverages the reduction/oxidation of O2 at an electrode surface, leading to modifications in electrical signals. Carbon-based conductive materials such as CNTs detect O2 by changing material resistance29. As an alternative, creating a hydrogel network with embedding ions allows the measurement of current generated by the movement of ions reacting between O2 and electrodes, providing a method for O2 detection (Fig. 2a)40. In cases of the electrochemical sensing method, there have been ongoing efforts to produce wearable devices, including mask-type and patch-type, primarily using flexible and stretchable materials or incorporating elastomers. This approach allows continuous O2 monitoring, facilitating immediate treatment and care before severe situations arise.
a Hydrogel-based electrochemical breath oxygen sensor. Reproduced with permission from ref. 40, copyright (John Wiley and Sons, 2023) b Wearable breath sensor for real-time carbon dioxide detection utilizing the optical method. Reproduced with permission from ref. 45, copyright (Springer Nature, 2022) c A wearable and disposable breath ammonia sensor based on paper comprised of PEDOT:PSS/Iron(III) chloride composite. Reproduced with permission from ref. 51, copyright (John Wiley and Sons, 2022) d Nanofiber-based breath ammonia sensor with dual-sensing mechanisms of colorimetric and electrochemical. Reproduced with permission from ref. 52, copyright (American Chemical Society, 2023) e Disposable paper-based electrochemical breath hydrogen peroxide sensor. Reproduced with permission from ref. 54, copyright (American Chemical Society, 2019) f Electrochemical Hydrogen peroxide sensor with the microfluidic exhaled breath composite (EBC) collector. Reproduced with permission from ref. 55, copyright (Elsevier, 2023) g CRISPR-based SARS-CoV-2 detecting sensor integrated into the face mask. Reproduced with permission from ref. 56, copyright (Springer Nature, 2021) h Impedance-based breath pathogen sensor, especially for detecting SARS-CoV-2 spike protein. Reproduced with permission from ref. 57, copyright (Elsevier, 2021).
Carbon dioxide
Carbon dioxide (CO2) is a primary byproduct of oxidative metabolism. Hypercapnia, signifying an excessive of CO2, can result in respiratory acidosis, characterized by symptoms such as confusion, lethargy, and in severe cases, respiratory failure41. Conversely, hypocapnia, indicating insufficient CO2, can cause respiratory alkalosis, characterized by symptoms such as dizziness, lightheadedness, and, in severe cases, fainting42.
Non-dispersive infrared (NDIR) sensing mechanisms, which utilize CO2’s ability to absorb infrared light at specific wavelengths, are known for effective non-wearable CO2 detection43. In the NDIR sensing process, infrared light traverses the gas sample, and by measuring the intensity of light transmitted through the gas path, the concentration of CO2 can be obtained44. However, the NDIR sensing method faces limitations for wearable applications due to the challenges of reducing the size and weight of the NDIR sensor. Additionally, power consumption is substantial, making achieving the required energy efficiency for wearables difficult.
To address these limitations and achieve selectivity for CO2 in wearable applications, an alternative CO2 sensor utilizing luminescent materials becomes viable. Optical CO2 sensors, employing metal oxide semiconductors, utilize ultraviolet (UV) light to generate free electron-hole pairs. This action alters the potential barrier and reduces the depletion region. Additionally, photo-dissociation occurs, forming new chemical species that demonstrate changes in electrical characteristics or luminescence. Lanthanum oxide emerges as a notable luminescent material for CO2 sensing. Escobedo et al. developed a mask-shaped CO2 sensor utilizing a phosphor lanthanum oxysulfide:europium (La2O2S:Eu), with a wide energy gap (Eg~4.6 eV) (Fig. 2b)45. The emission response of this sensor to UV light exposure was used as an indicator of CO2 concentration. Another method employed TiO2(Eg~3.2 eV)/Al2O3 heterostructure, where UV light generates electron-hole pairs, enhancing the conductivity of the channel46.
Ammonia
NH3 is a byproduct generated through the digestion of proteins. In the liver, the produced NH3 undergoes processing and is subsequently converted into urea. In the presence of health issues like liver and kidney diseases or urea cycle disorder, NH3 levels in the blood would be elevated, resulting in an increased concentration of NH3 in exhaled breath47,48. Moreover, assessing NH3 levels in breath proves to be a valuable approach for evaluating halitosis49.
The continuous development of wearable breathing sensors involves the application of organic conducting polymers, for the electrochemical detection of NH350. Fujita et al. introduced a wearable and disposable NH3 sensor, where iron(III) chloride served as variant resistors, following the deposition of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) onto a flexible polypropylene film (Fig. 2c)51. The introduction of iron(III) chloride doping into PEDOT:PSS results in PEDOT:PSS:Fe3+, which exhibits a heightened response to NH3 compared to other VOCs. Furthermore, Chen et al. strategically enhanced sensitivity to NH3 by integrating dual-sensing mechanisms, employing a nanofiber-based hetero-structure that seamlessly combines both electrochemical and colorimetric approaches (Fig. 2d)52. The researchers also took a deliberate step to augment the porosity of the nanofiber film, aiming to further elevate the sensor’s sensitivity specifically to NH3.
Hydrogen peroxide
Hydrogen peroxide (H2O2) detection in exhaled breath holds significance due to its potential role as a non-invasive marker for inflammation and oxidative stress in the airways53. Recently, Prussian Blue-based electrochemical sensors were integrated into wearable breath sensors to detect the H2O2 level in exhaled breath. Maier et al. reported the disposable paper-based electrochemical sensor for continuous real-time monitoring of H2O2 in exhaled breath (Fig. 2e)54. This disposable paper-based was designed to be replaceable in commercial respiratory masks. Similarly, Cao et al. introduced a wearable system for monitoring breath H2O2 levels, consisting of a microfluidic EBC collector integrated with an electrochemical sensor utilizing a polyaniline/Prussian blue nanolayer (Fig. 2f)55. They employed a multi-microchannel design in the EBC collector and detected the H2O2 levels via a time-current curve analysis within a three-electrode system. These components were integrated into an N95 mask, enabling the measurement of H2O2 concentration in collected EBC and real-time monitoring displayed on an LED panel.
Pathogens
The recent SARS-CoV-2 pandemic has significantly heightened public consciousness regarding the importance of infectious disease prevention. Given the transmission characteristics of droplet-borne diseases, maintaining continuous vigilance in pathogen detection becomes critical. In this context, reports of wearable systems designed to detect SARS-CoV-2 by integrating droplet sampling technology with small, efficient pathogen detection sensors are worth noting. Nguyen et al. reported the synthetic biology sensor-based wearable SARS-CoV-2 monitoring device, which comprised a hydration reservoir, a sample pad, a microfluidic paper-based analytical device (µPAD), and a lateral flow assay strip (Fig. 2g)56. Following the collection of breath, capillary action facilitates the transfer of the gathered fluid and viral particles from the sample pad to the µPAD The µPAD encompasses freeze-dried reagents for viral lysis, transcription–recombinase polymerase amplification reaction, and a CRISPR-based Cas12a SHERLOCK sensor designed to target SARS-CoV-2 RNA. Triggered by a simple button press, the device operated autonomously, maintaining high sensitivity and specificity without requiring power or liquid handling. In addition, an impedance-based breath pathogen sensor was proposed to detect respiratory viruses, including the SARS-CoV-2 spike protein (Fig. 2h)57. Hydrophilic porous membrane efficiently collected droplets, and anti-spike functionalized nanowire arrays effectively captured the targeted viral proteins, resulting in detectable changes in impedance. By integrating this wearable sensor with an impedance circuit, the system enabled real-time wireless transmission of impedance data to a smartphone, demonstrating the promising potential for on-mask virus detection and monitoring.
Wearable breath sensor for respiratory parameter monitoring
Breakthroughs in material science and nano-microtechnology have led to the development of flexible, stretchable sensors specifically designed to measure pressure, temperature, and humidity58. These innovative sensors are now seamlessly integrated into face masks or attached as a patch to philtrum to continuously track key respiratory parameters of breath airflow, temperature, and humidity during inhalation and exhalation. This approach leads to the development of sophisticated respiration recognition systems. In this section, we delve into the mechanism of wearable breath sensors designed to monitor airflow, temperature, and humidity of breath meticulously. Furthermore, we explore the latest research developments in this rapidly evolving field.
Airflow monitoring
The expansion and contraction of the lungs create airflow, known as inhalation and exhalation. This generated airflow induces mechanical interaction with sensors crafted in the form of masks or patches (both within and outside the respiratory system), which is then converted into mechanical force or movement59,60. Subsequently, this is transformed into measurable electrical signals, allowing the measurement of patterns of respiratory changes.
The methods for measuring airflow in wearable form can be generally categorized into capacitive and resistive type. The capacitive type involves placing a dielectric material (e.g., high-k material, air gap) between parallel plates of conducting material. Changes in capacitance, particularly in the thickness of the dielectric layer due to airflow, are measured61,62. Yang et al. employed a thermoplastic polyurethane nanofiber membrane as the dielectric layer, incorporating silver nanowires for electrodes and poly(vinylidene fluoride) nanofiber membrane for encapsulation (Fig. 3a)63. These materials demonstrate air permeability, allowing airflow through the membranes, yet they exhibit impermeability to water, safeguarding against water droplets and ensuring stability, even in humid conditions.
a Capacitive breath airflow sensor. Reproduced with permission from ref. 63, copyright (John Wiley and Sons, 2017) b Resistive breath airflow sensor utilizing piezoresistive materials. Reproduced with permission from ref. 66, copyright (American Chemical Society, 2021) c Self-powered breath airflow sensors based on the piezoelectric effect. Reproduced with permission from ref. 67, copyright (John Wiley and Sons, 2023) d Self-powered breath airflow sensors based on the triboelectric effect. Reproduced with permission from ref. 68, copyright (American Chemical Society, 2018) e Temperature sensor based on nickel oxide nanoparticles as a negative temperature coefficient material. Reproduced with permission from ref. 75, copyright (John Wiley and Sons, 2019). f Colorimetric breath temperature sensor based on thermochromic dye-coated nanofiber membrane. Reproduced with permission from ref. 79, copyright (John Wiley and Sons, 2022) g Self-powered breath temperature sensors based on the pyroelectric effect. Reproduced with permission from ref. 80, copyright (Elsevier, 2017) h Resistive breath humidity sensors. Reproduced with permission from refs. 93,105, copyright (John Wiley and Sons, 2016), copyright (American Chemical Society, 2019) i Capacitive breath humidity sensors. Reproduced with permission from refs. 108,109, copyright (Springer Nature, 2021), copyright (Springer Nature, 2022).
On the other hand, the resistive type employs piezoresistive materials to detect changes in resistance due to breath airflow64. Networks of conducting nanomaterials, such as CNT or MXene, vary during inhalation and exhalation, causing changes in junction resistance and overall network resistance (Fig. 3b)65,66. Sun et al. utilized a crack-based strain sensor, significantly enhancing sensitivity to detect breath airflow65.
The focus has also shifted towards self-powered breath sensors, which simultaneously achieve sensing and energy harvesting for low power consumption of respiration monitoring systems. Airflow induces structural changes that activate the piezoelectric effect, generating alterations in electric dipoles and producing AC voltages for self-powering (Fig. 3c)67. The triboelectric effect provides another self-powering method, converting mechanical motion or friction into electrical energy by harnessing the charge imbalances resulting from contact and separation of the electrodes (Fig. 3d)64,68. Zhong, et al. demonstrated a dual approach, utilizing piezoelectric and triboelectric effects within a sandwiched structure (Au-Teflon AF-air gap-Teflon AF-Au)60. Teflon AF retains electrostatic charges via corona charging treatment, while the air gap boasts a high dielectric constant. The contact and separation between Teflon AF layers induce triboelectric charges, and breathing-induced alterations in dipole moments induce piezoelectric-based charges. This integration of airflow sensing and self-powering mechanisms enables continuous respiratory health monitoring, providing valuable insights without the need for frequent recharging.
Temperature monitoring
Exhaled breath temperature can serve as a non-invasive indicator of airway inflammation. It reflects changes in bronchial blood flow and inflammatory markers, making it a potentially essential tool for diagnosing and monitoring respiratory conditions, including asthma and lung cancer69,70. Several studies have demonstrated that the elevated exhaled breath temperature in asthma patients, compared to healthy subjects, suggests the potential of exhaled breath temperature as a valuable diagnostic marker71,72. Measuring breath temperature not only serves as an indicator of airway inflammation and respiratory condition but also allows for the analysis of breath patterns by detecting temperature variations during both exhalation and inhalation, providing a more comprehensive assessment of respiratory function73.
One approach to detecting the temperature information is using thermistor materials, particularly those exhibiting a negative thermal coefficient (NTC)74,75,76,77,78. For example, Shin et al. introduced a conformally attachable patch-like temperature sensor based on nickel oxide nanoparticles as an NTC material (Fig. 3e)75. This epidermal temperature sensor was placed on the nasal cavity and successfully monitored the temperature variation based on exhalation and inhalation. On the other hand, The colorimetric temperature sensor system, employing a highly sensitive nanofiber membrane with a thermochromic dye, showed a real-time monitoring system for measuring and detecting changes in breath temperature (Fig. 3f)79. This approach enhanced the practicality of continuous, unobtrusive monitoring, which is particularly valuable in healthcare and related applications. Another option is to utilize pyroelectric nanogenerators as wearable breath temperature-derived respiration sensors with energy harvesting capabilities (Fig. 3g)67,80,81,82. The pyroelectric effect is a phenomenon in which certain materials with spontaneous polarization experience a change in their electric dipole moment when their temperature changes83,84. This change in electric dipole moments generates an electric charge or voltage across the material.
Humidity monitoring
Breath humidity, the moisture content in the air we inhale and exhale during respiration, plays a pivotal role in our overall well-being and health. It is a crucial indicator, reflecting the complex interplay of environmental and physiological factors affecting our respiratory system. Environmental humidity, for instance, plays a pivotal role in respiratory health, as dry air exposure can lead to discomfort or respiratory issues, including cough, sore throat, nosebleeds, headache, and oral health problems85,86. Meanwhile, monitoring relative humidity (RH) variations in exhaled and inhaled breath allows assessing respiratory rate and intensity87. This capability proves invaluable in healthcare and clinical settings, aiding in diagnosing and managing various respiratory conditions and optimizing patient care. Moreover, research efforts have differentiated between nasal and oral breathing patterns88,89,90,91,92,93. This distinction unveils individual breathing habits, revealing potential concerns about mouth breathing, including impacts on respiratory health, sleep quality, hypertension, facial deformities, and poor growth in children94,95,96. The comprehensive exploration of breath humidity’s implications and applications is vital to advancing our understanding of respiratory health and enhancing healthcare outcomes.
The main sensing mechanism of humidity sensors typically involves the interaction between a sensing material and water vapor in the gas97. Most flexible humidity sensors operate based on a proton-hopping process known as the Grotthuss chain reaction, where water molecules and hydronium ions interact to facilitate a dynamic charge transfer at a specific RH98. The sensor’s signal strength reflects the number of water molecules on the active material’s surface, with the material’s hydrophilic nature being crucial for sensing performance. Water molecules are initially adsorbed on the material’s surface through chemical bonding with hydroxyl groups or surface defects, and as humidity increases, more water layers form, promoting ionization and hydronium ion transfer, which affects the sensor’s output.
Various studies utilized resistive humidity sensors for their wearable breath sensor, which is made from a variety of materials such as metal oxide99,100, carbon-based nanomaterials88,89,90,91,101,102, ZnIn2S4 nanosheet103, poly lactic glycolic acid104, gold nanowire92, and cellulose (Fig. 3h)93,105,106. For instance, the cellulose-based ionic film showed a resistive humidity sensing mechanism, reducing its resistance by dissolving potassium hydroxide while absorbing moisture106. On the other hand, capacitive humidity sensors have developed, which measure the humidity level via measuring the capacitance change concerning the RH (Fig. 3i)107,108,109,110,111,112. For example, a graphene oxide-based sensor exhibited the monotonic increase of capacitance concerning the RH, as the absorbed water molecules strengthened the polarization effect, which in turn raised the dielectric constant of the material108.
Applications of wearable breath sensors
Breathing pattern recognition using wearable breath sensor
The assessment of breathing patterns involves analyzing several factors, including respiratory rate, duration of inhalation and exhalation, breath intensity, and rhythm regularity113. Understanding these elements is crucial in evaluating respiratory health and guiding clinical interventions for improved healthcare management. Wearable breath sensors are employed to identify these patterns by measuring airflow, temperature, and humidity changes in exhaled and inhaled breath9. Utilizing the breathing pattern measured by wearable breath sensors, various applications such as breath classification, exercise monitoring, and respiratory condition diagnosis would be done.
Breathing pattern analysis through wearable sensors, capturing exhaled and inhaled breath characteristics, is instrumental in identifying distinct breath classes such as normal, fast, deep breathing, coughing, and apnea. These classes are typically differentiated by assessing respiratory ratio and breath intensity. Continuous wavelet transform analysis offers a detailed method enabling comprehensive scrutiny of frequency and magnitude parameters linked to various breathing conditions, accentuating the uniqueness of individual characteristics60.
Beyond the typical breath classes like normal, fast, deep breathing, and coughing, alternative approaches have emerged for a more diversified classification or analysis of breathing patterns. Wang et al. highlighted the importance of sensor recovery time in detecting high-frequency instances like coughing, choking, or asthma breathing, which exhibit significantly faster frequencies compared to normal breath (Fig. 4a)108. Additionally, Liu et al. differentiated breath patterns into five situations: grasp, frighten, meditate, sit, and sleep, analyzing sensor resistance deviation based on temperature and respiration rate to discern these diverse patterns (Fig. 4b)77. Another important utilization of breath pattern analysis is to unlock invaluable insights into understanding physical activities correlated to the dynamics of respiratory changes. Researchers have employed wearable breath sensors to monitor respiratory patterns in real-time during exercises, enabling assessment of training intensity and physiological status in diverse environmental conditions (Fig. 4c)75,77,102. Moreover, wearable breathing sensors demonstrate promise in monitoring sleep status and diagnosing sleep disorders, notably sleep apnea. Satoko et al. developed a wireless wearable respiration monitoring platform based on a humidity sensor and acceleration sensor to continuously capture instances of breathing cessation and variations in respiratory rates over an extended period of seven hours (Fig. 4d)103.
a High-frequency breathing pattern, including coughing, choking, and asthmatic breathing, recognized by fast-response breathing sensors. Reproduced with permission from ref. 108, copyright (Springer Nature, 2021) b Gasp, frighten, meditate, sit, and sleep breath patterns recognized by wearable breath sensor. Reproduced with permission from ref. 77, copyright (Elsevier, 2020) c Breath pattern analysis for recognizing physiological responses of the body during exercise. Reproduced with permission from refs. 75,102, copyright (John Wiley and Sons, 2019), copyright (American Chemical Society, 2018) d Continuous respiration monitoring for recording sleep apnea symptoms. Reproduced with permission from ref. 103, (Elsevier, 2022).
The combination of IoT technology and wearable breath sensors has enabled continuous monitoring through the immediate analysis of breath patterns and conveying their significance to the users. Various components like batteries, voltage boost circuitry, analog-to-digital converter, micro-controller, and Wi-Fi module could be integrated, thus transmitting digital breath data wirelessly to smartphones, enabling the real-time monitoring of breath patterns (Fig. 5a)60. Furthermore, machine learning algorithms have made significant strides in real-time classification of breath patterns78,109,114. Preprocessing, such as feature extraction and normalization with successive classification of machine learning algorithms, would be conducted. Combined with an IoT system, real-time classification of breath patterns could be utilized as a health monitoring system, which displayed SOS warnings and communicated these abnormal signals to healthcare providers through various networks, including phone calls, emails, and messages whenever abnormal breathing, such as rapid breathing, was detected (Fig. 5b)78.
a Breath sensor integrated with an IoT system for real-time monitoring of breathing patterns. Reproduced with permission from ref. 60, (John Wiley and Sons, 2021) b Utilization of a 1D-convolutional neural network algorithm to classify five breath classes (normal, fast, deep breathing, speaking, and coughing), along with the corresponding confusion matrix. Reproduced with permission from ref. 78, (American Chemical Society, 2023) c Chronic respiratory disease diagnosis by incorporating wearable breath sensor with decision tree algorithm. Asthma, bronchitis, chronic obstructive pulmonary disease (COPD), and healthy states were classified with 95.5% accuracy. Reproduced with permission from ref. 116, (American Chemical Society, 2022).
Furthermore, machine learning techniques can be utilized to diagnose chronic respiratory diseases. Identifying intricate breathing patterns by incorporating wearable sensors and machine learning algorithms demonstrates the potential to facilitate the diagnosis and differentiation of various chronic respiratory diseases115. For instance, Zhang et al. utilized a decision tree algorithm with a bagged ensemble strategy to classify the respiratory signals into four classes (asthma, bronchitis, chronic obstructive pulmonary disease, and healthy states) with an impressive accuracy of 95.5% (Fig. 5c)116. This application demonstrates the potential of machine learning in enhancing the diagnostic capabilities of respiratory health monitoring systems.
Face mask platform for healthcare
Integrating various sensors into masks through IoT technology has paved the way for developing wearable mask platforms extending beyond respiratory monitoring and catering to diverse healthcare applications. For instance, Pan et al. incorporated temperature and photoplethysmography sensors into masks to detect various physiological data, including body temperature, heart rate, blood O2 saturation, and blood pressure (Fig. 6a)117. Similarly, a mask platform equipped with temperature sensors, a 3-axis gyroscope, and pressure sensors allowed continuous monitoring of respiratory, heartbeat rates, mask fit, and wear time118. Another study by Kim et al. proposed a comprehensive system integrating accelerometers, temperature sensors, humidity sensors, and capacitance sensor pads (Fig. 6b)119. This system successfully monitored the respiratory rate, coughing, and speaking along with other physiological data, including skin temperature and humidity. In addition, they successfully employed machine learning to classify four distinct mask positions (fully covered, nose exposed, nose and mouth exposed, taken off). These advancements underscore the evolution of wearable mask platforms into sophisticated healthcare tools capable of real-time monitoring of a broad spectrum of physiological parameters. The integration of various sensors and the application of machine learning techniques are expected to increase the versatility and effectiveness of these platforms in providing comprehensive health insights.
a Wearable face mask platform consisting of temperature and photoplethysmography sensors for real-time detection of various physiological data. Reproduced from ref. 117. This is an unofficial adaptation of an article that appeared in an ACS publication. ACS has not endorsed the content of this adaptation or the context of its use. b Wearable face mask platform integrating accelerometer, temperature sensor, humidity sensor, and capacitance sensor pads. k-means clustering was utilized to classify four distinct mask positions (fully covered, nose exposed, nose and mouth exposed, taken off). Reproduced with permission from ref. 119, copyright (Springer Nature, 2022).
Real-time disease detection using wearable breath sensor
The breath contains a multitude of biomarkers that offer valuable insights into the detection of various diseases120. Conventionally, efforts have been made to identify diseases through various exhaled breath analysis methods, focusing on respiratory diseases and expanding to cover a broader spectrum of health conditions, including cardiovascular diseases121,122. The emergence of wearable breath sensors opens the potential to assess the presence or absence of diseases in everyday life in real-time.
Our previous discussion explored wearable breath sensors capable of detecting key biomarkers such as O2, CO2, NH3, H2O2, and pathogens. These biomarkers act as pivotal indicators, and understanding their concentrations provides meaningful insights into potential disease implications. However, addressing more intricate disease scenarios necessitates the simultaneous analysis of multiple biomarkers. An e-nose system, inspired by the human olfactory system, offers a promising solution for this challenge. A sensor array, capable of detecting intricate species in breath, integrated with a pattern recognition algorithm, could identify various components of breath and further decode complex disease implications by applying machine learning algorithms123. A compact and wearable design of the e-nose system should be discussed for real-time monitoring. A notable example is the wearable optical e-nose system (Fig. 7a)124. This system incorporates a mask with 32 colorimetric sensor arrays, enabling the rapid detection of various exhaled metabolites. Through image analysis of the color response, this system successfully differentiated between patients, healthy individuals, and those who had recovered from the infection. This example exemplifies the potential of wearable e-nose systems in advancing our understanding of diseases through comprehensive and real-time breath analysis.
a Wearable optical electronic nose (e-nose) system utilizing 32 colorimetric sensor arrays for rapid detection of various exhaled metabolites. Reproduced with permission from ref. 124, copyright (Elsevier, 2022) b Synthetic breath biomarkers, protease-derived volatile reporters, were released into the breath and collected and analyzed for sensitive detection of lung inflammation. Reproduced with permission from ref. 126, copyright (Springer Nature, 2020).
As previously highlighted, the valuable information within our breath’s biomarkers offers insights into various diseases. However, detecting these biomarkers during the early stages is challenging due to their low and variable concentrations125,126. Recent advancements in synthetic biomarker technology have proven effective in addressing this challenge. Unlike endogenic biomarkers, synthetic biomarkers are artificially generated by introducing biological and chemical probes into the body, amplifying signals specific to particular diseases125. Beyond the blood and urine, synthetic biomarker technology has evolved to be found in exhaled breath, providing a non-invasive approach to diagnosing pathogenic infections or cancer. VOC reporters from exhaled breath are amplified through probes with pathogen-specific and metabolism-specific properties127. For example, Lange et al. demonstrated a cancer diagnosis by intravenously administering the D5-ethyl-β-D-glucuronide probe to mice and then detecting D5-ethanol released by the tumor’s β-glucuronidase enzyme in the exhaled breath128. Chan et al. coined the term “synthetic breath biomarker” to describe synthetic biomarkers released in exhaled breath126. They designed a synthetic breath biomarker to detect respiratory disease by utilizing protease-sensing nanoparticles (Fig. 7b). In a mouse model of acute lung inflammation, they delivered these nanoparticles locally to the lungs. They detected the resulting volatile reporter by mass spectrometry, achieving high sensitivity in identifying diseased mice. This novel method shows potential for improving the accuracy and precision of disease detection, particularly during the early stages. The expansion of synthetic biomarkers into breath analysis represents a significant stride towards non-invasive diagnostic methods, offering a potential breakthrough in the timely identification of pathogenic infections and cancer.
Outlook
In this review, we have explored the recent advances in wearable breath sensor technology, shedding light on its diverse applications in healthcare monitoring. The advancements outlined in this paper showcase the transformative potential of wearable breath sensors, from conventional breath sampling and analysis techniques to the real-time detection of crucial biomarkers and monitoring of respiratory parameters utilizing wearable breath sensors. The discussed applications, including breathing pattern recognition, face mask platforms for healthcare, and real-time disease detection, underscore the versatility and impact of these devices in shaping the landscape of personalized and proactive healthcare. Integrating IoT systems and machine learning techniques has been highlighted as a transformative approach to analyzing the respiratory parameters and biomarkers of breath leading to more personalized and efficient healthcare solutions.
In perspective for the future, several key areas require further development of these sensors to be more widely adopted in everyday life:
-
1.
Innovative sensor design for long-term use: Current wearable breath sensors, predominantly designed as patches or integrated into face masks, face challenges in continuous wearability, including comfort, cosmetic concerns, skin inflammation, and breathability. Developing sensor designs that facilitate comfortable, long-term wear while enabling real-time monitoring is crucial. This advancement would greatly enhance these technologies’ feasibility and user acceptance for daily-life health monitoring.
-
2.
Deepening analysis of breath characteristics: Present methods mainly focus on distinguishing breathing patterns by analyzing differences between inhalation and exhalation. A deeper exploration of the intrinsic significance of breath humidity and temperature is necessary. Additionally, understanding the influence of external environmental factors and addressing motion artifacts is crucial. Hardware and software solutions for calibrating data from external environmental and internal breath measurements, along with noise removal techniques, should be explored to enhance the accuracy of breath analysis.
-
3.
Combining multifunctional sensors and machine learning: Advancements in multifunctional sensors and sophisticated machine learning algorithms could open new avenues in breath analysis. A broader range of clinical approaches, catering to diverse races, genders, and ages, will enhance the universality of device performance. This approach offers the potential to monitor health status and early diagnose disease by deciphering the complex context within the breath.
-
4.
Reliability and multi-use capability in sensors: Effective continuous monitoring requires reliable and suitable sensors for multiple uses. Developing robust sensor designs resistant to biofouling and contamination is essential. Strategies for mitigating these issues may involve incorporating antifouling treatments or implementing self-cleaning mechanisms on sensor surfaces129,130. Maintaining these sensors’ longevity and consistent performance is paramount for their ongoing practical application in health assessment. While disease detection using biomarkers holds potential due to their high correlation with diseases, existing sensing mechanisms like electrochemical and molecular biological methods may face limitations in multi-use capability and continuous real-time detection.
-
5.
Limitations of synthetic breath biomarkers: While synthetic breath biomarkers show promise for significantly improving disease diagnostic sensitivity and selectivity through targeted manipulation of VOCs in the breath, this technology has several challenges. The major drawback is the inconvenience of injecting or ingesting probes that generate volatile reporters. In addition, a comprehensive understanding of the release mechanisms of these probes or reporters, as well as the released reporters’ toxicity and human health effects, is essential for the further development and clinical application of synthetic breath biomarker technology.
In conclusion, wearable breath sensors are at a pivotal stage, with immense potential for impacting healthcare monitoring and disease diagnosis. The path forward involves technological innovations and a deeper understanding of respiratory data and its implications. As we progress, the synergy of advanced sensor design, utilization of multifunctional sensors, and intelligent data analysis will be key in realizing the full potential of these devices in transforming healthcare practices.
References
Winters, B. R. et al. Standardization of the collection of exhaled breath condensate and exhaled breath aerosol using a feedback regulated sampling device. J. Breath Res. 11, 047107 (2017).
Risby, T. H. & Solga, S. Current status of clinical breath analysis. Appl. Phys. B 85, 421–426 (2006).
Minh, T. D. C., Blake, D. R. & Galassetti, P. R. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res. Clin. Pract. 97, 195–205 (2012).
Jin, H., Abu‐Raya, Y. S. & Haick, H. Advanced materials for health monitoring with skin‐based wearable devices. Adv. Healthc. Mater. 6, 1700024 (2017).
Gao, Y., Yu, L., Yeo, J. C. & Lim, C. T. Flexible hybrid sensors for health monitoring: materials and mechanisms to render wearability. Adv. Mater. 32, 1902133 (2020).
Yin, R., Wang, D., Zhao, S., Lou, Z. & Shen, G. Wearable sensors‐enabled human–machine interaction systems: from design to application. Adv. Funct. Mater. 31, 2008936 (2021).
Muthu, B. et al. IOT based wearable sensor for diseases prediction and symptom analysis in healthcare sector. Peer-to-Peer Netw. Appl. 13, 2123–2134 (2020).
Dai, N. et al. Recent advances in wearable electromechanical sensors—Moving towards machine learning-assisted wearable sensing systems. Nano Energy 105, 108041 (2022).
Dinh, T. et al. Stretchable respiration sensors: advanced designs and multifunctional platforms for wearable physiological monitoring. Biosens. Bioelectron. 166, 112460 (2020).
Ates, H. C. & Dincer, C. Wearable breath analysis. Nat. Rev. Bioeng. 1, 80–82 (2023).
Righettoni, M., Amann, A. & Pratsinis, S. E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 18, 163–171 (2015).
Lawal, O., Ahmed, W. M., Nijsen, T. M., Goodacre, R. & Fowler, S. J. Exhaled breath analysis: a review of ‘breath-taking’methods for off-line analysis. Metabolomics 13, 1–16 (2017).
Holden, K. A. et al. Use of the ReCIVA device in breath sampling of patients with acute breathlessness: a feasibility study. ERJ Open Res. 6, 00119–02020 (2020).
Horváth, I., Hunt, J. & Barnes, P. J. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur. Respir. J. 26, 523–548 (2005).
Konstantinidi, E. M., Lappas, A. S., Tzortzi, A. S. & Behrakis, P. K. Exhaled breath condensate: technical and diagnostic aspects. Sci. World J. 2015, 435160 (2015).
Soto, F. et al. Wearable collector for noninvasive sampling of SARS-CoV-2 from exhaled breath for rapid detection. ACS Appl. Mater. Interfaces 13, 41445–41453 (2021).
Cai, S.-H., Di, D., Yuan, Z.-C., Chen, W. & Hu, B. Paper-in-facemask device for direct mass spectrometry analysis of human respiratory aerosols and environmental exposures via wearable continuous-flow adsorptive sampling: a proof-of-concept study. Anal. Chem. 93, 13743–13748 (2021).
Yuan, Z.-C. et al. Solid-phase microextraction fiber in face mask for in vivo sampling and direct mass spectrometry analysis of exhaled breath aerosol. Anal. Chem. 92, 11543–11547 (2020).
Daniels, J. et al. A mask-based diagnostic platform for point-of-care screening of Covid-19. Biosens. Bioelectron. 192, 113486 (2021).
Kim, K.-H., Jahan, S. A. & Kabir, E. A review of breath analysis for diagnosis of human health. TrAC Trends Anal. Chem. 33, 1–8 (2012).
Sola Martínez, R. A. et al. Data preprocessing workflow for exhaled breath analysis by GC/MS using open sources. Sci. Rep. 10, 22008 (2020).
Smith, D. & Španěl, P. Direct, rapid quantitative analyses of BVOCs using SIFT-MS and PTR-MS obviating sample collection. TrAC Trends Anal. Chem. 30, 945–959 (2011).
Vendel, I., Hertog, M. & Nicolaï, B. Fast analysis of strawberry aroma using SIFT-MS: a new technique in postharvest research. Postharvest Biol. Technol. 152, 127–138 (2019).
Metsälä, M. Optical techniques for breath analysis: from single to multi-species detection. J. Breath. Res. 12, 027104 (2018).
Thorpe, M. J., Balslev-Clausen, D., Kirchner, M. S. & Ye, J. Cavity-enhanced optical frequency comb spectroscopy: application to human breath analysis. Opt. Express 16, 2387–2397 (2008).
Banik, G. D. & Mizaikoff, B. Exhaled breath analysis using cavity-enhanced optical techniques: a review. J. Breath. Res. 14, 043001 (2020).
Liang, Q. et al. Ultrasensitive multispecies spectroscopic breath analysis for real-time health monitoring and diagnostics. Proc. Natl. Acad. Sci. 118, e2105063118 (2021).
Huang, L. et al. Noninvasive diagnosis of gastric cancer based on breath analysis with a tubular surface-enhanced Raman scattering sensor. ACS Sens. 7, 1439–1450 (2022).
Rajavel, K., Lalitha, M., Radhakrishnan, J. K., Senthilkumar, L. & Rajendra Kumar, R. T. Multiwalled carbon nanotube oxygen sensor: enhanced oxygen sensitivity at room temperature and mechanism of sensing. ACS Appl. Mater. Interfaces 7, 23857–23865 (2015).
Jin, L. et al. Polymeric Ti3C2T x MXene composites for room temperature ammonia sensing. ACS Appl. Nano Mater. 3, 12071–12079 (2020).
Maity, D. & Kumar, R. T. R. Polyaniline anchored MWCNTs on fabric for high performance wearable ammonia sensor. ACS Sens. 3, 1822–1830 (2018).
Kim, S. J. et al. Metallic Ti3C2T x MXene gas sensors with ultrahigh signal-to-noise ratio. ACS Nano 12, 986–993 (2018).
Assen, A. H., Yassine, O., Shekhah, O., Eddaoudi, M. & Salama, K. N. MOFs for the sensitive detection of ammonia: Deployment of fcu-MOF thin films as effective chemical capacitive sensors. ACS Sens. 2, 1294–1301 (2017).
Pandey, S. & Nanda, K. K. Au nanocomposite based chemiresistive ammonia sensor for health monitoring. Acs Sens. 1, 55–62 (2016).
Shahmoradi, A., Hosseini, A., Akbarinejad, A. & Alizadeh, N. Noninvasive detection of ammonia in the breath of hemodialysis patients using a highly sensitive ammonia sensor based on a polypyrrole/sulfonated graphene nanocomposite. Anal. Chem. 93, 6706–6714 (2021).
Houspie, L. et al. Exhaled breath condensate sampling is not a new method for detection of respiratory viruses. Virol. J. 8, 1–7 (2011).
Zheng, Y., Chen, H., Yao, M. & Li, X. Bacterial pathogens were detected from human exhaled breath using a novel protocol. J. Aerosol Sci. 117, 224–234 (2018).
Singer, M. et al. Dangers of hyperoxia. Crit. Care 25, 1–15 (2021).
Webster, W. S. & Abela, D. The effect of hypoxia in development. Birth Defects Res. C Embryo Today Rev. 81, 215–228 (2007).
Wu, Z. et al. A humidity‐resistant, sensitive, and stretchable hydrogel‐based oxygen sensor for wireless health and environmental monitoring. Adv. Funct. Mater. 34, 2308280 (2023).
Weinberger, S. E., Schwartzstein, R. M. & Weiss, J. W. Hypercapnia. N. Engl. J. Med. 321, 1223–1231 (1989).
Laffey, J. G. & Kavanagh, B. P. Hypocapnia. N. Engl. J. Med. 347, 43–53 (2002).
Hodgkinson, J., Smith, R., Ho, W. O., Saffell, J. R. & Tatam, R. P. Non-dispersive infra-red (NDIR) measurement of carbon dioxide at 4.2 μm in a compact and optically efficient sensor. Sens. Actuators B Chem. 186, 580–588 (2013).
Swinehart, D. F. The beer-lambert law. J. Chem. Educ. 39, 333 (1962).
Escobedo, P. et al. Smart facemask for wireless CO2 monitoring. Nat. Commun. 13, 72 (2022).
Eswaran, A., Thirumalainambi, M., Subramaniam, R. & Annadurai, G. Highly selective CO 2 sensing response of lanthanum oxide nanoparticle electrodes at ambient temperature. Nanoscale Adv. 5, 3761–3770 (2023).
Dimski, D. S. Ammonia metabolism and the urea cycle: function and clinical implications. J. Vet. Intern. Med. 8, 73–78 (1994).
Ricci, P. P. & Gregory, O. J. Sensors for the detection of ammonia as a potential biomarker for health screening. Sci. Rep. 11, 7185 (2021).
Amano, A., Yoshida, Y., Oho, T. & Koga, T. Monitoring ammonia to assess halitosis. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endodontol. 94, 692–696 (2002).
Wu, G. et al. A wearable mask sensor based on polyaniline/CNT nanocomposites for monitoring ammonia gas and human breathing. Sens. Actuators B Chem. 375, 132858 (2023).
Fujita, H. et al. Paper‐based wearable ammonia gas sensor using organic–inorganic composite PEDOT: PSS with Iron (III) compounds. Adv. Mater. Technol. 7, 2101486 (2022).
Chen, H. et al. Wearable dual-signal NH3 sensor with high sensitivity for non-invasive diagnosis of chronic kidney disease. Langmuir 39, 3420–3430 (2023).
Stolarek, R., Bialasiewicz, P., Krol, M. & Nowak, D. Breath analysis of hydrogen peroxide as a diagnostic tool. Clin. Chim. Acta 411, 1849–1861 (2010).
Maier, D. et al. Toward continuous monitoring of breath biochemistry: a paper-based wearable sensor for real-time hydrogen peroxide measurement in simulated breath. ACS Sens. 4, 2945–2951 (2019).
Cao, Y. et al. Polyaniline/Prussian blue nanolayer enhanced electrochemical sensing of H2O2 in EBC using an integrated condensation facemask. Sens. Actuators B Chem. 393, 134189 (2023).
Nguyen, P. Q. et al. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat. Biotechnol. 39, 1366–1374 (2021).
Xue, Q. et al. An intelligent face mask integrated with high density conductive nanowire array for directly exhaled coronavirus aerosols screening. Biosens. Bioelectron. 186, 113286 (2021).
Trung, T. Q. & Lee, N. E. Flexible and stretchable physical sensor integrated platforms for wearable human‐activity monitoringand personal healthcare. Adv. Mater. 28, 4338–4372 (2016).
Ghosh, R. et al. Fabrication of piezoresistive Si nanorod-based pressure sensor arrays: a promising candidate for portable breath monitoring devices. Nano Energy 80, 105537 (2021).
Zhong, J. et al. Smart face mask based on an ultrathin pressure sensor for wireless monitoring of breath conditions. Adv. Mater. 34, 2107758 (2022).
Joo, Y. et al. Highly sensitive and bendable capacitive pressure sensor and its application to 1 V operation pressure‐sensitive transistor. Adv. Electron. Mater. 3, 1600455 (2017).
Ji, S. et al. High dielectric performances of flexible and transparent cellulose hybrid films controlled by multidimensional metal nanostructures. Adv. Mater. 29, 1700538 (2017).
Yang, W. et al. A breathable and screen‐printed pressure sensor based on nanofiber membranes for electronic skins. Adv. Mater. Technol. 3, 1700241 (2018).
Meng, K. et al. Wearable pressure sensors for pulse wave monitoring. Adv. Mater. 34, 2109357 (2022).
Sun, Z. et al. Skin-like ultrasensitive strain sensor for full-range detection of human health monitoring. ACS Appl. Mater. Interfaces 12, 13287–13295 (2020).
Yang, L. et al. Wearable pressure sensors based on MXene/tissue papers for wireless human health monitoring. ACS Appl. Mater. Interfaces 13, 60531–60543 (2021).
Dai, J. et al. A wearable self‐powered multi‐parameter respiration sensor. Adv. Mater. Technol. 8, 2201535 (2023).
Zheng, H. et al. Concurrent harvesting of ambient energy by hybrid nanogenerators for wearable self-powered systems and active remote sensing. ACS Appl. Mater. Interfaces 10, 14708–14715 (2018).
Popov, T. A. Human exhaled breath analysis. Ann. Allergy Asthma Immunol. 106, 451–456 (2011).
Carpagnano, G. E. et al. Exhaled breath temperature home monitoring to detect NSCLC relapse: results from a pilot study. BioMed. Res. Int. 2022, 1515274 (2022).
Paredi, P., Kharitonov, S. A. & Barnes, P. J. Faster rise of exhaled breath temperature in asthma: a novel marker of airway inflammation? Am. J. Respir. Crit. Care Med. 165, 181–184 (2002).
García, G., Bergna, M., Uribe, E., Yañez, A. & Soriano, J. Increased exhaled breath temperature in subjects with uncontrolled asthma. Int. J. Tuberc. Lung Dis. 17, 969–972 (2013).
AL‐Khalidi, F. Q., Saatchi, R., Burke, D., Elphick, H. & Tan, S. Respiration rate monitoring methods: a review. Pediatr. Pulmonol. 46, 523–529 (2011).
Liao, F. et al. Ultrafast response flexible breath sensor based on vanadium dioxide. J. Breath. Res. 11, 036002 (2017).
Shin, J. et al. Sensitive wearable temperature sensor with seamless monolithic integration. Adv. Mater. 32, 1905527 (2020).
Gandla, S. et al. Highly linear and stable flexible temperature sensors based on laser‐induced carbonization of polyimide substrates for personal mobile monitoring. Adv. Mater. Technol. 5, 2000014 (2020).
Liu, Y. et al. Epidermal electronics for respiration monitoring via thermo-sensitive measuring. Mater. Today Phys. 13, 100199 (2020).
Zhao, T. et al. Tracing the Flu Symptom Progression via a Smart Face Mask. Nano Lett. 23, 8960–8969 (2023).
Kim, D. H. et al. Porous nanofiber membrane: rational platform for highly sensitive thermochromic sensor. Adv. Funct. Mater. 32, 2200463 (2022).
Xue, H. et al. A wearable pyroelectric nanogenerator and self-powered breathing sensor. Nano Energy 38, 147–154 (2017).
Roy, K. et al. A self-powered wearable pressure sensor and pyroelectric breathing sensor based on GO interfaced PVDF nanofibers. ACS Appl. Nano Mater. 2, 2013–2025 (2019).
Kim, M. J. et al. Breathing‐driven self‐powered pyroelectric ZnO integrated face mask for bioprotection. Small 19, 2200712 (2023).
Whatmore, R. Pyroelectric devices and materials. Rep. Prog. Phys. 49, 1335 (1986).
Bowen, C. R. et al. Pyroelectric materials and devices for energy harvesting applications. Energy Environ. Sci. 7, 3836–3856 (2014).
Kaminsky, D. A., Bates, J. H. & Irvin, C. G. Effects of cool, dry air stimulation on peripheral lung mechanics in asthma. Am. J. Respir. Crit. Care Med. 162, 179–186 (2000).
Davis, R. E., McGregor, G. R. & Enfield, K. B. Humidity: a review and primer on atmospheric moisture and human health. Environ. Res. 144, 106–116 (2016).
Duan, Z., Jiang, Y. & Tai, H. Recent advances in humidity sensors for human body related humidity detection. J. Mater. Chem. C 9, 14963–14980 (2021).
Pan, T. et al. Flexible humidity sensor with high sensitivity and durability for respiratory monitoring using near-field electrohydrodynamic direct-writing method. ACS Appl. Mater. Interfaces 15, 28248–28257 (2023).
Kim, H.-S., Kang, J.-H., Hwang, J.-Y. & Shin, U. S. Wearable CNTs-based humidity sensors with high sensitivity and flexibility for real-time multiple respiratory monitoring. Nano Converg. 9, 1–14 (2022).
Zhu, J. et al. High‐sensitivity and low‐hysteresis GO–NH2/Mesoporous SiO2 nanosphere‐fabric‐based humidity sensor for respiratory monitoring and noncontact sensing. Adv. Mater. Interfaces 9, 2101498 (2022).
Pang, Y. et al. Wearable humidity sensor based on porous graphene network for respiration monitoring. Biosens. Bioelectron. 116, 123–129 (2018).
Adhyapak, P. V., Kasabe, A. M., Bang, A. D., Ambekar, J. & Kulkarni, S. K. Highly sensitive, room temperature operated gold nanowire-based humidity sensor: adoptable for breath sensing. RSC Adv. 12, 1157–1164 (2022).
Duan, Z. et al. Facile, flexible, cost-saving, and environment-friendly paper-based humidity sensor for multifunctional applications. ACS Appl. Mater. Interfaces 11, 21840–21849 (2019).
Fitzpatrick, M. F. et al. Effect of nasal or oral breathing route on upper airway resistance during sleep. Eur. Respir. J. 22, 827–832 (2003).
Watso, J. C. et al. Acute nasal breathing lowers diastolic blood pressure and increases parasympathetic contributions to heart rate variability in young adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 325, R797–R808 (2023).
Jefferson, Y. Mouth breathing: adverse effects on facial growth, health, academics, and behavior. Gen. Dent. 58, 18–25 (2010).
Arman Kuzubasoglu, B. Recent studies on the humidity sensor: a mini review. ACS Appl. Electron. Mater. 4, 4797–4807 (2022).
Lu, Y., Yang, G., Shen, Y., Yang, H. & Xu, K. Multifunctional flexible humidity sensor systems towards noncontact wearable electronics. Nano Micro Lett. 14, 150 (2022).
Jin, X., Zha, L., Wang, F., Wang, Y. & Zhang, X. Fully integrated wearable humidity sensor for respiration monitoring. Front. Bioeng. Biotechnol. 10, 1070855 (2022).
Deb, M. et al. SnO2-based ultra-flexible humidity/respiratory sensor for analysis of human breath. Biosensors 13, 81 (2023).
Yao, X. et al. High‐performance flexible humidity sensors for breath detection and non‐touch switches. Nano Sel. 3, 1168–1177 (2022).
He, J. et al. High performance humidity fluctuation sensor for wearable devices via a bioinspired atomic-precise tunable graphene-polymer heterogeneous sensing junction. Chem. Mater. 30, 4343–4354 (2018).
Honda, S., Hara, H., Arie, T., Akita, S. & Takei, K. A wearable, flexible sensor for real-time, home monitoring of sleep apnea. Iscience 25, 104163 (2022).
Soomro, A. M. et al. All-range flexible and biocompatible humidity sensor based on poly lactic glycolic acid (PLGA) and its application in human breathing for wearable health monitoring. J. Mater. Sci. Mater. Electron. 30, 9455–9465 (2019).
Güder, F. et al. Paper‐based electrical respiration sensor. Angew. Chem. Int. Ed. 55, 5727–5732 (2016).
Wang, Y., Zhang, L., Zhou, J. & Lu, A. Flexible and transparent cellulose-based ionic film as a humidity sensor. ACS Appl. Mater. Interfaces 12, 7631–7638 (2020).
Sinha, A., Stavrakis, A. K., Simic, M. & Stojanovic, G. M. Polymer-thread-based fully textile capacitive sensor embroidered on a protective face mask for humidity detection. ACS Omega 7, 44928–44938 (2022).
Wang, X. et al. An ultrafast-response and flexible humidity sensor for human respiration monitoring and noncontact safety warning. Microsyst. Nanoeng. 7, 99 (2021).
Chen, G. et al. A nanoforest-based humidity sensor for respiration monitoring. Microsyst. Nanoeng. 8, 44 (2022).
Li, B. et al. High sensitivity portable capacitive humidity sensor based on In2O3 nanocubes-decorated GO nanosheets and its wearable application in respiration detection. Sens. Actuators B Chem. 299, 126973 (2019).
Kanaparthi, S. Pencil‐drawn paper‐based non‐invasive and wearable capacitive respiration sensor. Electroanalysis 29, 2680–2684 (2017).
Simić, M. et al. Portable respiration monitoring system with an embroidered capacitive facemask sensor. Biosensors 12, 339 (2022).
Benchetrit, G. Breathing pattern in humans: diversity and individuality. Respir. Physiol. 122, 123–129 (2000).
Fang, Y. et al. A deep‐learning‐assisted on‐mask sensor network for adaptive respiratory monitoring. Adv. Mater. 34, 2200252 (2022).
Tobin, M. J. et al. Breathing patterns: 2. Diseased subjects. Chest 84, 286–294 (1983).
Zhang, K. et al. Biodegradable smart face masks for machine learning-assisted chronic respiratory disease diagnosis. ACS Sens. 7, 3135–3143 (2022).
Pan, L. et al. Lab-on-mask for remote respiratory monitoring. ACS Mater. Lett. 2, 1178–1181 (2020).
Curtiss, A. et al. FaceBit: smart face masks platform. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 5, 1–44 (2021).
Kim, J.-H. et al. A conformable sensory face mask for decoding biological and environmental signals. Nat. Electron. 5, 794–807 (2022).
Pham, Y. L. & Beauchamp, J. Breath biomarkers in diagnostic applications. Molecules 26, 5514 (2021).
Pereira, J. et al. Breath analysis as a potential and non-invasive frontier in disease diagnosis: an overview. Metabolites 5, 3–55 (2015).
Cikach, F. S. Jr & Dweik, R. A. Cardiovascular biomarkers in exhaled breath. Prog. Cardiovasc. Dis. 55, 34–43 (2012).
Li, Y., Wei, X., Zhou, Y., Wang, J. & You, R. Research progress of electronic nose technology in exhaled breath disease analysis. Microsyst. Nanoeng. 9, 129 (2023).
Bordbar, M. M. et al. Mask assistance to colorimetric sniffers for detection of Covid-19 disease using exhaled breath metabolites. Sens. Actuators B Chem. 369, 132379 (2022).
Kwong, G. A. et al. Synthetic biomarkers: a twenty-first century path to early cancer detection. Nat. Rev. Cancer 21, 655–668 (2021).
Chan, L. W. et al. Engineering synthetic breath biomarkers for respiratory disease. Nat. Nanotechnol. 15, 792–800 (2020).
Djago, F., Lange, J. & Poinot, P. Induced volatolomics of pathologies. Nat. Rev. Chem. 5, 183–196 (2021).
Lange, J. et al. Volatile organic compound based probe for induced volatolomics of cancers. Angew. Chem. Int. Ed. 58, 17563–17566 (2019).
Damodaran, V. B. & Murthy, N. S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 20, 1–11 (2016).
Blossey, R. Self-cleaning surfaces—virtual realities. Nat. Mater. 2, 301–306 (2003).
Acknowledgements
This study was supported by the National Research Foundation of Korea (grant number 2021R1A2B5B03001691) and the Interdisciplinary Research Initiatives Program by College of Engineering and College of Medicine Seoul National University.
Author information
Authors and Affiliations
Contributions
D.K., J.L., M.P. and S.K. contributed to all aspects of this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Materials thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Onur Parlak and John Plummer.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, D., Lee, J., Park, M.K. et al. Recent developments in wearable breath sensors for healthcare monitoring. Commun Mater 5, 41 (2024). https://doi.org/10.1038/s43246-024-00480-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43246-024-00480-w