Abstract
Controlling the fast electrophoresis of nano-objects in solid-state nanopores is a critical issue for achieving electrical analysis of single-particles by ionic current. In particular, it is crucial to slow-down the translocation dynamics of nanoparticles. We herein report that a focused electric field and associated water flow in a surround-gate nanopore can be used to trap and manipulate a nanoscale object. We fine-control the electroosmosis-induced water flow by modulating the wall surface potential via gate voltage. We find that a nanoparticle can be captured in the vicinity of the conduit by balancing the counteracting electrophoretic and hydrodynamic drag forces. By creating a subtle force imbalance, in addition, we also demonstrate a gate-controllable motion of single-particles moving at an extremely slow speed of several tens of nanometers per second. The present method may be useful in single-molecule detection by solid-state nanopores and nanochannels.
Similar content being viewed by others
Introduction
Solid-state nanopore is a sensor capable of analyzing individual particles and molecules of various sizes1,2,3 such as viruses4,5, extracellular vehicles6,7, proteins8,9,10, and genomes11,12,13 dispersed in electrolyte solution. It probes temporal blockage of the ion flow through a hole sculpted in a thin dielectric membrane upon a substance passing through there by electrophoresis14. While proven to possess great potential as a versatile nanosensor, the excessively fast and stochastic nature of the translocation dynamics remains to be the central challenge that generally poses critically short residence time along with spatially random positions of analytes in the sensing zone for implementing the electrical analysis8,15,16,17. For instance, whereas biological nanochannels were able to achieve ultraslow DNA translocation speed of about 1 nucleotide per ms by employing a polymerase reaction-derived ratcheting mechanism18,19, the motion is generally orders of magnitude faster in solid-state nanopores15 imposing difficulties for single-nucleotide discriminations. Electrophoresis of single-molecule proteins in a nanopore was also reported to be too rapid for detecting the associated ionic blockade current under the given temporal resolution of the ionic current measurements8. Meanwhile, despite the significant efforts devoted in the past to add external probes for handling the capture-to-translocation kinetics of single-particles and -molecules, such as optoelectronic20 and dielectrophoretic approaches21,22,23, their abilities are still insufficient to slow-down the nanoscopic objects for the goal of single-protein analyses10 and nanopore sequencing15.
Here, aiming to realize an active control of electrophoretically-driven mass transport inside a nanopore, therefore, we employed a field-effect transistor approach24,25 to regulate the single-nanoparticle translocation dynamics, where we found its capability for holding an object in confined nanospace and manipulating the motions at a 10 nm s−1 level by tailoring the interplay between the electrostatic and hydrodynamic forces via a gate control.
Results
Ion transport in surround-gate nanopores
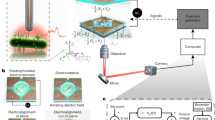
Our device is a gating nanopore comprised of a lithographically-defined 300 nm-sized hole sculpted in a SiNx membrane of thickness 50 nm with an embedded Pt nano-ring and 20 nm-thick SiO2 gate dielectrics (Fig. 1a–d, see also Supplementary Figs. S1 and S2). The top surface was covered with 5 μm-thick polyimide layer except a 50 μm region around the nanopore to ensure better temporal response26 of the ionic current as well as to reduce the capacitance-derived noise27,28. The cross-membrane ionic current Iion versus voltage Vb characteristics measured in phosphate buffer saline (PBS) showed ohmic behavior with the open conductance Gopen of 427 nS (Fig. 1e) in agreement to the analytical expression of Ropen (=Gopen−1) = Rpore + Racc = 2.3 MΩ within 2% error where Rpore = 4ρLpore/πdpore2 and Racc = ρ/dpore are the resistance inside and outside the pore29,30, respectively, for the channel geometry characterized by the depth Lpore = 90 nm and the diameter dpore = 300 nm with the resistivity ρ = 0.5 Ωm of the electrolyte buffer used. Meanwhile, application of the gate voltage VG in a range from −1 to 1 V to the Pt electrode caused a slight change in the Iion-Vb characteristics manifesting non-negligible influence of the transverse electrostatics on the longitudinal ion transport31,32.
a A schematic model depicting electrophoretic translocation of a 200 nm-sized carboxylated polystyrene nanobead through a 300 nm diameter surround-gate nanopore under the applied cross-membrane Vb and gate voltage VG. Optical images of the nanopore chip showing an overview (b) and a magnified view at the center (c). The gate voltage was added to the Pt surround gate via the microelectrodes. Entire surface except the 50 μm region around the nanopore was coated with a thick polyimide layer for lowering capacitance-induced high-frequency noise of the cross-membrane ionic current Iion. d A scanning electron micrograph of the 300 nm-sized gating nanopore. e Iion versus Vb characteristics in 1 × PBS under different VG conditions. A partial Iion versus time (t) trace (f) and a close view of a resistive pulse (g) occurred upon a nanoparticle passed through the nanopore. The width of the signal td represents the time required for the particle to move through the channel. The base current is offset to zero.
Single-nanoparticle detections by ionic current measurements
Resistive pulses were observed under positive Vb when one side of the chambers was filled with PBS containing 200 nm-sized carboxylated polystyrene nanoparticles at concentration of 1 pM (Fig. 1f, see also Supplementary Fig. S3). As the Iion traces were featureless under negative voltage, the characteristic signals are naturally ascribed to transient ion blockage by electrophoretically-driven translocation of the negatively-charged polymeric nanobeads (Fig. 1g). Here, the width td of the resistive pulses reflects the time single nanoparticles spent to pass through the nanopore, which was as short as 127 μs in case of Fig. 1g under Vb = 0.5 V and VG = +0.2 V.
Gate voltage-controlled single-nanoparticle translocation speeds
Single-nanoparticle motion control via transverse electric field is examined by evaluating VG dependence of td. More than a hundred of resistive pulses were recorded at each gate voltage condition from –1.2 to +1.2 V at 0.2 V step while keeping Vb at 0.5 V. The pulse widths revealed unimodal distributions (Fig. 2a) except at elevated VG wherein significantly wider signals were sporadically observed with the longest one being 2.01 s under VG = −1.05 V. In order to see the gating effect more clearly, we exhibited Gaussian fitting and plotted the peak values tp as a function of the gate voltage (Fig. 2b). The diagram displayed three distinct regimes in terms of the transverse field effect: almost constant tp at −0.6 V ≤ VG ≤ +0.8 V (regime I); rise in the translocation time at larger |VG | for both the positive (regime II) and negative polarities (regime III). The overall tendency was found to be similar when we extended the experiment to 100 nm-sized nanoparticles (Supplementary Fig. S4) except the absence of the prolonged translocation time at VG ≥ +1.0 V (Supplementary Fig. S5).
The VG-derived single-particle dynamics can be interpreted as a consequence of varying contributions of hydrodynamic dragging and columbic interactions under the gate-modulated surface potential at the nanopore wall (Fig. 3a–d)33,34,35,36,37. When VG < 0, the negatively-charged nanoparticles are repelled from the wall via the columbic forces37. In the meantime, Vb-excited migration of mobile counter cations generates water flow in the direction opposite to the electrophoresis of the polymeric beads (Fig. 3b)34,35. Thus, under large negative VG, the hydrodynamic drag would become comparable to the electrophoretic counterpart, thereby enabling long-term trap of the nanobeads in the nanopore for longer than 0.1 s. At the other extreme, the positive wall surface temporarily adsorbs particles by the attractive coulomb forces (Fig. 3d), which is seen as long plateaus at a certain Iion level (It is worth noting that only a scarce number of nanoparticles were actually immobilized under the positive gate voltages. This reflects the spatially-confined transverse electric field in the nanopore by ion screening (Debye length is about 0.3 nm) bringing strong columbic interactions to only those approached in vicinity of the wall surface. The absence of the gating effect at positive VG for 100 nm-sized nanospheres (Fig. 2b) is hence attributed to less chance for the smaller particles to pass through the off-axial positions.). In contrast, the gating effects become weaker at lower |VG | causing little influence against the cross-membrane electric-field-dominated single-nanoparticle kinetics (Fig. 3c).
a Typical Iion traces observed for 200 nm-sized nanoparticles in the three different VG regimes under Vb = 0.5 V (orange: VG = −0.20 V (I); pink: VG = + 1.20 V (II); cyan: VG = −1.05 V(III)). The open pore current is offset to zero. Schematic explanations of the underlying mechanism. Green and pink balls describe, respectively, negative and positive electrical potentials induced at the SiO2 wall surface by the gating. Under large negative VG, the strong electroosmotic flow in direction opposite to the electrophoresis of the negatively-charged nanoparticles retard the translocation speed (b). When VG is small, the electroosmotic flow becomes too weak to cause any notable influence on the electrophoretically-driven fast translocation motions of the particles irrespective of the voltage polarity (c). In case of large positive gate voltage conditions, the strong columbic interaction tends to temporarily trap the nanobeads when they come across the nanopore wall surface.
Electrical trapping of single-nanoparticles via gating control
Direct comparisons of the resistive pulses show marked difference in the motion degrees of freedom of the trapped nanoparticles. The Iion curves reveal similar retention time of the particles irrespective of the gate polarity (Fig. 4a) but much larger fluctuations under negative VG (Fig. 4b), which signifies more pronounced up-down movements of the nanobeads. Indeed, unlike the strong columbic attraction that immobilizes the nanoparticles on the wall surface, the counteracting hydrodynamic drag and electrophoretic forces are anticipated to induce stochastic motions in analogous to the objects trapped by gradient forces in laser focus38,39. These features were also observed in the corresponding noise spectra (Fig. 4c) as stronger 1/f characteristics at VG = −1.05 V attributed to the more prominent stochastic motions of the trapped particles.
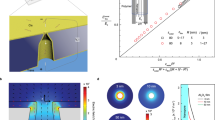
We further investigated the vibration behavior by deriving the time-course change in the particle position z from the Iion signals. We conducted a three-dimensional finite element analysis of the ionic current blockade where we numerically estimated the cross-membrane ionic current through the gating nanopore with a rigid sphere of 200 nm diameter positioned at z along the pore axis (Fig. 5a)40. The obtained z versus Iion pulse was then normalized by the height (Inorm) and utilized as a reference to convert the experimental resistive pulses (Fig. 5b) into z versus t traces. For instance, the motion track elucidated the nanoparticles being adsorbed at 130 nm above the pore center under VG = +1.20 V (Fig. 5c inset). In addition, the subsequent drop in the ionic current at around t = 0.38 s (Fig. 5b inset) suggested electrophoretic transits of the particles after desorption.
a Finite element analysis of ionic current blockade in the 300 nm-sized surround-gate nanopore by a 200 nm-sized nanoparticle under Vb = 0.5 V. z denotes the position of the center-of-mass of the spherical particle with respect to the middle of the nanopore. Open pore current is offset to zero. Inorm is the normalized ionic current by the height of the pulse. b A typical Iion signal.at VG = −1.20 V. The inset shows a close view at the end of the signal wherein a sharp drop in the ionic current represents fast translocation of the surface-adsorbed nanoparticle after desorption. c Motion track of the nanoparticle in b. Inset image illustrates the location of the nanobead adsorbed on the positively-biased nanopore wall surface via the coulomb interactions. d A Iion signal at VG = −1.05 V and corresponding particle motions. The absence of the sharp edge at the end of the signal indicates repelling of the nanoparticle instead of electrophoretic translocation as depicted in the insets. Illustrative models depicting the motions of the 200 nm- (e) and 100-nm-sized nanoparticles (f) trapped at the surround-gate nanopore orifice under VG = −1.05 V. g The electrophoretic (FEP) and the electroosmotic drag forces (FEOF) on the nanoparticle positioned at zps. σwall is the surface charge density at the pore wall. The sign of FEOF is inverted for the sake of clarity. h A schematic illustration describing the force balance at the nanopore orifice.
Vibration modes of electrically trapped nanoparticles
This single-nanoparticle tracking also led us to visualize the fluctuations under VG = −1.05 V that took place at 187 nm and 166 nm above the channel with the amplitudes of 42 nm and 90 nm for the 200 nm- and 100 nm-sized particles, respectively (Fig. 5d–f). The underlying mechanism can be understood as oscillation of the spherical objects under the counteracting electrophoretic force FEP and hydrodynamic drag force FEOF by the electroosmosis-induced fluid flow coming out of the gating nanopore. To shed further light on the physical picture, we extended the numerical simulations to estimate the spatial distributions of the two forces acting on the nanoparticle (Supplementary Figs. S6–S8). Specifically, the amount of surface charge q on the nanoparticles was assessed from the zeta potentials of −31 and −36 mV measured by a zeta sizer for the 200 nm- and 100 nm-sized particles, respectively, through Graham’s equation41,42. The particle-position-dependent FEP was then obtained as –qE(z) where E(z) is the electric field profile along the pore axis deduced by numerically solving Poisson, Nernst-Plank, and Navier–Stokes equations43,44. At the same time, the electroosmotically-driven fluid velocity U(z) under the negative VG was utilized to calculate the Stokes drag force FEOF as 3πηdPSU. As a result, we found a crossover point at around z = 200 nm where FEP becomes weaker than FEOF as the 200 nm-sized nanobead moves toward the nanopore (Figs. 5g and 5h). The nanobeads were thus anticipated to be trapped at the position where FEP balanced with FEOF.
Here we remind that whereas the nanobead is anticipated to undergo harmonic oscillation under the electrophoretic and hydrodynamic drag forces at the orifice, the motion is in fact efficiently damped via viscous drag letting it to rest at the equilibrium point, which is akin to the principle of optical tweezer capable of trapping a transparent microscale object in a laser focal point via the gradient force emerging by the interplay between the induced dipole and the local electromagnetic field profile45. For example, the electrokinetics can be roughly described as a nanosphere of mass m vibrating in water with a spring constant k. This anticipates the particle to oscillate at a natural frequency f given as,
Meanwhile, with the deduced k of 1.66 pN nm−1 from FEP and FEOF (Supplementary Fig. S9a, b), we find that k/m < (3πηdps/2 m)2, thus predicting overdamping of the harmonic oscillation. More directly, we also simulated the vibration motion by solving a Langevin equation with the Euler-Maruyama approach (Supplementary Fig. S9c-d). The results displayed random fluctuations of the particle positions by time instead of harmonic oscillations at a well−defined frequency, thereby corroborated negligible contributions of inertia due to the prominent influence of viscous drag. This in turn elucidated that the Iion fluctuations at VG = −1.05 V were of Brownian motions of the trapped nanobeads.
Ultrafine manipulation of single-nanoparticle translocation speed
The field-effect nanopore can be implemented not only for trapping particles but also to let them slowly move toward one direction. Even small deviations of VG from −1.05 V were confirmed to induce unbalanced forces (Fig. 6a): VG ≥ −0.9 V offered inadequate hydrodynamic dragging to stop the electrophoretically-captured nanobeads (Supplementary Fig. S10) while VG ≤ −1.1 V yielded too strong electroosmotic flow that completely blocked the nanoparticles at the orifice (see also Supplementary Fig. S11 for the gating effect on 100 nm-sized nanoparticles demonstrating the trapping at –0.9 V). Yet, a subtle increase in the gate voltage from −1.05 V still allowed the nanoparticles to be hold in the trap zone with the center of mass now moving toward the pore under the slightly weaker water flow. The actual single-nanoparticle velocity vPS was estimated by linear fitting to the z–t curve to be −59 nm s−1 at VG = −1.00 V (Fig. 6b), which could be fastened by 17% to −69 nm s−1 through further decreasing the gate voltage to −0.96 V (Fig. 6c, d, see also Supplementary Fig. S12). The extremely low range of vPS marks more than four orders of magnitude reduction in the single-particle velocity under no gate-control. This manifests the fact that while the gating control of electroosmotic flow36 and particle trapping by hydrodynamic drag and electrophoretic forces35 were separately reported previously, the combination of these effects enables the ultra-fine control of single-nanoparticle translocation dynamics.
a The effective range of VG for the particle trap. The ionic current signals (top) and the corresponding z–t characteristics for 200 nm-sized nanoparticles captured under VG = −1.00 V (b) and VG = −0.96 V (c). Gray lines are linear fits to the curves whose slope signify the average velocity vps of the nanoparticles. d VG-dependence of vPS.
Discussion
It is worth discussing whether interference between the gate and the cross-membrane voltage can be a cause of the nanoparticle oscillation in the nanopore. Indeed, numerical studies predicted significant modulation of the electrostatic potential profile31,32 that may create energy minima to trap a charged object inside the pore. To verify this point, we exhibited a control experiment using a SiNx nanopore without any gate electrode (Supplementary Fig. S13). It in fact revealed emergence of the corrugated resistive pulses suggestive of the particle traps inside the conduit under a specific Vb and pH conditions, the finding of which unambiguously prove the predominant role of electroosmosis on the trapping of nanoparticles demonstrated in the surround-gate nanopores.
As for the manipulation capability, the field-effect nanopore approach can be potentially used to accomplish 0.2 nt ms−1, which is the speed achievable only in biological nanochannels15,18,19, when tentatively comparing with the translocation speed of single molecule DNA that generally passes through a solid-state nanopore at a rate faster than 100 nucleotides per ms (100 nt ms−1). While it may require tagging of DNA on a nanocarrier surface for implementing genome sequencing46, due to the soft nature of polynucleotides undergoing deformations by the counter-acting forces47, the technique has an advantage over optical tweezers in sense that the mechanism involves no additional structure thus allowing compact device for mass production. Besides, it promises direct applications for resistive pulse analyses of small molecules such as proteins10 and micro-RNA48 where it is generally a prerequisite to slow-down their translocation dynamics for reliably detecting the associated ionic current blockade8,17.
Methods
Fabrication of gating nanopores
A 4-in. 525 μm-thick Si wafer both sides coated with low-pressure chemical vapor deposition-grown 50 nm-thick SiNx layers was diced into 30 mm square chips. On the chip, Au microelectrodes were formed by photolithography followed by radio-frequency magnetron sputtering of Au (20 nm)/Cr (5 nm) and lift-off in N,N-dimethylformamide (DMF, Wako) under ultrasonication. After that, the SiNx layer at the other side was partially removed by reactive ion etching with CF4 etchant gas. The thus exposed Si layer was immersed in KOH aq. and heated to 80 °C for wet etching. As a result of the anisotropic nature of the etching process of the single-crystal Si led to a formation of a deep trench with ~50 nm-thick SiNx membrane at the bottom. Here, the membrane was arranged to be formed in the gap space between the Au microeletrodes by using the pre-designed external markers on the wafer surface. Subsequently, a gate electrode was fabricated by first delineating the pattern by an electron beam lithography using a resist ZEP-520A (Zeon). After development, Pt (20 nm) was deposited by the sputtering. The substrate was then put in DMF overnight for lift-off without sonication.
Integration of a polyimide micropore
The surround gate nanopore chip was covered by a photo-sensitive 5 μm-thick polyimide layer (Toray) by spin-coating and pre-baking. Then, the polyimide 50 μm around the nanopore was removed by photolithography. By the low-k polymer coating, we can ensure high temporal response of the ionic current as well as low noise platform by the reduced cross-plane net capacitance, which is a pre-requisite for reliable analyses of fast translocation motions of nanoparticles by the resistive pulse measurements. Meanwhile, the thus formed polymeric micropore causes negligible influence on the cross-membrane ionic current due to the more two orders of magnitude larger size compared to the surround gate nanopore. Finally, the top surface including the exposed Pt electrode was coated with 10 nm-thick SiO2 at the top and bottom sides by chemical vapor deposition. Here, the SiO2 layer served as gate dielectrics as well as active surface to bond polydimethylsiloxane (PDMS) blocks for device sealing as explained in the following section.
Nanopore sealing
The polyimide-coated surround gate nanopore chip was sealed from the both sides by two PDMS blocks. The polymer blocks were obtained by first curing PDMS on a SU-8 mold on a Si wafer. The mold was fabricated in advance by spin coating SU-8 3000 (MICROCHEM), pre-baking, and photolithography. The SU-8 pattern had “I” shape so that after hardening the cured PDMS at 100 °C in oven, each block cut-out from the mold has I-shaped trench at one side of the surfaces. This trench served as a fluidic channel for letting nanoparticle dispersion solution into the nanopore after the PDMS blocks were adhered at both sides of the nanopore chip, which was exhibited by contacting the both surfaces after an oxygen plasma treatment.
Ionic current measurements
Electrolyte buffer 1 × PBS (Wako) was added from one side of the nanopore through inlet and outlet holes punched in the PDMS blocks in advance via the PDMS channel. At the other side, the same buffer containing 200 nm- or 100 nm-sized carboxylated nanobeads (Polysciences) was injected. Then, Ag/AgCl rods were inserted into the remaining holes in the PDMS blocks at the both sides. Using these as electrodes, the cross-membrane ionic current Iion was recorded under the dc voltage Vb applied. Meanwhile, metal pins were contacted to the microelectrode pads to add the gate voltage. For the Iion–Vb characteristics measurements, pico-ammeters Keithley6487 (Keithley) were used for adding voltage ramps as well as to measure the ionic current. On the other hand, battery-based potentiostats were utilized for the resistive pulse measurements for the sake of better noise performance as voltage sources. Also, the current sampling was implemented at 1 MHz speed by using a home-built amplifier40 backed by a fast digitizer (NI-5922, National Instruments) under a LabVIEW program control.
Data analytics
In order to extract resistive pulses from Iion traces, the moving open pore current was first offseted to zero by subtracting the linearly-fitted base current in each of the 0.5 s sections. The positions of the pulses were then searched by finding local minima in the offseted curve using a threshold level set at −1 nA. Subsequently, resistive pulse signals were extracted by saving the data 0.01 s before and after the pulse apexes.
Finite element analysis
The ionic current flowing through the 300 nm-sized surround gate electrode embedded nanopore was simulated by a finite element method using COMSOL multiphysics 5.4 with AC/DC, Chemical Reaction Engineering, and Computational Fluid Dynamics modules. The nanopore structure was modeled as a cylinder of 8 μm-radius and 16.05 μm-height wherein a 50 nm-thick Si3N4 membrane (electrical conductivity of σ = 1.4 × 10−14 S m−1 and relative permittivity of εr = 9.7) covered with 30 nm-thick Pt and 20 nm-thick SiO2 layers was placed at the middle. In the membrane, a through-hole with a radius of 150 nm was opened at the center. The nanopore as well as the cylinder was filled with water containing Na+ and Cl- at a concentration of 137 mM with the mobility of, respectively, 5.2 × 10−8 m2 V−1 s−1 and 7.4 × 10−8 m2 V−1 s−1. The ionic current was calculated by simultaneously solving Poisson equation, continuity equation at steady-state current, Nernst-Planck equation, and Naiver–Stokes equation under the potential difference of 0.5 V set between the top and bottom surface of the micrometer-scale cylinder and the wall surface potential to VG.
The electrophoretic (FEP) and electroosmotic drag force (FEOF) were then estimated based on the obtained distributions of electric-field E(r) and fluid velocity u(r) as follows:
In the above, E is the electric field calculated from the above electrokinetic model, σ is the density of the negative charges on the nanoparticle surface with zeta potential of –31 mV as measured using a zeta sizer, and the integration was over the nanoparticle surface \(\oiint {ds}\). On the other side, FEOF was estimated through an integration of the viscous force density fEOF on the nanoparticle surface:
where η is the viscosity of the solution and the partial differential ∂u/∂r represents the fluid velocity gradient along the nanoparticle-centered radial direction around the particle surface. Here we remind that a simplified estimation of FEOF was provided through the Stokes equation FEOF = 3πdnpu where dnp is the diameter of the spherically-shaped nanoparticle and u is the average fluid velocity around the nanoparticle.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
Howorka, S. & Siwy, Z. Nanopore analytics: sensing of single molecules. Chem. Soc. Rev. 38, 2360–2384 (2009).
Luo, L. et al. Resistive pulse analysis of nanoparticles. Annu. Rev. Anal. Chem. 7, 513–535 (2014).
Shi, W., Friedman, A. K. & Baker, L. A. Nanopore sensing. Anal. Chem. 89, 157–188 (2017).
McMullen, A., de Haan, H. W., Tang, J. X. & Stein, D. Stiff filamentous virus translocations through solid-state nanopores. Nat. Comm. 5, 4171 (2014).
Arima, A. et al. Identifying single viruses using biorecognition solid-state nanopores. J. Am. Chem. Soc. 140, 16834–16841 (2018).
Nawaz, M. et al. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat. Rev. Urol. 11, 688–701 (2014).
Coumans, F. A. W. et al. Reproducible extracellular vesicle size and concentration determination with tunable resistive pulse sensing. J. Extracel. Ves. 140, 16834–16841 (2018).
Plesa, C. et al. Fast translocation of proteins through solid state nanopores. Nano Lett. 13, 658–663 (2013).
Si, W. & Aksimentiev, A. Nanopore sensing of protein folding. ACS Nano 11, 7091–7100 (2017).
Varongchayakul, N., Song, J., Meller, A. & Grinstaff, M. W. Single-molecule protein sensing in a nanopore: a tutorial. Chem. Soc. Rev. 47, 8512–8524 (2018).
Branton, D. et al. The potential and challenges of nanopore sequencing. Nat. Biotechnol. 26, 1146–1153 (2008).
Heerema, S. J. & Dekker, C. Graphene nanodevices for DNA sequencing. Nat. Nanotechnol. 11, 127–136 (2016).
Feng, J. et al. Identification of single nucleotides in MoS2 nanopores. Nat. Nanotechnol. 10, 1070–1076 (2015).
Coulter, W. H. Means of counting particles suspended in a fluid. US Patent No. 2656508 (1953).
Venkatesan, B. M. & Bashir, R. Nanopore sensors for nucleic acid analysis. Nat. Nanotechnol. 6, 615–624 (2011).
Keyser, U. F. Controlling molecular transport through nanopores. J. Roy. Soc. Interf. 8, 1369–1378 (2011).
Acharya, S. et al. Improved measurement of proteins using a solid state nanopore coupled with a hydrogel. ACS Sens. 5, 370–376 (2020).
Cherf, G. M. et al. Automated forward and reverse ratcheting of DNA in a nanopore at 5-Å precision. Nat. Biotechnol. 30, 344–348 (2012).
Nivala, J., Marks, D. B. & Akeson, M. Unfoldase-mediated protein translocation through an α-hemolysin nanopore. Nat. Biotechnol. 31, 247–250 (2013).
Fiori, N. D. et al. Optoelectronic control of surface charge and translocation dynamics in solid-state nanopores. Nat. Nanotechnol. 8, 946–951 (2013).
Chou, C.-F. et al. Electrodeless dielectrophoresis of single- and double-stranded DNA. Biophys. J. 83, 2170–2179 (2002).
Tanaka, S. et al. Tailoring particle translocation via dielectrophoresis in pore channels. Sci. Rep. 6, 31670 (2016).
Luan, B. et al. Base-by-base ratcheting of single stranded DNA through a solid-state nanopore. Phys. Rev. Lett. 104, 238103 (2010).
Nam, S.-W., Rooks, M. J., Kim, K.-B. & Rossnagel, S. M. Ionic field effect transistors with sub-10 nm multiple nanopores. Nano Lett. 9, 2044–2048 (2009).
Ren, R. et al. Nanopore extended field-effect transistor for selective single-molecule biosensing. Nat. Comm. 8, 586 (2017).
Tsutsui, M. et al. Temporal response of ionic current blockade in solid-state nanopores. ACS Appl. Mater. Interf. 10, 34751 (2018). 34757.
Rosenstein, J. K., Wanunu, M., Merchant, C. A., Drndic, M. & Shepard, K. L. Integrated nanopore sensing platform with sub-microsecond temporal resolution. Nat. Methods 9, 487–492 (2012).
Tabard-Cossa, V., Trivedi, D., Wiggin, M., Jetha, N. N. & Marziali, A. Noise analysis and reduction in solid-state nanopores. Nanotechnology 18, 305505 (2007).
Garaj, S. et al. Graphene as a subnanometre trans-electrode membrane. Nature 467, 190–193 (2010).
Tsutsui, M. et al. Single-nanoparticle detection using a low-aspect-ratio pore. ACS Nano 6, 3499–3505 (2012).
Liu, Y., Huber, D. E., Tabard-Cossa, V. & Dutton, R. W. Descreening of field effect in electrically gated nanopores. Appl. Phys. Lett. 97, 143109 (2010).
Liu, Y., Huber, D. E. & Dutton, R. W. Limiting and overlimiting conductance in field-effect gated nanopores. Appl. Phys. Lett. 96, 253108 (2010).
He, Y. et al. translocation through gate modulation of nanopore wall surface charges. ACS Nano 5, 5509–5518 (2011).
Yeh, L.-H., Xue, S., Joo, S. W., Qian, S. & Hsu, J.-P. Field effect control of surface charge property and electroosmotic flow in nanofluidics. J. Phys. Chem. C 116, 4209–4216 (2012).
Stein, D. et al. Electronic concentration of DNA polymers in nanofluidic channels. Nano Lett. 10, 766–772 (2010).
Schasfoort, R. B. M., Schlautmann, S., Hendrikse, J. & van den Berg, A. Field-effect flow control for microfabricated fluidic networks. Science 286, 942–945 (1999).
Chen, P. et al. Atomic layer deposition to fine-tune the surface properties and diameters of fabricated nanopores. Nano Lett. 4, 1333–1337 (2004).
Block, S. M. Making light work with optical tweezers. Nature 360, 493–495 (1992).
Grigorenko, A. N., Roberts, N. W., Dickinson, M. R. & Zhang, Y. Nanometric optical tweezers based on nanostructured substrates. Nat. Photon. 2, 365–370 (2008).
Tsutsui, M. et al. Particle trajectory-dependent ionic current blockade in low-aspect-ratio pores. ACS Nano 10, 803–809 (2016).
O’Brien, R. W. & White, L. R. Electrophoretic mobility of a spherical colloidal particle. J. Chem. Soc. Farad. Trans. 2, 1607–1626 (1978).
Arjmandi, N., Roy, W. V., Lagae, L. & Borghs, G. Measuring the electric charge and zeta potential of nanometer-sized objects using pyramidal-shaped nanopores. Anal. Chem. 84, 8490–8496 (2012).
Laohakunakorn, N., Thacker, V. V., Muthukumar, M. & Keyser, U. Nano Lett. 15, 695–702 (2015).
German, S. R., Luo, L., Henry, H. S. & Mega, T. L. Controlling nanoparticle dynamics in conical nanopores. J. Phys. Chem. C 117, 703–711 (2013).
Li, T., Kheifets, S., Medellin, D. & Raizen, M. G. Measurement of the instantaneous velocity of a Brownian particle. Science 328, 1673–1675 (2010).
Keyser, U. F. et al. Direct force measurements on DNA in a solid-state nanopore. Nat. Phys. 2, 473–477 (2006).
Aksimentiev, A. Deciphering ionic current signatures of DNA transport through a nanopore. Nanoscale 2, 468–483 (2010).
Wanunu, M. et al. Rapid electronic detection of probe-specific microRNAs using thin nanopore sensors. Nat. Nanotechnol. 5, 807–814 (2010).
Acknowledgements
A part of this work was supported by ImPACT Program of Council for Science, Technology, and Innovation (Cabinet Office, Government of Japan), JST PRESTO Grant Number JPMJPR17HC, JST CREST Grant Number JPMJCR1666, and the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 15H05417 and 18H01846.
Author information
Authors and Affiliations
Contributions
M.T., T.W., K.T., and T.K. planned experiments. M.T. and S.R. fabricated Surround-gate nanopores and performed resistive pulse measurements of single-nanoparticles. K.Y. and Y.H. performed finite element analyses of ion transport and nanoparticle translocation dynamics in the nanopores. M.T. and T.K. co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary handling editor: Aldo Isidori.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsutsui, M., Ryuzaki, S., Yokota, K. et al. Field effect control of translocation dynamics in surround-gate nanopores. Commun Mater 2, 29 (2021). https://doi.org/10.1038/s43246-021-00132-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43246-021-00132-3
This article is cited by
-
Localised solid-state nanopore fabrication via controlled breakdown using on-chip electrodes
Nano Research (2022)
-
Solid-state nanopore systems: from materials to applications
NPG Asia Materials (2021)