Abstract
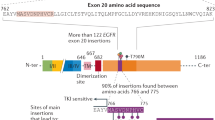
Oncogenic alterations in human epidermal growth factor receptor 2 (HER2) occur in approximately 2% of patients with non-small cell lung cancer and predominantly affect the tyrosine kinase domain and cluster in exon 20 of the ERBB2 gene. Most clinical-grade tyrosine kinase inhibitors are limited by either insufficient selectivity against wild-type (WT) epidermal growth factor receptor (EGFR), which is a major cause of dose-limiting toxicity or by potency against HER2 exon 20 mutant variants. Here we report the discovery of covalent tyrosine kinase inhibitors that potently inhibit HER2 exon 20 mutants while sparing WT EGFR, which reduce tumor cell survival and proliferation in vitro and result in regressions in preclinical xenograft models of HER2 exon 20 mutant non-small cell lung cancer, concomitant with inhibition of downstream HER2 signaling. Our results suggest that HER2 exon 20 insertion-driven tumors can be effectively treated by a potent and highly selective HER2 inhibitor while sparing WT EGFR, paving the way for clinical translation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
CRISPR screening and expression data generated and analyzed in this study are deposited in the Gene Expression Omnibus with the accession code GSE181673. Structural data were deposited into the PDB with the accession code 7PCD. Source data have been provided as Source Data files. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
Plots and statistical analyses were conducted with the statistical programming language R (v.3.5.0.). The code of the respective scripts is available upon request.
References
Citri, A. & Yarden, Y. EGF–ERBB signalling: towards the systems level. Nat. Rev. Mol. Cell Biol. 7, 505–516 (2006).
Hynes, N. E. & MacDonald, G. ErbB receptors and signaling pathways in cancer. Curr. Opin. Cell Biol. 21, 177–184 (2009).
Roskoski, R. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 79, 34–74 (2014).
Wang, Z. ErbB receptor signaling, methods and protocols. Methods Mol. Biol. 1652, 3–35 (2017).
Yasuda, H., Kobayashi, S. & Costa, D. B. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 13, e23–e31 (2012).
Yan, M., Parker, B. A., Schwab, R. & Kurzrock, R. HER2 aberrations in cancer: Implications for therapy. Cancer Treat. Rev. 40, 770–780 (2014).
Stephens, P. et al. Intragenic ERBB2 kinase mutations in tumours. Nature 431, 525–526 (2004).
Roy, K. et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc. Natl Acad. Sci. USA 104, 8131–8136 (2007).
Mei, L. & Nave, K.-A. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron 83, 27–49 (2014).
Barok, M., Joensuu, H. & Isola, J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 16, 209 (2014).
Baselga, J. et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 366, 109–119 (2012).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 372, 724–734 (2015).
Franklin, M. C. et al. Insights into ErbB signaling from the structure of the ErbB2–pertuzumab complex. Cancer Cell 5, 317–328 (2004).
Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 25–32 (2012).
Phillips, G. D. L. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Res. 68, 9280–9290 (2008).
Verma, S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791 (2012).
Nguyen, X., Hooper, M., Borlagdan, J. P. & Palumbo, A. A review of Fam-trastuzumab deruxtecan-NXKI in HER2-positive breast cancer. Ann. Pharmacother. https://doi.org/10.1177/1060028021998320 (2021).
Li, D. et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 27, 4702–4711 (2008).
Geyer, C. E. et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 355, 2733–2743 (2006).
Burstein, H. J. et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J. Clin. Oncol. 28, 1301–1307 (2010).
Murthy, R. K. et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 382, 597–609 (2019).
Wang, S. E. et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 10, 25–38 (2006).
Cocco, E., Lopez, S., Santin, A. D. & Scaltriti, M. Prevalence and role of HER2 mutations in cancer. Pharmacol. Therapeut. 199, 188–196 (2019).
Minami, Y. et al. The major lung cancer-derived mutants of ERBB2 are oncogenic and are associated with sensitivity to the irreversible EGFR/ERBB2 inhibitor HKI-272. Oncogene 26, 5023–5027 (2007).
Connell, C. M. & Doherty, G. J. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open 2, e000279 (2017).
Perera, S. A. et al. HER2YVMA drives rapid development of adenosquamous lung tumors in mice that are sensitive to BIBW2992 and rapamycin combination therapy. Proc. Natl Acad. Sci. USA 106, 474–479 (2009).
Shih, A. J., Telesco, S. E. & Radhakrishnan, R. Analysis of somatic mutations in cancer: molecular mechanisms of activation in the ErbB family of receptor tyrosine kinases. Cancers 3, 1195–1231 (2011).
Bertelsen, V. & Stang, E. The mysterious ways of ErbB2/HER2 trafficking. Membranes 4, 424–446 (2014).
Shi, Y. & Wang, M. Afatinib as first‐line treatment for advanced lung adenocarcinoma patients harboring HER2 mutation: a case report and review of the literature. Thorac. Cancer 9, 1788–1794 (2018).
Lai, W.-C. V. et al. Afatinib in patients with metastatic HER2 -mutant lung cancers: an international multicenter study. J. Clin. Oncol. 35, 9071–9071 (2017).
Zhou, J. et al. Clinical outcomes of patients with HER2-mutant advanced lung cancer: chemotherapies versus HER2-directed therapies. Ther. Adv. Med. Oncol. 12, 1758835920936090 (2020).
Li, B. T. et al. HER2 insertion YVMA mutant lung cancer: long natural history and response to afatinib. Lung Cancer 90, 617–619 (2015).
Liu, S. et al. Targeting HER2 aberrations in non-small cell lung cancer with osimertinib. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.ccr-17-1875 (2018).
Lynette, A. S. & Ian, C. Measuring and interpreting the selectivity of protein kinase inhibitors. J. Chem. Biol. 2, 131 (2009).
Roskoski, R. Orally effective FDA-approved protein kinase targeted covalent inhibitors (TCIs). Pharmacol. Res. 165, 105422 (2021).
Qingsong, L. et al. Developing irreversible inhibitors of the protein kinase cysteinome. Chem. Biol. https://doi.org/10.1016/j.chembiol.2012.12.006 (2013).
Tomoyasu, I. et al. Design and synthesis of novel human epidermal growth factor receptor 2 (HER2)/epidermal growth factor receptor (EGFR) dual inhibitors bearing a pyrrolo[3,2-d]pyrimidine scaffold. J. Med. Chem. 54, 8030 (2011).
Kathleen, A. et al. Structural analysis of the mechanism of inhibition and allosteric activation of the kinase domain of HER2 protein. J. Biol. Chem. 286, 18756 (2011).
Robichaux, J. P. et al. Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity. Cancer Cell 36, 444–457 (2019).
Mohr, S. E., Smith, J. A., Shamu, C. E., Neumüller, R. A. & Perrimon, N. RNAi screening comes of age: improved techniques and complementary approaches. Nat. Rev. Mol. Cell Bio. 15, 591–600 (2014).
Su, X., Lin, Z. & Lin, H. The biosynthesis and biological function of diphthamide. Crit. Rev. Biochem. Mol. 48, 515–521 (2013).
Inoki, K., Li, Y., Zhu, T., Wu, J. & Guan, K.-L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657 (2002).
Inoki, K., Zhu, T. & Guan, K.-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 (2003).
Buttitta, F. et al. Mutational analysis of the HER2 gene in lung tumors from Caucasian patients: mutations are mainly present in adenocarcinomas with bronchioloalveolar features. Int. J. Cancer 119, 2586–2591 (2006).
Liu, Z. et al. Clinical characterization of ERBB2 exon 20 insertions and heterogeneity of outcomes responding to afatinib in Chinese lung cancer patients. Oncotargets Ther. 11, 7323–7331 (2018).
Zheng, Y.-B. et al. Inhibitor response to HER2 G776YVMA in-frame insertion in HER2-positive breast cancer. Cancer Invest. 34, 123–129 (2016).
Prelaj, A. et al. Poziotinib for EGFR and HER2 exon 20 insertion mutation in advanced NSCLC: results from the expanded access program. Eur. J. Cancer https://doi.org/10.1016/j.ejca.2021.02.038 (2021).
Le, X. et al. Poziotinib in non-small-cell lung cancer harboring HER2 exon 20 insertion mutations after prior therapies: ZENITH20-2 trial. J. Clin. Oncol. https://doi.org/10.1200/jco.21.01323 (2021).
Ramalingam, S. S. et al. Overall Survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 382, 41–50 (2020).
Soria, J.-C. et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N. Engl. J. Med. 378, 113–125 (2018).
Yadav, B., Wennerberg, K., Aittokallio, T. & Tang, J. Searching for drug synergy in complex dose–response landscapes using an interaction potency model. Comput. Struct. Biotechnol. J. 13, 504–513 (2015).
Köferle, A. et al. Interrogation of cancer gene dependencies reveals paralog interactions of autosome and sex chromosome-encoded genes. Cell Rep. 39, 110636 (2022).
Hörmann, A. et al. RIOK1 kinase activity is required for cell survival irrespective of MTAP status. Oncotarget 9, 28625–28637 (2018).
Shi, J. et al. Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nat. Biotechnol. 33, 661–667 (2015).
Han, H. et al. Targeting HER2 exon 20 insertion-mutant lung adenocarcinoma with a novel tyrosine kinase inhibitor mobocertinib. Cancer Res. 81, 5311–5324 (2021).
Hofmann, M. H. et al. BI-3406, a potent and selective SOS1–KRAS interaction inhibitor, is effective in KRAS-driven cancers through combined MEK inhibition. Cancer Discov. 11, 142–157 (2021).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Acknowledgements
We thank all colleagues at Boehringer Ingelheim RCV Oncology Research for discussions and critical feedback throughout the study. The project was funded by the Austrian Promotion Agency in 2019 with two grants within the general program (872827 and 0879012). We acknowledge Proteros Biostructures for their contributions to X-ray crystallography.
Author information
Authors and Affiliations
Contributions
The medicinal chemistry sub-team was led by B.W. The biology sub-team was led by R.A.N. Design of inhibitors and synthetic routes was conducted by B.W., D.B., M.T., S.Z. and J.E.F. Inhibitor synthesis was performed by S.K., P.K., G.S., J. Bruchhaus, M.S., J. Balla, B.P.-S., J. Zimmer, S.M. and T.F. Selection of compounds for further profiling was carried out by B.W., D.S. and R.A.N. A. Bergner, D.B. and H.E. were responsible for the screening library. X-ray crystallography was the responsibility of G.B. The DMPK study design was conducted by D.S. The DMPK assays were performed by A. Beran. The biology sub-team consisted of A. Baum, V.S., P.C., R.S., D.A.B., C.R., A.H., A.K., M.C., S.L., L.L. and T.G. Setup of gene–drug screening pipeline was carried out by J. Zuber. Computational work and visualization of data was performed by D.G., M.B., A.S. and R.A.N. The study idea was conceived by B.W., P.E., M. Pearson, M. Petronczki, N.K., D.M., F.S. and R.A.N. The manuscript was written by B.W. and R.A.N.
Corresponding authors
Ethics declarations
Competing interests
All authors except J. Zuber are or were full time employees of Boehringer Ingelheim.
Peer review
Peer review information
Nature Cancer thanks Ryohei Katayama, Antonio Passaro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 DMPK parameters.
(a) Ba/F3 IC50 values in HER2YVMA vs. HER2YVMA,C805S cell lines. All results are quoted as the geometric mean ± SEM, pIC50 = -log IC50 (M). The number of repeats, n, are described in parentheses. (b) In vitro DMPK data for selected HER2YVMA inhibitors. PPB, plasma protein binding, solubility indicates kinetic solubility measured via an in-house HPLC method, ER, efflux ratio. n = 3 technical replicates. (c) In vivo mouse PK data for BI-1622 and BI-4142. Data shown are the geometric mean of n = 3 individual mice. MRT, mean residence time.
Extended Data Fig. 2 Structural information.
(a) Chemical structure of the HER2 TKI used as ligand in X-Ray crystallography in PDB 7PCD. (b) Ligand interaction diagram of the ligand shown in panel a with HER2. (c) Cellular IC50 data of the HER2 TKI used as ligand in the X-Ray crystal structure PDB 7PCD in comparison to BI-1622 and BI-4142. All results are quoted as the geometric mean ± SEM, pIC50 = -log IC50 (M). The number of repeats, n and the IC50, are described in parentheses. (d) X-Ray crystallography data collection, processing and refinement statistics.
Extended Data Fig. 3 Resistance and tumor cell data.
(a) N-ethyl-N-nitrosourea (ENU) resistance screen in Ba/F3 cells dependent on HER2YVMA. Compounds and concentrations as x-fold IC50 are indicated. Bar-plot indicates absolute count for number of times the resistance allele was observed. n = 2 for BI-4142, BI-1622, Afatinib and Poziotinib. (b) Left table: Cell proliferation assay in Ba/F3 cells (parental, or dependent on HER2YVMA or HER2YVMA,T862A) with indicated compounds. Right table: Cell proliferation assay in Ba/F3 cells (dependent on HER2YVMA or HER2YVMA,L800F) with indicated compounds. All results are quoted as the geometric mean ± SEM, pIC50 = -log IC50 (M). The number of repeats, n, and the IC50 are described in parentheses. (c) Confirmation of EGFR knockout and overexpression of exogenous EGFR variants by Western Blot in PC-9 cells. Representative image of two independent experiments. (d) Selectivity Index plot for proliferation assay in human tumor cells dependent on different oncogenic EGFR and HER2 variants in reference to EGFRWT dependent A431 cells. Number of n repeats is described in panel e of Extended Data Fig. 3. (e) Table summarizing the cell proliferation experiments in various tumor cell lines with indicated compounds. All results are quoted as the geometric mean ± SEM, pIC50 = -log IC50 (M). The number of repeats, n, and the IC50 are described in parentheses.
Extended Data Fig. 4 Biomarker modulation and gene expression changes.
(a) Heatmap visualizing gene expression profiling of pharmacodynamics biomarkers using BI-1622 and Poziotinib in NCI-H2170 HER2YVMA dependent cells sampling at 0.5 h, 2 h and 16 h (n = 3 independent experiments). Median gene expression values for each group are shown and z-score normalized across all samples with red colors represented high gene expression and blue colors showing low expression. (b) Reduction of pHER2 levels in NCI-H2170 HER2YVMA cells treated with increasing concentrations of BI-4142. Representative image of two independent experiments. (c) Dose response curves for phosphorylated HER2, phosphorylated ERK and DUSP6 mRNA levels. Representative image of two independent experiments. (d) Heatmap visualizing gene expression profiling of pharmacodynamics biomarkers using BI-4142 and Poziotinib in three cancer cell lines and sampling at 6 h and 24 h (n = 3 independent experiments). Median gene expression values for each group are shown and z-score normalized across all samples (n = 3) with red colors represented high gene expression and blue colors showing low expression.
Extended Data Fig. 5 Poziotinib efficacy and body weight data.
(a) Xenotransplantation experiment using NCI-H2170 HER2YVMA cells with Poziotinib at doses indicated in figure panel legend. Natrosol: n = 10 animals; Poziotinib 1 mg/kg qd n = 10 animals; Poziotinib 0.5 mg/kg bid n = 10 animals. The plot displays mean ± SEM. (b) Corresponding body weight changes for efficacy experiment shown in panel Extended Data Fig. 5a. The plot displays mean ± SEM.
Extended Data Fig. 6 Synthetic route for BI-1622 and BI-4142.
(a) Overview of the synthetic route to BI-1622. Reagents and conditions: i) i-PrOH, 45 °C, ii) mCPBA, DCM, 5 °C to r.t., iii) Boc-piperazine, DIPEA, DMF, 70 °C, iv) HCl in dioxane, DCM, methanol, r.t., v) acryloyl chloride, DCM, Et3N, 4 °C to r.t. (b) Overview of the synthetic route to BI-4142. Reagents and conditions: vi) Boc-piperazine, DIPEA, DMF, reflux, vii) oxalyl chloride, DCM, DMF, r.t., viii) i-PrOH, 45 °C, ix) HCl in dioxane, DCM, methanol, r.t., x) acrylic anhydride, DMAP, Et3N, DCM, r.t. or acryloyl chloride, DCM, Et3N, 4 °C to r.t.
Extended Data Fig. 7 1H NMR spectrum for BI-1622.
Graphical 1H NMR spectrum of BI-1622.
Extended Data Fig. 8 1H NMR spectrum BI-4142.
Graphical 1H NMR spectrum of BI-4142.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 3
Unprocessed western blots and gels.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wilding, B., Scharn, D., Böse, D. et al. Discovery of potent and selective HER2 inhibitors with efficacy against HER2 exon 20 insertion-driven tumors, which preserve wild-type EGFR signaling. Nat Cancer 3, 821–836 (2022). https://doi.org/10.1038/s43018-022-00412-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-022-00412-y