Abstract
Liver metastasis (LM) confers poor survival and therapy resistance across cancer types, but the mechanisms of liver-metastatic organotropism remain unknown. Here, through in vivo CRISPR–Cas9 screens, we found that Pip4k2c loss conferred LM but had no impact on lung metastasis or primary tumor growth. Pip4k2c-deficient cells were hypersensitized to insulin-mediated PI3K/AKT signaling and exploited the insulin-rich liver milieu for organ-specific metastasis. We observed concordant changes in PIP4K2C expression and distinct metabolic changes in 3,511 patient melanomas, including primary tumors, LMs and lung metastases. We found that systemic PI3K inhibition exacerbated LM burden in mice injected with Pip4k2c-deficient cancer cells through host-mediated increase in hepatic insulin levels; however, this circuit could be broken by concurrent administration of an SGLT2 inhibitor or feeding of a ketogenic diet. Thus, this work demonstrates a rare example of metastatic organotropism through co-optation of physiological metabolic cues and proposes therapeutic avenues to counteract these mechanisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Gene expression data generated in this study have been deposited in the Gene Expression Omnibus under accession number GSE188391. Previously published RNA-seq data for the Arriaga et al. cohort are accessible under accession number GSE143812, and MET500 data are accessible at https://xenabrowser.net/datapages/?cohort=MET500%20(expression%20centric).
The deidentified DNA-sequencing and RNA-seq data are owned by Caris Life Sciences and cannot be publicly shared due to the data usage agreement signed by B. Izar at Columbia University Iriving Medical Center. Qualified researchers can apply for access to these data by contacting J. Xiu (jxiu@carisls.com), submitting a brief proposal and signing a data usage agreement. Metabolomics data have been deposited in the Metabolomics Workbench (the NIH Common Fund’s National Metabolomics Data Repository website, https://www.metabolomicsworkbench.org) under study ID ST002851 and can be accessed directly via its project ID (https://doi.org/10.21228/M8043D).
The remaining data are available within the article or as source data. Source data are provided with this paper.
Code availability
No custom algorithms were used in this study.
References
Chaffer, C. L. & Weinberg, R. A. A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011).
Gao, Y. et al. Metastasis organotropism: redefining the congenial soil. Dev. Cell 49, 375–391 (2019).
Tsilimigras, D. I. et al. Liver metastases. Nat. Rev. Dis. Primers 7, 27 (2021).
Lee, J. C. et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci. Immunol. 5, eaba0759 (2020).
Tumeh, P. C. et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol. Res. 5, 417–424 (2017).
Bergers, G. & Fendt, S.-M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 21, 162–180 (2021).
Priestley, P. et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature 575, 210–216 (2019).
Biermann, J. et al. Dissecting the treatment-naive ecosystem of human melanoma brain metastasis. Cell 185, 2591–2608 (2022).
Broadfield, L. A. et al. Fat induces glucose metabolism in nontransformed liver cells and promotes liver tumorigenesis. Cancer Res. 81, 1988–2001 (2021).
Moris, D., Lu, L. & Qian, S. Mechanisms of liver-induced tolerance. Curr. Opin. Organ Transplant. 22, 71–78 (2017).
Lee, J. W. et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature 567, 249–252 (2019).
Landsberg, J. et al. Autochthonous primary and metastatic melanomas in Hgf-Cdk4 R24C mice evade T-cell-mediated immune surveillance. Pigment Cell Melanoma Res. 23, 649–660 (2010).
Bald, T. et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 507, 109–113 (2014).
& Rogava, M. et al. Tumor cell intrinsic Toll‐like receptor 4 signaling promotes melanoma progression and metastatic dissemination.Int. J. Cancer 150, 142–151 (2021).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Zaretsky, J. M. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016).
Bakhoum, S. F. et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472 (2018).
Brognard, J., Zhang, Y.-W., Puto, L. A. & Hunter, T. Cancer-associated loss-of-function mutations Implicate DAPK3 as a tumor‐suppressing kinase. Cancer Res. 71, 3152–3161 (2011).
Chen, S. et al. Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis. Cell 160, 1246–1260 (2015).
LaLonde, D. P., Brown, M. C., Bouverat, B. P. & Turner, C. E. Actopaxin Interacts with TESK1 to Regulate Cell Spreading on Fibronectin. J. Biol. Chem. 280, 21680–21688 (2005).
Yang, C.-S. et al. The protein kinase C super-family member PKN is regulated by mTOR and influences differentiation during prostate cancer progression. Prostate 77, 1452–1467 (2017).
Yang, M. et al. MYLK4 promotes tumor progression through the activation of epidermal growth factor receptor signaling in osteosarcoma. J. Exp. Clin. Cancer Res. 40, 166 (2021).
Cunningham, J. T., Moreno, M. V., Lodi, A., Ronen, S. M. & Ruggero, D. Protein and nucleotide biosynthesis are coupled by a single rate-limiting enzyme, PRPS2, to drive cancer. Cell 157, 1088–1103 (2014).
Wang, D. G. et al. PIP4Ks suppress insulin signaling through a catalytic-independent mechanism. Cell Reports 27, 1991–2001 (2019).
Kim, T. et al. TRIB1 regulates tumour growth via controlling tumour-associated macrophage phenotypes and is associated with breast cancer survival and treatment response. Theranostics 12, 3584–3600 (2022).
Soubeyrand, S., Martinuk, A., Lau, P. & McPherson, R. TRIB1is regulated post-transcriptionally by proteasomal and non-proteasomal pathways. PLoS One 11, e0152346 (2016).
Lafitte, M. et al. FGFR3 has tumor suppressor properties in cells with epithelial phenotype. Mol. Cancer 12, 83 (2013).
Hopkins, B. D. et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499–503 (2018).
Arriaga, J. M. et al. A MYC and RAS co-activation signature in localized prostate cancer drives bone metastasis and castration resistance. Nat Cancer 1, 1082–1096 (2020).
Robinson, D. R. et al. Integrative clinical genomics of metastatic cancer. Nature 548, 297–303 (2017).
Sridhar, S. et al. Prognostic significance of liver metastasis in durvalumab-treated lung cancer patients. Clinical Lung Cancer 20, e601–e608 (2019).
Tumeh, P. C. et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC.Cancer Immunol. Res. 7, 282 (2017).
Birkbak, N. J. & McGranahan, N. Cancer genome evolutionary trajectories in metastasis. Cancer Cell 37, 8–19 (2020).
El-Kebir, M., Satas, G. & Raphael, B. J. Inferring parsimonious migration histories for metastatic cancers. Nat. Genet. 50, 718–726 (2018).
Sivanand, S. et al. Cancer tissue of origin constrains the growth and metabolism of metastases. Preprint at bioRxiv https://doi.org/10.1101/2022.08.17.504141 (2022).
Yu, J. et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 27, 152–164 (2021).
Reichert, M. et al. Regulation of epithelial plasticity determines metastatic organotropism in pancreatic cancer. Dev. Cell 45, 696–711 (2018).
Kalaany, N. Y. & Sabatini, D. M. Tumours with PI3K activation are resistant to dietary restriction. Nature 458, 725–731 (2009).
Nencioni, A., Caffa, I., Cortellino, S. & Longo, V. D. Fasting and cancer: molecular mechanisms and clinical application. Nat. Rev. Cancer 18, 707–719 (2018).
Zhang, Y. et al. C24-ceramide drives gallbladder cancer progression through directly targeting phosphatidylinositol 5-phosphate 4-kinase type-2 gamma to facilitate mammalian target of rapamycin signaling activation. Hepatology 73, 692–712 (2021).
Triscott, J. et al. PI5P4Kα supports prostate cancer metabolism and exposes a survival vulnerability during androgen receptor inhibition. Sci. Adv. 9, eade8641 (2023).
Wang, B. et al. Integrative analysis of pooled CRISPR genetic screens using MAGeCKFlute. Nat. Protoc. 14, 756–780 (2019).
Frangieh, C. J. et al. Multi-modal pooled Perturb-CITE-Seq screens in patient models define novel mechanisms of cancer immune evasion. Preprint at bioRxiv https://doi.org/10.1101/2020.09.01.267211 (2020).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Love, M. I. et al. Tximeta: Reference sequence checksums for provenance identification in RNA-seq. PLoS Comput. Biol. 16, e1007664 (2020).
Beyaz, S. et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531, 53–58 (2016).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Wolock, S. L., Lopez, R. & Klein, A. M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cell Syst. 8, 281–291 (2019).
Han, X. et al. Mapping the Mouse Cell Atlas by Microwell-Seq. Cell 172, 1091–1107 (2018).
Sikkema, L. et al. An integrated cell atlas of the lung in health and disease. Nat. Med. 29, 1563–1577 (2023).
Rock, J. R. et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl Acad. Sci. USA 106, 12771–12775 (2009).
Rawlins, E. L. et al. The Role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4, 525–534 (2009).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 1, 417–425 (2015).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Zhang, Y., Parmigiani, G. & Johnson, W. E. ComBat-seq: batch effect adjustment for RNA-seq count data. NAR Genom. Bioinform. 2, lqaa078 (2020).
Korotkevich, G. et al. Fast gene set enrichment analysis. Preprint at bioRxiv https://doi.org/10.1101/060012 (2021).
Kanehisa, M. & Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Sellick, C. A., Hansen, R., Stephens, G. M., Goodacre, R. & Dickson, A. J. Metabolite extraction from suspension-cultured mammalian cells for global metabolite profiling. Nat. Protoc. 6, 1241–1249 (2011).
Acknowledgements
We sincerely thank S. H. Davis, who sadly passed away during the completion of this study, for her dedication to excellence in cancer biology research and her passion for conducting humane animal research. We also thank J. Doench (genome perturbation platform at the Broad Institute) for technical guidance on the in vivo CRISPR screen, A. V. Parent from UCSF for technical advice, and E. Choi, A. Hung, S.-H. Wilhelm and L. Hsiauo-Yun from Columbia University for technical advice. B.I. is supported by National Institute of Health grants R37CA258829, R01CA280414, R01CA266446 and U54CA274506 and by the Pershing Square Sohn Cancer Research Alliance Award, the Burroughs Wellcome Fund Career Award for Medical Scientists, a Tara Miller Melanoma Research Alliance Young Investigator Award, the Louis V. Gerstner, Jr. Scholars Program and the V Foundation Scholars Award. B.N. was supported by K00CA234950. M. Röcken is supported by Wilhelm-Sander-Stiftung (2020.100.1); DFG RO 764 15/2; Cluster of Excellence iFIT (EXC 2180) “Image-Guided and Functionally Instructed Tumor Therapies”, University of Tübingen, Germany, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germanyʼs Excellence Strategy EXC 2180-390900677. E.M.E. partially supported by NIH T32 (grant GM132083). L.C. is supported by NIH/NCI grant F30CA281104. S.F.B. is supported by Mark Foundation for Cancer Research (20-028-EDV), Oliver S. and Jennie R. Donaldson Charitable Trust, Mathers Foundation, CSHL Cancer Center Shared Resources (Animal and Histology Core Facilities) and acknowledges support by NCI Cancer Center Support grant P30CA045508. L.C.C. is supported by NCI R35 CA197588. This work was supported by NIH/NCI Cancer Center Support Grant P30CA013696 and MSTP training grant T32GM007367 (for MD/PHD Students). Metabolomics Workbench is supported by NIH grant U2C-DK119886 and OT2-OD030544 grants. The following illustrations were prepared using BioRender.com: Fig. 1a,h, Fig. 3h and Fig. 6a.
Author information
Authors and Affiliations
Contributions
B.I. conceived of and supervised the work. M. Rogava designed, performed and analyzed all key experiments. J.C.M., S.H.D., C.C., S. Tang, P.H., A.D.A., L.C., M.J.L., G.Z., B.N., S.C., R.J.C. and O.S. performed and supported experiments. M. Rogava and C.H. analyzed CRISPR screens. and in vitro RNA-seq data. T.J.A., S.K.D., S.W., C.G., S. Tagore, Y.W., W.G. and D.L. analyzed patient RNA-seq data newly generated here, assembled from publicly available data, or the Caris Life Sciences data. W.-Y.C., E.M.E. and A.M.L. analyzed scRNA-seq data. L.M., N.M., G.A., J.R., S.W.M. and A.T. generated and analyzed metabolomics data. A. Molotkov and A. Mintz performed PET-CT imaging studies. G.S., G.T.G., T.T., D.S., M. Röcken, T.K.E. and S.F.B. provided key materials, reagents or data. M.S., S.B., L.C.C., P.K.S., A.T., D.L. and A.M.L. provided additional study supervision. M. Rogava and B.I. wrote the initial and revised manuscripts with input and contributions from all authors. All authors approved the revised manuscript.
Corresponding author
Ethics declarations
Competing interests
B.I. has received consulting fees/honoraria from Volastra Therapeutics, Merck, AstraZeneca, Eisai and Janssen Pharmaceuticals and has received research funding to Columbia University from Alkermes, Arcus Biosciences, Checkmate Pharmaceuticals, Compugen, Immunocore and Synthekine. None of these are relevant to the current work. C.G. has received consulting fees from Watershed Informatics. S.F.B. owns equity in, receives compensation from and serves as a consultant and the Scientific Advisory Board and Board of Directors of Volastra Therapeutics. L.C.C. is a co-founder, member of the scientific advisory board and holds equity in Agios, Petra, Volastra Therapeutics, Faeth and Larkspur. These companies are developing novel therapies for cancer, though drugs from these companies are not discussed in this paper. D.S. reports grants (to institution) from Amgen, Array/Pfizer, Bristol-Myers Squibb, MSD, Novartis and Roche; consulting fees/honoraria from 4SC, Amgen, Array Biopharma, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Haystick, Immunocore, InFlarX, Innocent, LabCorp, Merck Serono, MSD, Nektar, NeraCare, Novartis, OncoSec, Pfizer, Philogen, Pierre Fabre, Replimune, Roche, Sandoz, Sanofi/Regeneron and Sun Pharma; support for attendings meetings or travel support from Bristol-Myers Squibb, MSD, Merck Serono, Novartis, Pierre Fabre and Sanofi; participation on drug safety monitoring or advisory boards for 4SC, Amgen, Array Biopharma, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Immunocore, InFlarX, Merck Serono, MSD, Nektar, NeraCare, Novartis, OncoSec, Pfizer, Philogen, Pierre Fabre, Replimune, Roche, Sandoz, Sanofi/Regeneron and SunPharma; and leadership roles for DeCOG, German Cancer Society, Hiege-Stiftung, Deutsche Hautkrebsstiftung, Nationale Versorgungskonferenz Hautkrebs (NVKH) and European Melanoma Registry (EuMelaReg). S.K.D., S.W. and G.S. are employees of Caris Life Sciences. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cas9 expressing HCmel12 melanoma cell line generation, mouse kinome (Brie) library titration and kinome (Brie) sgRNA representation at different steps of tumor cell editing, primary tumor growth, and metastasis.
a, Assessment of Cas9-activity using an EGFP/EGFP-sgRNA-reporter using flow cytometry in HCmel12 melanoma with Cas9-expression, alongside HCmel12 melanoma cells expressing Cas9 without reporter (negative control), and parental HCmel12 cells without Cas9 but with reporter (positive control). EGFP-negative cells (rightmost plot) indicated activity of ~94%. b, Transduction of lentiviral library in Cas9-expressing HCmel12 melanoma cells. Percentage of tumor cells (y axis) transduced with the Brie library at virus dilutions (x axis). c, Proportion of cells calculated to be infected by one sgRNA-containing viral particle (y axis) at different dilutions of the Brie library (x axis). Filled graphs in (b,c) indicate viral concentration/dilution used for the large-scale CRISPR screen. d, Pearson correlation coefficient of the normalized sgRNA read counts from Brie plasmid pool, transduced cells in vitro edited over time (10, 14, 21, 28, 35, 42, 49, 56 days after spin infection), from primary tumors (n = 8), from liver (n = 14), lung (n = 3), and lymph node (n = 8) metastases. For each biological sample type, biological replicates (R1, R2, R3… R8), for technical replicates (R3.1, R3.2) are shown. n = 8 mice for primary tumors, n = 7 mice with Liver mets, n = 3 mice with lung mets, n = 7 mice with LN mets. e, Boxplot of the normalized sgRNA read counts as in (d). Outliers are shown as colored dots for each respective sample. f, Principal-component analysis (PCA) of normalized sgRNA read counts. X- and y-axis with indicated explained variance of 49.7% and 5.4%, respectively. Samples from Brie plasmid, cells and primary tumors are shown in black while metastases are shown in red. Data is representative of two independent experiments.
Extended Data Fig. 2 CRIPSR/Cas9 viability screen in HCmel12 melanoma cells in vitro, Identification of essential genes and genes affecting engraftment of tumor cells in vivo followed by Identification of genes affecting liver tropism.
a, b, LFC for all four individual sgRNAs targeting genes enriched (red lines) or depleted (blue lines) in cells vs plasmid (a), and primary tumor vs cells before transplantation (b), are depicted. The top and bottom 10 enriched or depleted genes are labelled with gene symbols. c, Tumor growth curves in mice transplanted with Cas9 (n = 5 mice) or Brie-transduced (Cas9 + Brie) HCmel12 melanomas (n = 10 mice) are shown; mean± s.e.m. d, Bar graphs showing enrichment of sgRNAs targeted genes in among sgRNAs in liver metastasis. For each of the 14 individual liver metastasis samples (rows) harvested from 7 individual mice, the abundances of sgRNAs reads as a proportion of all reads are shown. Only target genes with at least 2% of the total reads in a sample are shown. e, Venn diagram showing the overlap of enriched sgRNAs in indicated comparisons with FDR < 0.06. Data is representative of two independent experiments.
Extended Data Fig. 3 Generation and validation of CRISPR knockout cells in vitro.
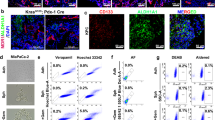
a, Immunoblot showing efficacy of CRISPR-Cas9-mediated knockout of Pip4k2c using four sgRNAs. b, Resulting insertions/deletions for Pip4k2c sgRNA #194 analyzed by Sanger sequencing with Tracking of Indels by Decomposition (TIDE https://tide.nki.nl) and estimated efficiency of Cas9-mediated cuts. c, Immunoblots of phosphorylated and total AKT at two phosphorylation sites pAktS473 and pAktT308 in parental and two of Pip4k2c KO clones over time. d,e, Immunoblot showing efficacy of CRISPR-Cas9-mediated knockout of PIP4K2C using guide #5 in human A375 melanoma cell lines (d) and resulting indels (e) as in (b). f, Immunoblot of HCmel12 parental or Pip4k2c KO cells treated with insulin, and/or GDC-0941 (0.1 µM) or BYL-719 (1 µM). Blotted is the total abundance of insulin receptor (Insr) and phosrpho-Insr. g, Effect of insulin treatment on migration potential measured by transwell assay. Shown is the mean number of migrated parental and Pip4k2c KO cells with and without insulin treatment. n = 3 replicates per condition; mean ± s.e.m. h, Same as in (g) for A375 melanoma cell lines. i,j, Proliferation assay of HCmel12 (i) or A375 (j) Pip4k2c WT and Pip4k2c KO melanoma cells at different insulin concentrations over time. Cell counts were normalized to t0 proliferation is shown as fold change. n = 3 replicates per condition; mean± s.e.m. k, Gene set enrichment analysis of RNA-seq data comparing Pip4k2c KO with parental cells stimulated with insulin. Exemplary pathway enrichment for mTORC1 is shown. l, Amino acid sequence of wild-type ORF (top) and allosteric domain-deficient (AD) Pip4k2c. m,n, Immunoblots showing phosphor-AKT in parental, Pip4k2c KO, and Pip4k2c KO rescued with either wild-type Pip4k2c allele (Pip4k2c Rec) or allosteric domain-deficient (Pip4k2c AD) and combinatorial exposure to insulin (250 ng/ml, first row) and PI3K inhibitor GDC-0941 (0.1 µM, second row) or BYL-719 (1 µM, third row) in murine HCmel12 (m) or human A375 (n) melanoma models. Samples are derived from the same experiment and gels/blots were processed in parallel. o, Incidence of liver metastasis in mice bearing A375 PIP4K2C WT and PIP4K2C KO melanoma cells, n = 10 mice per group; p, C-peptide levels (in pM) for Pip4k2c WT and Pip4k2c KO HCmel12 tumor-bearing animals with and without GDC-0941 treatment. Plasma was collected after 2 hours of treatment with GDC-0941 and vehicle treatment was used as control, n = 5 mice per group. Statistical significance was determined using 2-way ANOVA Tukey’s multiple comparisons test for (g,h,p). Data is representative of two independent experiments (g,h,i,j,o,p). Significance levels as indicated.
Extended Data Fig. 4 Validation of findings in mouse and human metastatic samples using scRNA-seq and Bulk RNA-seq.
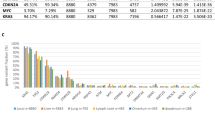
a-c, Quality control plots for single cell RNA sequence data. Cells were filtered based on (a) cumulative number of transcript counts, (b) fraction of mitochondrial mRNA detected per cell and (c) cell complexity as described in the Methods; shown here for one representative library. d, Kernel Density Estimate (KDE) plots showing distribution across cells of total number of transcripts (top) and mitochondrial fraction (bottom) for each sample. e,f, UMAP projections showing the tumor cell population, mean log-transformed gene expression of tumor cell marker gene signature, and log-transformed expression of select individual tumor cell marker genes. g, Top differentially expressed genes in liver metastasis (pink) vs lung metastasis (purple) tumor cells. Genes are projected by the −log10(p_adj) by the averagelog2(FC) and the most significant DEGs are labelled (padj < 0.05, Wilcoxon test). h, PCA of LM biopsy data across cancer types from Met500 and additional LM from melanoma patient biopsies (LM15) generated in this study pre- or post-batch correction with ComBat_seq (Methods). i, Differentially expressed genes in liver (n = 78, Met500; n = 15, LM15, pink) vs lung (n = 16, Met500, purple) metastases from patients across cancer types. Genes are projected by average log2 Fold Change by the -log10(FDR) with genes significantly enriched (FDR q-value < 0.05, Mann–Whitney test) in liver (pink) and in lung (purple) metastasis tumor samples, with specific genes of interest labelled. j, Pathway enrichment analysis (FDR q-value < 0.05, GSEA) in liver (n = 41, Met500; n = 15, LM15, pink) vs non-liver (n = 100, Met500, purple) metastases including only with 70% tumor cell purity. k, Pathway enrichment analysis (FDR q-value < 0.05, GSEA) in liver (n = 41, Met500; n = 15, LM15, pink) vs lung (n = 9, Met500, purple) metastases including only with 70% tumor cell purity. l,m, Expression levels of PIP4K2A/B in primary tumors vs. liver metastases (l) and liver vs. lung metastases (m). n, Frequency of genomic alterations across primary cutaneous melanomas and liver metastases from Caris melanoma patient cohort. The left table summarizes the frequencies between primary tumors and liver metastases for selected genes in the PI3K/AKT pathway, along with adjusted p and q values (methods), and the right table indicates unbiased analyses across all interrogated gene mutations among the same sites. o, Expression of Pip4k2c in primary prostate cancers, lymph node, lung, bone and liver metastases from the Arriaga et al. cohort; for all box plots, n refers to the number of samples, and p refers to the p-value. The center line indicates the median, the box limits denote the first and third quartiles, and the whiskers indicate the lowest or highest data points at the first quartile minus or plus 1.5 times the interquartile range. Statistical significance was determined using Frequency of genomic alterations: Mann–Whitney U Two-tailed test for (l, m) and chi-square or Fisher’s Exact for (n). Significance as indicated.
Extended Data Fig. 5 Metastatic site influences tumor cell metabolic state.
a, Score plot of a PCA analysis of all putatively identified metabolites by LC-MS in positive ionization mode. b, Score plot of a PLS-DA analysis of putatively identified metabolites by LC-MS in negative ionization mode comparing liver and lung metastatic samples from animals with A375 human melanomas with indicated genotypes (Methods). c, VIP score plot generated based on a PLS-DA of putatively identified metabolites by LC-MS in negative ionization mode comparing liver and lung metastatic samples from animals with A375 human melanomas with indicated genotypes, showing a strong change in lactic acid, glutamic acid and a hexose (for example glucose) that is causing the separation of the groups in (b). d, Heat map of results from a suspected targeted analysis. Compounds were manually identified in the LC-MS data based on MS1 accurate mass and MS2-spectrum. The corresponding peaks were integrated, and the results normalized by 13 C2-Citric acid that was spiked to the sample as an internal standard. The compounds were selected based on the multivariate data analysis indicating a change in the glycolysis and/or TCA cycle. disease site, liver (n = 8 specimens) vs. lung (n = 10 specimens); and genotype, parental [PIP4K2C WT] and PIP4K2C KO. Statistical significance was determined using Two-tailed t-test for (d), Lactate (p = 2.36727E-05), Pyruvate (p = 0.03), Citrate (p = 0.25), a-Ketoglutarate (p = 0.18), Succinate (p = 0.004), Fumarate (p = 0.04), Malate (p = 0.04), Hexose (p = 6.72275E-06). e, Extracted ion chromatograms (left) and corresponding MS2 spectra of the putatively identified hexose (for example glucose) with an accurate mass for the [M-H]- ion of 179.5610 (top) and of lactic acid with an accurate mass for the [M-H]- ion of 89.0244 (bottom). Much lower intensities were detected in the lung samples compared to the liver samples (Right).
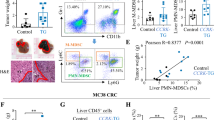
Extended Data Fig. 6 Role of Pip4k2c loss in primary tumor growth and response to systemic therapies.
a, Immunoblots in matched cell lines with indicated genotypes (top) and combinatorial exposure to insulin (250 ng/ml, first row), PI3K inhibitors GDC-0941 (0.1 µM, second row), and Doxycycline (1 µg/ml, third row). b, Combined tumor diameter at day 24 in treatment groups across genotypes; n = 10 mice per condition. Box plots denote the minima, maxima and median. Experiment was repeated twice with similar results. Statistical significance was determined using One-way ANOVA Tukey’s multiple comparisons test. Data is representative of two independent experiments (b). Significance levels as indicated.
Supplementary information
Supplementary Table 1
Supplementary information for the data provided in the article.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed full western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed full western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed full western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed full western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rogava, M., Aprati, T.J., Chi, WY. et al. Loss of Pip4k2c confers liver-metastatic organotropism through insulin-dependent PI3K-AKT pathway activation. Nat Cancer 5, 433–447 (2024). https://doi.org/10.1038/s43018-023-00704-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-023-00704-x