Abstract
Pancreatic ductal adenocarcinoma (PDAC) is considered non-immunogenic, with trials showing its recalcitrance to PD1 and CTLA4 immune checkpoint therapies (ICTs). Here, we sought to systematically characterize the mechanisms underlying de novo ICT resistance and to identify effective therapeutic options for PDAC. We report that agonist 41BB and antagonist LAG3 ICT alone and in combination, increased survival and antitumor immunity, characterized by modulating T cell subsets with antitumor activity, increased T cell clonality and diversification, decreased immunosuppressive myeloid cells and increased antigen presentation/decreased immunosuppressive capability of myeloid cells. Translational analyses confirmed the expression of 41BB and LAG3 in human PDAC. Since single and dual ICTs were not curative, T cell-activating ICTs were combined with a CXCR1/2 inhibitor targeting immunosuppressive myeloid cells. Triple therapy resulted in durable complete responses. Given similar profiles in human PDAC and the availability of these agents for clinical testing, our findings provide a testable hypothesis for this lethal disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Murine scRNA-seq and TCR sequencing data supporting the findings of this study have been deposited in the Sequence Read Archive under BioProject accession code PRJNA496487. Human PDAC genomic data were derived from the TCGA Research Network (http://cancergenome.nih.gov) and ICGC Research Network (https://dcc.icgc.org). Human PDAC scRNA-seq data were derived from the Genome Sequence Archive (accession codes CRA001160 and GSE155698). All of the other data are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. Cancer J. Clin. 72, 7–33 (2022).
Ying, H. et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 30, 355–385 (2016).
Rahib, L. et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74, 2913–2921 (2014).
Bear, A. S., Vonderheide, R. H. & O’Hara, M. H. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell 38, 788–802 (2020).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Royal, R. E. et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 33, 828–833 (2010).
O’Reilly, E. M. et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma. JAMA Oncol. 5, 1431–1438 (2019).
Ying, H. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012).
Elyada, E. et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9, 1102–1123 (2019).
Steele, N. G. et al. Multimodal mapping of the tumor and peripheral blood immune landscape in human pancreatic cancer. Nat. Cancer 1, 1097–1112 (2020).
Bjornson, Z. B., Nolan, G. P. & Fantl, W. J. Single-cell mass cytometry for analysis of immune system functional states. Curr. Opin. Immunol. 25, 484–494 (2013).
Bronte, V. et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 7, 12150 (2016).
Wang, G. et al. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov. 6, 80–95 (2016).
Vayrynen, S. A. et al. Composition, spatial characteristics, and prognostic significance of myeloic cell infiltration in pancreatic cancer. Clin. Cancer Res. 27, 1069–1081 (2021).
Liudahl et al. Leukocyte heterogeneity in pancreatic ductal adenocarcinoma: phenotypic and spatial features associated with clinical outcome. Cancer Discov. 11, 2014–2031 (2021).
Newman, A. et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 27, 773–782 (2019).
Zhang et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 181, 442–459 (2020).
Lu, X. et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543, 728–732 (2017).
Blank, C. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Winograd, R. et al. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol. Res. 3, 399–411 (2015).
Stromnes, I. M. et al. T-cell localization, activation, and clonal expansion in human pancreatic ductal adenocarcinoma. Cancer Immunol. Res. 5, 978–991 (2017).
Gubin, M. M. et al. High-dimensional analysis delineates myeloid and lymphoid compartment remodeling during successful immune-checkpoint therapy. Cell 175, 1014–1030 (2018).
Zilonis, R. et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity 50, 1317–1334 (2019).
Azizi, E. et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell 174, 1293–1308 (2018).
Hosein, A. N. et al. Cellular heterogeneity during mouse pancreatic ductal adenocarcinoma progression at single-cell resolution. JCI Insight 5, e129212 (2019).
Peng, J. et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 29, 725–738 (2019).
Chao, T., Furth, E. E. & Vonderheide, R. H. CXCR2-dependent accumulation of tumor-associated neutrophils regulates T-cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunol. Res. 4, 968–982 (2016).
Steele, C. W. et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 29, 832–845 (2016).
Zhang, L. et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature 564, 268–272 (2018).
Andersson, K. M. et al. Survivin coordinates formation of follicular T-cells acting in synergy with Bcl-6. Oncotarget 6, 20043–20057 (2015).
Sharief, M. K. & Semra, Y. K. Upregulation of the inhibitor of apoptosis proteins in activated T lymphocytes from patients with multiple sclerosis. J. Neuroimmunol. 119, 350–357 (2001).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Sade-Feldman, M. et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 175, 998–1013 (2018).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Carstens, J. L. et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat. Commun. 8, 15095 (2017).
Balachandran, V. et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551, 512–516 (2017).
Freed-Pastor, W. A. et al. The CD155/TIGIT axis promotes and maintains immune evasion in neoantigen expressing pancreatic cancer. Cancer Cell 39, 1342–1360 (2021).
Rajeshkumar, N. V. et al. Superior therapeutic efficacy of nab-paclitaxel over cremophor-based paclitaxel in locally advanced and metastatic models of human pancreatic cancer. Br. J. Cancer 115, 442–453 (2016).
Eriksson, E., Wenthe, J., Irenaeus, S., Loskog, A. & Ullenhag, S. Gemcitabine reduces MDSCs, Tregs and TGFβ-1 while restoring the Teff/Treg ratio in patients with pancreatic cancer. J. Transl. Med. 14, 282 (2016).
Shevchenko, I. et al. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int. J. Cancer 133, 98–107 (2013).
Chihara, N. et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 558, 454–459 (2018).
Van Der Leun, A. M., Thommen, D. S. & Schumacher, T. N. CD8+ T cell states in human cancer: insights from single cell analysis. Nat. Rev. Cancer 20, 218–232 (2020).
Braun, D. A. et al. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell 39, 632–648 (2021).
Pollok, K. E. et al. Inducible T cell antigen 4-1BB. Analysis of expression and function. J. Immunol. 150, 771–781 (1993).
Shuford, W. W. et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 186, 47–55 (1997).
Hernandez-Chacon, J. A. et al. Costimulation through the CD137/4–1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J. Immunother. 34, 236–250 (2011).
Sherwood, A. M. et al. Tumor-infiltrating lymphocytes in colorectal tumors display a diversity of T cell receptor sequences that differ from the T cells in adjacent mucosal tissue. Cancer Immunol. Immunother. 62, 1453–1461 (2013).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
Hopkins, A. C. et al. T cell receptor repertoire features associated with survival in immunotherapy-treated pancreatic ductal adenocarcinoma. JCI Insight 3, e122092 (2018).
Manuel, M. et al. Lymphopenia combined with low TCR diversity (divpenia) predicts poor overall survival in metastatic breast cancer patients. Oncoimmunology 1, 432–440 (2012).
Postow, M. A. et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J. Immunother. Cancer 3, 23 (2015).
Hundeyin, M. et al. Innate αβ T cells mediate antitumor immunity by orchestrating immunogenic macrophage programming. Cancer Discov. 9, 1288–1305 (2019).
Voelkl, S. et al. Characterization of MHC class-I restricted TCRαβ+ CD4−CD8− double negative T cells recognizing the gp100 antigen from a melanoma patient after gp100 vaccination. Cancer Immunol. Immunother. 58, 709–718 (2009).
Young, K. J., Kay, L. S., Phillips, M. J. & Zhang, L. Antitumor activity mediated by double-negative T cells. Cancer Res. 63, 8014–8021 (2003).
Castino, G. F. et al. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. Oncoimmunology 5, e1085147 (2015).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Markowitz, J. et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol. Immunother. 64, 149–159 (2015).
Stromnes, I. M. et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 63, 1769–1781 (2014).
Di Mitri, D. et al. Tumor-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 515, 134–137 (2014).
Nywening, T. M. et al. Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 67, 1112–1123 (2018).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Segal, N. H. et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin. Cancer Res. 23, 1929–1936 (2017).
Bartkowiak, T. et al. Activation of 4-1BB on liver myeloid cells triggers hepatitis via an Interleukin-27-dependent pathway. Clin. Cancer Res. 24, 1138–1151 (2018).
Li, J. et al. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity 49, 178–193 (2018).
Parra, E. R. et al. Validation of multiplex immunofluorescence panels using multispectral microscopy for immune-profiling of formalin-fixed and paraffin-embedded human tumor tissue. Sci. Rep. 7, 13380 (2017).
Xie, P. et al. SuperCT: a supervised-learning network for enhanced characterization of single-cell transcriptomic profiles. Nucleic Acids Res. 47, e48 (2019).
Lun, A. T. et al. EmptyDrops: distinguishing cells from empty droplets in droplet-based single-cell RNA sequencing data. Genome Biol. 20, 63 (2019).
Young, M. D. & Behjati, S. SoupX removes ambient RNA contamination from droplet-based single-cell RNA sequencing data. Gigascience 9, giaa151 (2020).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Benjamini, Y. Discovering the false discovery rate. J. R. Stat. Soc. 72, 405–416 (2010).
Acknowledgements
We thank the Small Animal Imaging Facility, Histology Core, Flow Cytometry and Cellular Imaging Core and Single Cell Genomics Core at the MD Anderson Cancer Center for assistance with these studies (NCI P30CA16672; CPRIT RP180684). We thank the members of the Multiplex Immunofluorescence and Image Analysis Laboratory in the Department of Translational Molecular Pathology for assistance with multiplex immunofluorescence and image analysis. We thank the Biospecimen Repository and Histopathology Service, Flow Cytometry and Cell Sorting Shared Resource, Biostatistics Shared Resource and Molecular Imaging Core at the Rutgers Cancer Institute of New Jersey (NCI P30CA072720). These studies were supported by NIH NCI P01 CA117969 (to R.A.D.), the Elsa Pardee Foundation Award, the Advanced Scholars Program, the Eleanor Russo Fund for Pancreatic Research, the Ralph A. Loveys Family Charitable Foundation, The Cultural & Charitable Club of Somerset Run and the New Jersey Health Foundation Award (to P.G.), NIH NCI R01CA240526 and R01CA236864 (to N.E.N.), NIH NCI R01CA231349 and 1R01CA258540 (to Y.A.W.) and NIH NCI R01CA220236 and P50CA221707, the Sheikh Khalifa Bin Zayed Foundation and MD Anderson’s Pancreatic Cancer Moon Shot (to A.M.). We thank V. Kulkarni for assistance with figure preparation.
Author information
Authors and Affiliations
Contributions
P.G. and R.A.D. conceived of and designed the study. P.G. performed most of the experiments and wrote the manuscript. A.S., E.S. and N.E.N. assisted with the design, performance and data analysis for the mouse scRNA-seq experiments. S.J. and X.S. assisted with the in vivo experiments and CyTOF. C.-J.W. and J.L. provided bioinformatics support for TCGA/ICGC and human scRNA-seq analysis. S.H.R., L.S.S. and E.P. assisted with the IHC and immunofluorescence of human PDAC specimens. P.H., H.Y., J.H., P. Dey and P. Deng assisted with the mouse colonies and in vivo experiments. D.Y.M. and J.A.Z. provided technical assistance with SX-682. D.J.S. assisted with manuscript editing, figure revisions and data review. M.K. and H.W. assisted with the procurement of human specimens. A.M. assisted with the procurement of human specimens and contributed expertise regarding PDAC and immunotherapy. K.C.-D. assisted with the flow cytometry experiments. D.M. assisted with the statistical analysis. All of the authors reviewed and edited the manuscript. Y.A.W. and R.A.D. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
R.A.D. is a founder, advisor and/or director of Tvardi Therapeutics, Asylia Therapeutics, Stellanova Therapeutics, Nirogy Therapeutics and Sporos Bioventures. J.A.Z. is the President and Chief Executive Officer at Syntrix Pharmaceuticals. D.Y.M. is the Director of Medicinal Chemistry and Preclinical Development at Syntrix Pharmaceuticals. A.M. receives royalties from Cosmos Wisdom Biotechnology and Thrive Earlier Detection, an Exact Sciences company. A.M. is also a consultant for Freenome and Tezcat Biotechnology. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Prominent infiltration of myeloid immunosuppressive cells in iKRAS tumors.
A. PDAC tumor development in syngeneic mouse model with representative images of tumor detected by bioluminescence, PET/CT and MRI at indicated timepoints. B. Tumor volume measured by MRI at indicated timepoints (top) and Kaplan-Meier curve depicting overall survival (bottom) for untreated iKRAS tumor bearing mice (n = 10 mice). C. Representative images of normal pancreas, orthotopic and autochthonous (GEMM) iKRAS tumors with H&E, Masson Trichrome, smooth muscle actin (SMA) and vimentin staining. Scale bars: 100 µm. D. Representative H&E images of iKRAS tumors invading into adjacent lymph nodes (left = 4x magnification, right=20x magnification). E. Representative coronal and axial MRI images of iKRAS tumor invading into duodenum. F. Representative images of normal pancreas and human PDAC tumors with SMA, Vimentin and CD45 staining. Scale bars: 100 µm. Red arrow indicates positively stained cells. G. Percentage of granulocytic (CD45+CD11b+Ly6G+Ly6C−) and monocytic MDSCs (CD45+CD11b+Ly6G−Ly6C+) within syngeneic iKRAS tumors (n = 10 tumors) assessed by CyTOF at 4 weeks after initial tumor detection. Two-sided Student’s t-test. H. Representative images (bottom) of normal pancreas, orthotopic and autochthonous (GEMM) iKRAS tumors with indicated staining. n = 6 biological replicates. Scale bars: 100 µm. The bar graph (top) shows quantification of each cell type as analyzed by IHC. Two-sided Student’s t-test. I. Representative images of normal pancreas and orthotopic iKRAS tumors with indicated staining. Scale bars: 100 µm. J. Percentage of Treg (CD45+CD3+TCRβ+CD4+FoxP3+) among CD4+ T cells within syngeneic iKRAS tumors (n = 10 tumors) assessed by CyTOF at 4 weeks after initial tumor detection. Two-sided Student’s t-test. K. Representative images of normal pancreas and human PDAC tumors with indicated staining. Scale bars: 100 µm. Red arrow indicates positively stained cells. Data in G,H,J are presented as mean ± s.e.m.
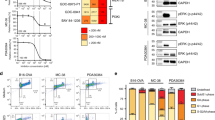
Extended Data Fig. 2 Prominent infiltration of myeloid immunosuppressive cells in human PDAC tumors.
A. Representative images of normal pancreas and human PDAC tumors with indicated staining. Scale bars: 100 µm. Red arrow indicates positively stained cells. B. Clustering of human TCGA PDAC samples (n = 178 patients) into MDSC-high, MDSC-low and MDSC-medium groups using a 39-gene MDSC signature13. C. CIBERSORTx quantification of monocyte/macrophage subset fraction in human PDAC samples; TCGA (n = 178 patients) and ICGC-AU (n = 92 patients). D. Representative images of normal pancreas and human PDAC tumors with indicated staining. Scale bars: 100 µm. Red arrow indicates positively stained cells. E. Kaplan-Meier plot depicting overall survival of TCGA PDAC patients (n = 178 patients) grouped by the gene expression signatures of C1q+ TAM (top) and Spp1+ TAM (bottom).
Extended Data Fig. 3 Heterogeneity of myeloid cells in iKRAS PDAC tumors identified by single cell gene expression profiling.
A. UMAP of all live CD45+ cells used for scRNA-seq analysis of untreated iKRAS tumors (n = 4,080 cells). B. Representative genes and functional markers used for identification of immune cell clusters. C. Heatmap of six immune cell clusters with unique signature genes. D. Representative genes and functional markers used for identification of myeloid cell clusters. E. Heatmap of myeloid cell clusters with unique signature genes. F. Representative genes and functional markers used for identification of dendritic cell clusters.
Extended Data Fig. 4 Dysfunctional phenotype of T cells in iKRAS PDAC tumors identified by single cell gene expression profiling.
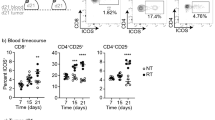
A. Representative genes and functional markers used for identification of T cell clusters. B. Heatmap of two CD4+ and four CD8+ T cell clusters with unique signature genes. C. Cell cycle scoring for two CD4+ and four CD8+ T cell clusters. D. Relative expression of select genes in CD8+ T cells as a function of pseudotime from Monocle2 inferred trajectory. Each point corresponds to a single cell, colored by CD8+ T cell cluster. Lines represent average expression at that location in the trajectory. E. Quantification of immune checkpoint expression on infiltrating CD4+ and CD8+ T cells in iKRAS tumors (n = 3 biological replicates), assessed by flow cytometry and analyzed by FlowJo.
Extended Data Fig. 5 Efficacy of immune checkpoint therapy (ICT) and treatment effects on immune microenvironment.
A. Treatment schedule and monitoring procedures for preclinical trials to evaluate effect of ICT on iKRAS PDAC bearing mice. B. Heatmap of immune checkpoint expression on T cells after 4−week treatment with control, anti-PD1 or anti-CTLA4 antibody (n = 3 mice/ group). C. UMAP demonstrating cell types in single-cell RNA sequencing of human PDAC samples from Peng et al.26 and Steele et al.10 (left), and expression of LAG3 and 41BB (TNFRSF9) on T cells (right). D. UMAP of all live CD45+ cells used for scRNA-seq analysis of iKRAS tumors treated with control, anti-PD1, anti-CTLA4, anti-41BB, anti-LAG3, SX-682 or combination (anti-LAG3 + anti-41BB + SX-682) treatment (n = 3 mice/group). E. UMAP projection of immune cell clusters (top) and cells with TCR detected (bottom). F. Violin plots displaying relative expression of representative genes and functional markers used for identification of immune cell clusters.
Extended Data Fig. 6 Efficacy of immune checkpoint therapy (ICT) and treatment effects on immune microenvironment.
A. Heatmap of six immune cell clusters with unique signature genes. B. UMAP projection of T cell clusters (top) and violin plots displaying relative expression of representative genes and functional markers used for identification of T cell clusters (bottom). C. Heatmap of ten T cell clusters with unique signature genes. D. UMAP projection of neutrophil/granulocyte clusters (top) and violin plots displaying relative expression of representative genes and functional markers used for identification of neutrophil/granulocyte clusters (bottom).
Extended Data Fig. 7 Efficacy of immune checkpoint therapy (ICT) and treatment effects on immune microenvironment.
A. Heatmap of five neutrophil/granulocyte clusters with unique signature genes. B. UMAP projection of monocyte/macrophage clusters (top) and violin plots displaying relative expression of representative genes and functional markers used for identification of monocyte/macrophage clusters (bottom). C. Heatmap of five monocyte/macrophage clusters with unique signature genes. D. Proportion of immune cell subtypes in single-cell sequencing analysis of established iKRAS tumors (tumor volume ~250mm3 prior to treatment initiation) treated with control, anti-PD1, anti-CTLA4, anti-41BB or anti-LAG3 antibody for 4 weeks (n = 3 tumors/group).
Extended Data Fig. 8 Effects of immune checkpoint therapy (ICT) treatment on immune microenvironment.
A. Proportion of T cell subtypes in scRNA-seq analysis of established iKRAS tumors (tumor volume ~250mm3 prior to treatment initiation) treated with control, anti-PD1, anti-CTLA4, anti-41BB or anti-LAG3 antibody for 4 weeks (n = 3 tumors/group). B. Top gene ontologies from GSEA of differential expression in T cells from anti-41BB and control antibody treated mice (n = 3 mice/group). C. Circos plots of T cell receptor clonotype frequencies and expression states of CD8+ T cells in iKRAS tumors after treatment with control (left) and anti-41BB antibody (right) for 4 weeks. Outer histogram is the frequency of each clonotype. Inner bars show the fraction of cells of particular clonotype in each expression state (colors correspond to the clusters in Extended Data Fig. 8a). Inner dendrograms are the hierarchical clustering of gene expression centroids for each clonotype. D. Multiple-testing corrected 95% binomial confidence intervals on the probability of a cell in each treatment group containing a TCR CD3R sequence which overlaps that of another cluster. (*p < 0.05) E. Violin plots showing CCR7 expression in CD4+, CD8+ and CD4-CD8− T cells. (*p < 0.05 two-sided unpaired Wilcox test) F. Proportion of CD4+, CD8+ and CD4−CD8− T cells with expression of CCR7 (left) and IL2Rβ (right). G. Expression of genes and functional markers on CD3+CD4−CD8− T cells. H. Violin plots showing expression of Stat6, Socs3 and Il1β among myeloid cells from control and anti-LAG3 antibody-treated tumors (n = 3 mice/group). (*p < 0.05 two-sided unpaired Wilcox test) I. Violin plots showing expression of Cxcl10, Stat1, Il10, Mrc1 and Socs3 among myeloid cells from control and anti-41BB antibody-treated tumors (n = 3 mice/group). (*p < 0.05 two-sided unpaired Wilcox test).
Extended Data Fig. 9 Efficacy of targeted therapy directed against Cxcr1/2 and treatment effects on immune microenvironment.
A. Kaplan-Meier plot depicting overall survival differences between patients with MDSC-high vs. MDSC-low signatures based on clustering of human TCGA PDAC samples (n = 178 patients) shown in Extended Data Fig. 2b. B. Representative images (left) of established iKRAS tumors treated with control and anti-Gr1 neutralizing antibody for 4 weeks with indicated staining. Scale bars: 100 µm. The bar graph (right) shows quantification of each cell type as analyzed by IHC. n = 6 biological replicates. Two-sided Student’s t-test. C. Tumor volume after 4 weeks of treatment with control or anti-Gr1 neutralizing antibody in mice bearing established (tumor volume ~250mm3 prior to treatment initiation) orthotopic iKRAS tumors (n = 10 mice/group). Two-sided Student’s t-test. D. Expression of Cxcr2 on granulocytic MDSCs in untreated iKRAS tumors, assessed by flow cytometry and analyzed by FlowJo (n = 3 tumors). E. Representative images of human PDAC tumors with indicated staining. Scale bars: 100 µm. Red arrow indicates positively stained cells in the same area of a core specimen. F. UMAP demonstrating cell types in single-cell RNA sequencing of human PDAC samples from Steele et al.10 with the expression of CXCR1 and CXCR2 on granulocytes/neutrophils and expression the of CSF1R, CCR2 and TREM2 on monocytes/macrophages. G. Migration of MDSCs toward conditioned medium from iKRAS tumor cells treated with control or SX-682 (n = 3 biological replicates). Student’s t-test. H. Tumor volume after 4 weeks of treatment with control or SX-682 in mice bearing established orthotopic iKRAS tumors (tumor volume ~250mm3 prior to treatment initiation) (n = 10 mice/group). Two-sided Student’s t-test. I. Stratification of infiltrating CD4+ and CD8+ T cells as naive (CD44lowCD62Lhigh), central memory (CD44highCD62Lhigh) and effector memory (CD44highCD62Llow), in established iKRAS tumors (tumor volume ~250mm3 prior to treatment initiation) treated with control, SX-682 or combination (anti-LAG3 + anti-41BB + SX-682) for 4 weeks assessed by flow cytometry and analyzed by FlowJo (n = 3 biological replicates). Two-sided Student’s t-test. J. Quantification of total tumor associated macrophages (TAM) and dendritic cells (DC) in established iKRAS tumors (tumor volume ~250mm3 prior to treatment initiation) treated with control, SX-682 or combination (anti-LAG3 + anti-41BB + SX-682) for 4 weeks assessed by flow cytometry and analyzed by FlowJo (n = 3 biological replicates). Two-sided Student’s t-test. K. Expression of Cxcr2 on myeloid cells in established iKRAS tumors (tumor volume ~250mm3 prior to treatment initiation) treated with control, SX-682 or combination (anti-LAG3 + anti-41BB + SX-682) for 4 weeks assessed by flow cytometry and analyzed by FlowJo (n = 3 biological replicates). L. Representative images (left) of control, SX-682 or combination (anti-LAG3 + anti-41BB + SX-682) treated iKRAS tumors with indicated staining. Scale bars: 100 µm. The bar graphs (right) show quantification of each cell type as analyzed by IHC. n = 6 biological replicates. Two-sided Student’s t-test. M. Quantification of change in the proportion of cells in cluster M_c2 as a proportion of total monocyte/macrophage cells in scRNA-seq analysis of iKRAS tumors following treatment with control, SX-682 or combination (anti-LAG3 + anti-41BB + SX-682) for 4 weeks (n = 3 mice/group). (*p < 0.05 mixed effect model) N. Tumor volume of mice bearing established orthotopic iKRAS tumors (tumor volume ~250mm3 prior to treatment initiation) treated with control, SX-682 or SX-682 with CD8 T cell depleting antibody (n = 10 mice/group). Two-sided Student’s t-test. Data in D,G,I,J,M are presented as mean ± s.e.m.
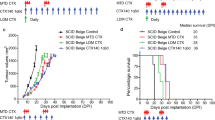
Extended Data Fig. 10 Efficacy of ICT in combination with targeted therapy directed against Cxcr1/2 and treatment effects on immune microenvironment.
A. Tumor volume of mice bearing established orthotopic iKRAS tumors (tumor volume ~250mm3 prior to treatment initiation) treated with control or anti-LAG3 + anti-41BB antibodies or combination (anti-LAG3 + anti-41BB + SX-682) for 4 weeks (n = 10 mice/group). Two-sided Student’s t-test. B. Tumor volume of mice bearing established orthotopic iKRAS tumors (tumor volume ~250mm3 prior to treatment initiation) treated with control or anti-PD1 + anti-CTLA4 antibodies or SX-682 or anti-PD1 + anti-CTLA4 + SX-682 for 4 weeks (n = 10 mice/group). Two-sided Student’s t-test. C. Body weight of mice (top left), before (pre-treatment), during (2 weeks) and after (4 weeks) treatment with control, SX-682 or combination (anti-LAG3 + anti-41BB + SX-682) for 4 weeks (n = 4 biological replicates). Mouse toxicity tests including creatinine, blood urea nitrogen (BUN), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin and alkaline phosphatase in the indicated treatment groups (n = 4 biological replicates). Representative images of H&E staining (middle) of the lung, heart, liver, kidney and spleen in the indicated treatment groups (n = 4 mice/group). Inset (bottom) shows representative H&E staining of liver tissues in the indicated treatment groups at higher magnification. D. CyTOF analysis of tumors from syngeneic iKRAS 2 and iKRAS 3 tumor bearing mice with equivalent tumor volume (~1000mm3) (n = 10 tumors/group). E. Quantification of tumor infiltrating CD45+ cells in syngeneic iKRAS 2 and iKRAS 3 tumors with equivalent tumor volume (~1000mm3) assessed by CyTOF (n = 10 tumors/group). F. Overall survival of mice bearing established orthotopic iKRAS 2 and iKRAS 3 tumors (tumor volume ~250mm3 prior to treatment initiation) treated with control or anti-LAG3 + anti-41BB + SX-682 for 4 weeks (n = 10 mice/group). Statistical differences were identified by Kaplan-Meier with log-rank test. G. Treatment schedule and monitoring procedures for preclinical trial to evaluate overall survival of mice bearing established autochthonous iKRAS tumors (tumor volume ~250mm3 prior to treatment initiation) treated with control or anti-PD1 + anti-CTLA4 antibodies or anti-LAG3 + anti-41BB antibodies or SX-682 or combination (anti-LAG3 + anti-41BB + SX-682) (n = 10 mice/group). Animals in the ‘extended’ treatment group received treatment with the combination regimen for 6 months or until death. Data in E are presented as mean ± s.e.m.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Tables 1–4.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gulhati, P., Schalck, A., Jiang, S. et al. Targeting T cell checkpoints 41BB and LAG3 and myeloid cell CXCR1/CXCR2 results in antitumor immunity and durable response in pancreatic cancer. Nat Cancer 4, 62–80 (2023). https://doi.org/10.1038/s43018-022-00500-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-022-00500-z
This article is cited by
-
The role of innate immune cells as modulators of the tumor microenvironment in the metastasis and treatment of pancreatic cancer
Clinical Cancer Bulletin (2023)