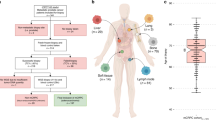
Abstract
De novo metastatic prostate cancer is highly aggressive, but the paucity of routinely collected tissue has hindered genomic stratification and precision oncology. Here, we leveraged a rare study of surgical intervention in 43 de novo metastatic prostate cancers to assess somatic genotypes across 607 synchronous primary and metastatic tissue regions plus circulating tumor DNA. Intra-prostate heterogeneity was pervasive and impacted clinically relevant genes, resulting in discordant genotypes between select primary restricted regions and synchronous metastases. Additional complexity was driven by polyclonal metastatic seeding from phylogenetically related primary populations. When simulating clinical practice relying on a single tissue region, genomic heterogeneity plus variable tumor fraction across samples caused inaccurate genotyping of dominant disease; however, pooling extracted DNA from multiple biopsy cores before sequencing can rescue misassigned somatic genotypes. Our results define the relationship between synchronous treatment-sensitive primary and metastatic lesions in men with de novo metastatic prostate cancer and provide a framework for implementing genomics-guided patient management.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The human reference genome hg38 was downloaded from the University of California, Santa Cruz. We used exon and transcription start site coordinates from the RefSeq Matched Annotation from NCBI and EMBL-EBI (MANE) database. All de-identified sequencing data have been deposited in the European Genome–Phenome Archive database under the accession code EGAS00001006466 and are available under standard controlled release. All other data supporting the findings of this study are available within the article (including its supplementary information and data files) and from the lead contact upon reasonable request. The source data for Figs. 1–5 and 7 and Extended Data Figs. 1–3, 5, 6, 9 and 10 are provided as source data files. Source data are provided with this paper.
Code availability
Custom computer code utilized for analysis is available on GitHub at https://github.com/amurth/de_novo_cspc_multifocal_sequencing.
References
Helgstrand, J. T. et al. Trends in incidence and 5-year mortality in men with newly diagnosed, metastatic prostate cancer-A population-based analysis of 2 national cohorts. Cancer 124, 2931–2938 (2018).
Hamid, A. A. et al. Metastatic hormone-sensitive prostate cancer: toward an era of adaptive and personalized treatment. Am. Soc. Clin. Oncol. Educ. Book 43, e390166 (2023).
Kyriakopoulos, C. E. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J. Clin. Oncol. 36, 1080–1087 (2018).
Gravis, G. et al. Burden of metastatic castrate naive prostate cancer patients, to identify men more likely to benefit from early docetaxel: further analyses of CHAARTED and GETUG-AFU15 studies. Eur. Urol. 73, 847–855 (2018).
Vapiwala, N., Hofman, M. S., Murphy, D. G., Williams, S. & Sweeney, C. Strategies for evaluation of novel imaging in prostate cancer: putting the horse back before the cart. J. Clin. Oncol. 37, 765–769 (2019).
Nyquist, M. D. et al. Combined TP53 and RB1 loss promotes prostate cancer resistance to a spectrum of therapeutics and confers vulnerability to replication stress. Cell Rep. 31, 107669 (2020).
Ku, S. Y. et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83 (2017).
Annala, M. et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 8, 444–457 (2018).
de Bono, J. et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 382, 2091–2102 (2020).
Abida, W. et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J. Clin. Oncol. 38, 3763–3772 (2020).
Abida, W. et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 5, 471–478 (2019).
Sweeney, C. et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet 398, 131–142 (2021).
Cooper, C. S. et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat. Genet. 47, 367–372 (2015).
Espiritu, S. M. G. et al. The evolutionary landscape of localized prostate cancers drives clinical aggression. Cell 173, 1003–1013 (2018).
Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015).
Schaeffer, E. M. et al. NCCN guidelines insights: prostate cancer, version 1.2023. J. Natl. Compr. Canc. Netw. 20, 1288–1298 (2022).
Mateo, J. et al. Genomics of lethal prostate cancer at diagnosis and castration resistance. J. Clin. Invest. 130, 1743–1751 (2020).
Stopsack, K. H. et al. Oncogenic genomic alterations, clinical phenotypes, and outcomes in metastatic castration-sensitive prostate cancer. Clin. Cancer Res. 26, 3230–3238 (2020).
Wyatt, A. W. et al. Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J. Natl. Cancer Inst. 109, djx118 (2017).
Herberts, C. et al. Deep whole-genome ctDNA chronology of treatment-resistant prostate cancer. Nature 608, 199–208 (2022).
Vandekerkhove, G. et al. Circulating tumor DNA abundance and potential utility in de novo metastatic prostate cancer. Eur. Urol. 75, 667–675 (2019).
Kohli, M. et al. Clinical and genomic insights into circulating tumor DNA-based alterations across the spectrum of metastatic hormone-sensitive and castrate-resistant prostate cancer. eBioMedicine 54, 102728 (2020).
Van der Eecken, K. et al. Tissue- and blood-derived genomic biomarkers for metastatic hormone-sensitive prostate cancer: a systematic review. Eur. Urol. Oncol. 4, 914–923 (2021).
Sweeney, C. J. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 373, 737–746 (2015).
Fizazi, K. et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 377, 352–360 (2017).
Fizazi, K. et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 399, 1695–1707 (2022).
Hussain, M. et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J. Clin. Oncol. 41, 3595–3607 (2023).
Gilson, C. et al. Genomic profiles of de novo high- and low-volume metastatic prostate cancer: results from a 2-stage feasibility and prevalence study in the STAMPEDE trial. JCO Precis. Oncol. 4, 882–897 (2020).
Hussain, M. et al. Tumor genomic testing for >4,000 men with metastatic castration-resistant prostate cancer in the phase III trial PROfound (Olaparib). Clin. Cancer Res. 28, 1518–1530 (2022).
Annala, M. et al. Evolution of castration-resistant prostate cancer in ctDNA during sequential androgen receptor pathway inhibition. Clin. Cancer Res. 27, 4610–4623 (2021).
Jayaram, A. et al. Plasma tumor gene conversions after one cycle abiraterone acetate for metastatic castration-resistant prostate cancer: a biomarker analysis of a multicenter international trial. Ann. Oncol. 32, 726–735 (2021).
Annala, M. et al. Cabazitaxel versus abiraterone or enzalutamide in poor prognosis metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2 trial. Ann. Oncol. 32, 896–905 (2021).
Mayrhofer, M. et al. Cell-free DNA profiling of metastatic prostate cancer reveals microsatellite instability, structural rearrangements and clonal hematopoiesis. Genome Med. 10, 85 (2018).
Bielski, C. M. et al. Genome doubling shapes the evolution and prognosis of advanced cancers. Nat. Genet. 50, 1189–1195 (2018).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 162, 454 (2015).
Black, J. R. M. & McGranahan, N. Genetic and non-genetic clonal diversity in cancer evolution. Nat. Rev. Cancer 21, 379–392 (2021).
Dentro, S. C. et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell 184, 2239–2254 (2021).
Løvf, M., Zhao, S., Axcrona, U. & Johannessen, B. Multifocal primary prostate cancer exhibits high degree of genomic heterogeneity. Eur. Urol. 75, 498–505 (2019).
Boysen, G. et al. SPOP-mutated/CHD1-deleted lethal prostate cancer and abiraterone sensitivity. Clin. Cancer Res. 24, 5585–5593 (2018).
Swami, U. et al. Association of SPOP mutations with outcomes in men with de novo metastatic castration-sensitive prostate cancer. Eur. Urol. 78, 652–656 (2020).
Litchfield, K. et al. Representative sequencing: unbiased sampling of solid tumor tissue. Cell Rep. 31, 107550 (2020).
Emmett, L. et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): a prospective multicentre study. Eur. Urol. 80, 682–689 (2021).
Tomlins, S. A. et al. Real-world performance of a comprehensive genomic profiling test optimized for small tumor samples. JCO Precis. Oncol. 5, PO.20.00472 (2021).
Adalsteinsson, V. A. et al. Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat. Commun. 8, 1324 (2017).
Hamid, A. A. et al. Compound genomic alterations of TP53, PTEN, and RB1 tumor suppressors in localized and metastatic prostate cancer. Eur. Urol. 76, 89–97 (2019).
Nava Rodrigues, D. et al. RB1 heterogeneity in advanced metastatic castration-resistant prostate cancer. Clin. Cancer Res. 25, 687–697 (2019).
van de Haar, J. et al. Limited evolution of the actionable metastatic cancer genome under therapeutic pressure. Nat. Med. 27, 1553–1563 (2021).
Clarke, N. W. et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid. 1, EVIDoa2200043 (2022).
Agarwal, N. et al. TALAPRO-2: phase 3 study of talazoparib (TALA) + enzalutamide (ENZA) versus placebo (PBO) + ENZA as first-line (1L) treatment in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 41, LBA17 (2023).
Herberts, C. & Wyatt, A. W. Technical and biological constraints on ctDNA-based genotyping. Trends Cancer. 7, 995–1009 (2021).
Deek, M. P. et al. The mutational landscape of metastatic castration-sensitive prostate cancer: the spectrum theory revisited. Eur. Urol. 80, 632–640 (2021).
Kumar, A. et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 22, 369–378 (2016).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015).
Parker, C. C. et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 392, 2353–2366 (2018).
Lumen, N. et al. The role of cytoreductive radical prostatectomy in the treatment of newly diagnosed low-volume metastatic prostate cancer. results from the local treatment of metastatic prostate cancer (LoMP) registry. Eur. Urol Open Sci. 29, 68–76 (2021).
Phillips, R. et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol 6, 650–659 (2020).
Reiter, J. G. et al. Lymph node metastases develop through a wider evolutionary bottleneck than distant metastases. Nat. Genet. 52, 692–700 (2020).
Nguyen, B. et al. Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell 185, 563–575 (2022).
Berglund, E. et al. Spatial maps of prostate cancer transcriptomes reveal an unexplored landscape of heterogeneity. Nat. Commun. 9, 2419 (2018).
Mottet, N. et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. part 1: screening, diagnosis, and local treatment with curative intent. Eur. Urol. 79, 243–262 (2020).
Buelens, S. et al. Multicentre, prospective study on local treatment of metastatic prostate cancer (LoMP study). BJU Int. 129, 699–707 (2022).
van Leenders, G. J. L. H. et al. The 2019 International Society of Urological Pathology (ISUP) consensus conference on grading of prostatic carcinoma. Am. J. Surg. Pathol. 44, e87–e99 (2020).
Turkbey, B. et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur. Urol. 76, 340–351 (2019).
Verbeke, S. L. J. et al. A reappraisal of hemangiopericytoma of bone; analysis of cases reclassified as synovial sarcoma and solitary fibrous tumor of bone. Am. J. Surg. Pathol. 34, 777–783 (2010).
Roth, A. et al. PyClone: statistical inference of clonal population structure in cancer. Nat. Methods 11, 396–398 (2014).
Satas, G., Zaccaria, S., El-Kebir, M. & Raphael, B. J. DeCiFering the elusive cancer cell fraction in tumor heterogeneity and evolution. Cell Systems 12, 1004–1018 (2021).
Niknafs, N., Beleva-Guthrie, V., Naiman, D. Q. & Karchin, R. Subclonal hierarchy inference from somatic mutations: automatic reconstruction of cancer evolutionary trees from multi-region next generation sequencing. PLoS Comput. Biol. 11, e1004416 (2015).
Tarabichi, M. et al. A practical guide to cancer subclonal reconstruction from DNA sequencing. Nat. Methods 18, 144–155 (2021).
Acknowledgements
This work was funded by the Canadian Institutes of Health Research through a Project Grant to A.W.W. and P.O. and Kom op tegen Kanker (Stand up to Cancer—Flemish Cancer Society; P.O.). Other support for the study was provided by the Prostate Cancer Foundation (including via Young Investigator Awards to E.M.K. and N.M.F.) and the Terry Fox Research Institute New Frontiers Program. Part of the collaborative work between A.W.W. and P.O. was enabled by ‘PrIOMiC’, an International Thematic Network, funded by Ghent University and coordinated by ‘Cancer Research Institute Ghent’. No funding sources were involved in the design or execution of the study. We are grateful to all participating patients and their families.
Author information
Authors and Affiliations
Contributions
Conceptualization was the responsibility of N.L., J.V.D., P.O. and A.W.W. Methodology was the responsibility of E.W.W., K.V.d.E., A.J.M., C.H., J.S., N.M.F., E.R., S.V., N.L., J.V.D., M.A., G.V., P.O. and A.W.W. Software was the responsibility of E.W.W., A.J.M., E.M.K., J.S., S.W.S.N., X.E.C., E.R. and M.A. Formal analysis was conducted by E.W.W., K.V.d.E., A.J.M., E.M.K., C.H., J.S., S.W.S.N., X.E.C., N.M.F., E.R., A.M., K.P., S.V., N.L., J.V.D., B.D.L., M.A. and G.V. Investigation was carried out by E.W.W., K.V.d.E., A.J.M., E.M.W., E.S., C.Q.B., G.D., K.B., A.W., S.V., N.L. and J.V.D. Resources were the responsibility of N.L., P.O., J.S., M.A. and A.W.W. Data curation was carried out by E.W.W., K.V.d.E., A.J.M., E.M.K., S.W.S.N., X.E.C., N.M.F., E.R., E.S., C.Q.B., G.D., K.B., A.W., S.V. and M.A. Writing of the original draft was carried out by E.W.W., K.V.d.E., A.J.M., E.M.K., C.H., J.S., G.V. and A.W.W. Review and editing was conducted by E.W.W., K.V.d.E., A.J.M., E.M.K., C.H., J.S., S.W.S.N., X.E.C., N.M.F., E.R., B.D.L., M.A., G.V., P.O. and A.W.W. Visualization was carried out by E.W.W., K.V.d.E., A.J.M., E.M.K., C.H., J.S., S.W.S.N., X.E.C., N.M.F., E.R. and M.A. Supervision was the responsibility of B.D.L., M.A., G.V., P.O. and A.W.W. Project administration was carried out by N.L., J.V.D., G.V., P.O. and A.W.W. Funding acquisition was the responsibility of N.L., J.V.D., P.O. and A.W.W.
Corresponding author
Ethics declarations
Competing interests
E.W.W. is an employee at Guardant Health (although only contributed to this article before commencing employment with Guardant Health in April 2023). E.M.K. has consulted or served in an advisory role for Astellas Pharma, Janssen and Ipsen, received travel funding from Astellas Pharma, Pfizer, Ipsen and Roche, received honoraria from Janssen, Ipsen, Astellas Pharma and Research Review and research funding from Astellas Pharma (institutional) and AstraZeneca. M.A. is a shareholder in Fluivia. The laboratory of N.L. has research funding from Bayer (Inst) and Janssen (Inst) and has received travel funding from Ipsen (Inst). P.O. has consulted or served in an advisory role for Janssen-Cilag, Bayer, Astellas Pharma, Curium Pharma, Telix Pharmaceuticals and Novartis and has received travel funding from Ferring. The laboratory of P.O. has received research funding from Varian Medical Systems (Inst) and Bayer (Inst). A.W.W. has served on advisory boards and/or received honoraria from AstraZeneca, Astellas, Bayer, EMD Serono, Janssen, Merck and Pfizer. The laboratory of A.W.W. has a contract research agreement with ESSA Pharma. All other authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Sequencing metrics and tumor fraction estimation.
a. DNA yield, median coverage, and tumor fraction (TF) estimates derived from targeted sequencing. Numbers of independent samples per category annotated in brackets. b. Hierarchical approach for TF estimation. Mutations deemed ineligible for TF estimation in Group 1 (that is mutations not shared across most same-patient samples, and are therefore potentially subclonal) can underestimate TF compared to heterozygous single-nucleotide polymorphism (SNP) allele frequency applied in Group 2 (middle scatter plot). c. Comparison of TF by targeted (left) and whole-exome sequencing (WES; right) sequencing versus pathology tumor cellularity (TC; n = 521). Bars show the fraction of samples with higher pathology-derived than sequencing-derived TF estimates. d. Sequencing-derived TF estimates versus median targeted sequencing coverage (for all tissue samples; n = 523). e. High median coverage of all ISUP grade 5 samples with sequencing TF of 0% indicating that sequencing insufficiencies do not explain low tumor fraction results. f. Sequencing-derived TF estimates for each tissue sample (n are annotated), grouped by patient along the x-axis. ISUP (International Society of Urological Pathology) high and low refer to primary grade ≥4 and <4, respectively). Asterisks denote patients who received preoperative androgen-deprivation therapy (ADT). g. Variant allele frequencies of somatic mutations by targeted sequencing and whole-exome sequencing (WES) are highly correlated. Each dot represents a mutation detected via either sequencing method with jointly sufficient locus-specific depth (n = 234). Mutations with <30x coverage by either method are excluded. h. Coverage log ratio (LR) correlation between 73-gene targeted panel and WES (in samples sequenced with both modalities; n = 152). Each dot (n = 11271) represents a gene shared across panels. i. Few somatic mutations detected solely by WES are within established cancer genes. Of 7757 mutations detected in genes not covered by the targeted sequencing panel, only six were pathogenic coding mutations in key cancer driver genes (moving left to right, each subsequent bar is a subset of the previous bar). cfDNA: cell-free DNA, FFPE: formalin-fixed paraffin-embedded, MLN: metastatic lymph node, PB: prostate biopsy, RP: radical prostatectomy.
Extended Data Fig. 2 Concurrent metastatic prostate and small-cell lung cancer in ID19.
a. Clinical timeline of cancer diagnoses and treatment for patient ID19 from prostate cancer (PCa) diagnosis until death. b. Variant allele fraction (VAF) of all somatic mutations detected in the cell-free DNA (cfDNA) sample at time of radical prostatectomy and pelvic lymph node (LN) dissection (RP/PLND). Mutations in red were independently detected in prostate tumor tissue. c. Pairwise VAF comparisons between 5 samples with whole-exome sequencing data. Each dot represents the VAF of one mutation independently detected in at least one sample. SCLC: small-cell lung cancer, ADT: androgen-deprivation therapy, CCF: cancer cell fraction, ctDNA: circulating tumor DNA, PLND: pelvic lymph node dissection, PSA: prostate-specific antigen.
Extended Data Fig. 3 Per-patient aggregate driver gene alteration status.
a. Summary of patient genotypes (n = 43) aggregated from all same-patient samples (n = 607). Variants are shown if they were present in >2 samples (for SNVs/indels and deep deletions/amplifications) or >3 high tumor fraction (<20%) samples (for shallow gains and deletions). ID31 and ID39 did not have sufficient high tumor fraction samples for copy number analysis and ID24 did not have any tumor positive samples for mutation or copy number analysis. Asterisks denote patients who received preoperative androgen-deprivation therapy. b-c. Copy number and tumor fraction model from whole-exome sequencing for patient ID5 with whole-genome doubling (b) and ID23 with diploid genome (c), where the top plot of each panel shows coverage log ratio (left axis) and copy number (right axis), and the bottom plot shows germline heterozygous single-nucleotide polymorphism (SNP) allele frequency (HSAF, left axis) and allelic ratio (right axis). Median coverage log ratio and HSAF for each segment are shown in colored thick bars, where colors indicate copy number and allelic ratio. Green segments did not fit to a specific copy number and allelic ratio. d-e. Comparisons of alteration frequencies between our cohort and public cohorts of localized castration-sensitive prostate cancer (CSPC)15, metastatic CSPC (mCSPC)18, and metastatic castration-resistant prostate cancer (mCRPC)35. Patient numbers and alteration frequencies are identical to Fig. 4a. Top (d): circles show the detection rate across all patients in our cohort with variants detected in >1 sample (for single-nucleotide substitutions/indels) or >2 high tumor fraction (>20%) samples (for copy number variants). Bottom (d): circles show the expected frequency of somatic mutations, amplifications, and homozygous losses in our cohort (using an average of single randomly-selected samples per patient) compared to published cohorts. e. Frequency of biallelic TP53, RB1, and PTEN loss across cohorts. TCGA: The Cancer Genome Atlas, MSKCC: Memorial Sloan Kettering Cancer Center, SU2C: Stand Up 2 Cancer.
Extended Data Fig. 4 Immunohistochemistry staining of prostate tissue to confirm tumor-suppressor gene loss.
Staining of PTEN and RB1 protein in eight representative tumor tissue samples, plus two positive control samples derived from benign tonsil tissue. A total of 26 tumor tissue regions were tested, and genomic and immunohistochemistry results were concordant in 24 of 26 (see Supplementary Table 10). Images were captured at 20× magnification. All scale bars represent 50 µm. Asterisk denotes the patient who received preoperative androgen-deprivation therapy. Experiments were performed once per sample. RP: radical prostatectomy, LOH: loss of heterozygosity, MLN: metastatic lymph node.
Extended Data Fig. 5 Evidence of genomic heterogeneity across same-patient tumor regions.
a. Private versus shared mutations between the primary and metastatic niche. b. Clonal status of all mutations (All muts), and for the subset that are primary-exclusive (P. Private), metastatic-exclusive (M. Private), or shared. Mutation n are provided at the top of each bar. Mutations detected in multiple same-patient samples were defined as clonal if they had a CCF ≥ 0.8 in any sample. c. Per-gene mutation count, with color denoting mutation heterogeneity (private to primary or metastatic niche, or shared). Only data from patients (n = 25 [targeted], n = 29 [whole-exome sequencing; WES]) with both tumor fraction (TF)-positive primary and metastatic samples are included. Top and bottom rows of (a-c) utilize samples subjected to targeted and WES, respectively. Patient (ID8) with mismatch repair deficient disease was excluded. d. Most mutations detected across same-patient samples with WES data are not shared across all tumor regions. 36 patients with multiple TF-positive samples were included. Patient (ID8) with mismatch repair deficient disease was excluded. In-set stripplot: fraction of mutations in each patient that were detected in all TF-positive samples versus number of same-patient samples with WES data, suggesting that sample availability does not impact the proportion of mutations that are detected in all same-patient WES samples. e. Heatmap showing whole-exome sequencing derived copy number profiles for all samples with ≥30% TF (n = 127). Blue denotes copy losses while red denotes copy gains, both relative to base copy number (four copies in samples with evidence of whole-genome duplication [WGD], two for presumed diploid samples). Same-patient samples and WGD are annotated on the left. Asterisks denote patients who received preoperative ADT.
Extended Data Fig. 6 Evidence for independent primary prostate tumors in three patients.
See Supplementary Fig. 5 for the four other patients with multiple independent cancers. The prostate schematics show the approximate location of profiled prostate biopsy (PB) cores and radical prostatectomy (RP) regions (no map was available for ID38). Estimated extent of tumor involvement (as determined by histopathological review) is shown, and independent tumors (labeled A, B, and C) are indicated. For select representative (‘model’) samples from each independent tumor, the highest observed International Society of Urological Pathology (ISUP) grade and key genomic alterations are shown. Different patterns of copy number alterations in genes (middle) from targeted sequencing for the model samples; coloring indicates deep deletion (dark blue), shallow deletion (light blue), copy-neutral (black), copy-gain (red) and amplification (dark red). Scatterplots (right) and Pearson correlation of all mutations detected via whole-exome sequencing in select sample pairs depict no overlap between independent tumors. Del: deletion, Mut: mutation. ADT: androgen-deprivation therapy, TF: tumor fraction, UCC: urothelial carcinoma, VAF: variant allele fraction.
Extended Data Fig. 7 Phylogenetic tree topologies and metastatic dissemination patterns.
Evolutionary tree diagrams for all additional patients not provided in Fig. 6 and/or Extended Data Figure 8. Branch colors correspond to PyClone-identified clones, and to patient-specific matrices shown in Supplementary Fig. 7. Patients without metastatic clones shown had no available tumor fraction positive metastatic sample. a. Patients with evidence of multiple independent primary tumors. Localized independent tumors generally display less branching than metastatic lineages. b. Evolutionary trees of all other de novo mCSPC cases. Del: copy number deletion, mut: mutation (single-nucleotide substitution / small insertion or deletion). Equally parsimonious trees for ID17, ID18, ID29, and ID4 are shown in Supplementary Fig. 8e. c. Results for ID8 demonstrating heterogeneous mismatch repair defective status and extensive phylogenetic branching. Each shape (circle = primary, diamond = metastasis) is a Pyclone-identified clone assignment for each sample (n = 12), where shape size represents median cancer cell fraction of mutations within the cluster. MSH2/6 status is shown on the left. Mutations per megabase (Mut/Mb) of each sample are shown on the right and cluster mutation count is shown above. Colors are assigned to the truncal clone and phylogenetically distinct branches. d. Output from PyClone and SCHISM, colored by distinct branches, for ID8. Number of mutations per branch are annotated (branch length does not reflect mutation count). Each colored branch has a unique MSH2/6 status and Mut/Mb in associated samples. All samples with exclusively pink populations, including metastatic lymph nodes, are notably devoid of hypermutation and have intact MSH6, while the two other clusters (yellow and green) exhibit hypermutation and losses of both MSH2 and MSH6. The green clone likely contains a second hit not detectable by WES (for example intronic truncating rearrangement). All three populations colonized the metastatic niche. LR: log ratio, MLN: metastatic lymph node, PB: prostate biopsy, RP: radical prostatectomy, WDG: whole-genome duplication.
Extended Data Fig. 8 Parsimonious migration models depicting intersample clonal seeding.
Plausible clonal dissemination patterns between samples of all patients included in Fig. 6 or patients with multiple metastatic samples (total patient n = 14). Pie plot proportion is not representative of clonal proportion within samples. Clinically-relevant somatic alterations, whole-genome duplication status, and select clinical characteristics are annotated. Unlabeled nodes represent hypothetical monoclonal samples (that is not directly observed/sequenced) to assist with interpretation (via showing stepwise clonal evolution where possible). ADT: androgen-deprivation therapy, del: copy number deletion, mut: mutation (single-nucleotide substitution / small insertion or deletion), MLN: metastatic lymph node, PB: prostate biopsy, RP: radical prostatectomy.
Extended Data Fig. 9 Concordance between plasma ctDNA and same-patient tumor tissue samples.
a. Oncoprint of all same-patient formalin-fixed paraffin-embedded (FFPE) samples and the cell-free DNA (cfDNA) sample with the highest ctDNA fraction collected prior to androgen-deprivation therapy (ADT) initiation, for the 10 patients with detected ctDNA pre-ADT. Asterisks indicate the patient who received ADT prior to radical prostatectomy. Total sample n = 156 (146 tissue; 10 cfDNA). b. ctDNA fraction and prostate-specific antigen (PSA) levels (ng/mL) of 3 patients with pre- and post-radical prostatectomy cfDNA collections. c. Composite oncoprint of all same-patient FFPE samples and the cfDNA sample with the highest ctDNA sample fraction collected after progression to metastatic castration-resistant prostate cancer (mCRPC), for 8 patients. Asterisks indicate patients who received androgen-deprivation theory (ADT) prior to radical prostatectomy. Total sample n = 129 (121 tissue; 9 cfDNA). CNV: copy number variant, LR: log ratio.
Extended Data Fig. 10 In vitro pooled and sequenced biopsy material recapitulates the average genotypes of constituent input samples and in silico merged samples.
a. Concordance between somatic mutation variant allele frequency (VAF, left column), gene copy number log ratios (center column), and tumor fraction (right column) between in vitro combined versus mean input sample (black), in vitro versus in silico combined samples (blue), and in silico combined versus mean input sample (green). Note that in vitro log ratio values were normalized using white blood cell DNA (rather than source-matched pooled benign tissue as per the other comparator samples). Seven patients are included and the number of mutations assessed is indicated on each plot. b. Genomic status of TP53, PTEN, and RB1 in primary, in silico combined, and in vitro combined samples. Copy number is corrected for tumor fraction. Heterozygous single-nucleotide polymorphism (SNP) variant allele fraction greater than 60% was considered a heterozygous loss. LOH: loss of heterozygosity.
Supplementary information
Supplementary Information
Supplementary Note & Supplementary Figs. 1–9.
Supplementary Table 1
Supplementary Tables 1–17.
Source data
Source Data Fig. 1
Processed data for figure reproduction.
Source Data Fig. 2
Processed data for figure reproduction.
Source Data Fig. 3
Processed data for figure reproduction.
Source Data Fig. 4
Processed data for figure reproduction.
Source Data Fig. 5
Processed data for figure reproduction.
Source Data Fig. 7
Processed data for figure reproduction.
Source Data Extended Data Fig. 1
Processed data for figure reproduction.
Source Data Extended Data Fig. 2
Processed data for figure reproduction.
Source Data Extended Data Fig. 3
Processed data for figure reproduction.
Source Data Extended Data Fig. 5
Processed data for figure reproduction.
Source Data Extended Data Fig. 6
Processed data for figure reproduction.
Source Data Extended Data Fig. 9
Processed data for figure reproduction.
Source Data Extended Data Fig. 10
Processed data for figure reproduction.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Warner, E.W., Van der Eecken, K., Murtha, A.J. et al. Multiregion sampling of de novo metastatic prostate cancer reveals complex polyclonality and augments clinical genotyping. Nat Cancer 5, 114–130 (2024). https://doi.org/10.1038/s43018-023-00692-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-023-00692-y
This article is cited by
-
Stellenwert der lokalen Therapien beim oligometastasierten, hormonsensitiven Prostatakarzinom
Die Urologie (2024)
-
Impact of disease volume on survival efficacy of triplet therapy for metastatic hormone-sensitive prostate cancer: a systematic review, meta-analysis, and network meta-analysis
International Journal of Clinical Oncology (2024)