Abstract
Evaporitic technology for lithium mining from brines has been questioned for its intensive water use, protracted duration and exclusive application to continental brines. In this Review, we analyse the environmental impacts of evaporitic and alternative technologies, collectively known as direct lithium extraction (DLE), for lithium mining, focusing on requirements for fresh water, chemicals, energy consumption and waste generation, including spent brines. DLE technologies aim to tackle the environmental and techno–economic shortcomings of current practice by avoiding brine evaporation. A selection of DLE technologies has achieved Li+ recovery above 95%, Li+/Mg2+ separation above 100, and zero chemical approaches. Conversely, only 30% of DLE test experiments were performed on real brines, and thus the effect of multivalent ions or large Na+/Li+ concentration differences on performance indicators is often not evaluated. Some DLE technologies involve brine pH changes or brine heating up to 80 oC for improved Li+ recovery, which require energy, fresh water and chemicals that must be considered during environmental impact assessments. Future research should focus on performing tests on real brines and achieving competitiveness in several performance indicators simultaneously. The environmental impact of DLE should be assessed from brine pumping to the production of the pure solid lithium product.
Key points
-
Fresh water consumption of direct lithium extraction (DLE) needs to be urgently quantified. Many DLE technologies might require larger freshwater volumes than current evaporative practices, compromising their applicability in arid locations.
-
Chemical processing is not completed until a pure solid product is obtained. Energy consumption of DLE should be estimated for the overall process, including potential water extraction or evaporation from pure but dilute LiCl solutions, as is the case with many DLE technologies.
-
Lithium ions are only a minor component in continental, geothermal and oilfield brines. Thus, from a circular economy perspective, there is potential for extraction of more than one valuable mineral, notably, borates, magnesium, potassium and sodium salts.
-
Knowledge of the precise number, distribution and depths of brine and fresh water wells is vital for hydrogeological modelling of lithium brine deposits. The distinct hydrogeology of each salar means that each deposit should be modelled independently, and results from one exploitation cannot be directly extrapolated to another.
-
Environmental monitoring should be permanent and precede the start of the exploitation as environmental impacts might only be observable in the long term. Water monitoring requires gathering precipitation data, river flows and a sufficient number of observation wells to follow water tables at different locations.
-
Environmental monitoring guidelines have been drafted with evaporitic technology in mind, but they should also be applied to the implementation of any DLE technology, which still consumes brine, uses fresh water and produces residues, the latter two hopefully at considerably lower volumes.
Similar content being viewed by others
Introduction
Lithium is a fundamental raw material for the renewable energy transition owing to its widespread use in rechargeable batteries and the deployment of electric vehicles1,2,3,4. The electric vehicle stock has increased strongly from a few thousands in 2010 to 11.3 million in 2020, and 142 million electric vehicles are forecast to be on the road by 20305. Global lithium production has tripled between 2010 and 20206. Different projections estimate that the demand for lithium will grow by 18–20-fold by 2050 if existing extraction policies are followed. However, if new, more sustainable extraction policies are implemented, demand is estimated to increase by as much as 40-fold by 2050 (refs. 7,8).
Currently, lithium extraction is exclusively from hard-rock ores and continental brines6,9, with continental brine resources being more abundant than hard-rock ores6,9,10,11. The evaporitic technology currently used to extract lithium from continental brine deposits relies on open air evaporation to concentrate the brine. Large volumes of water, 100–800 m3 per tonne of lithium carbonate, depending on the deposit, are lost through evaporation, raising concerns about the overall sustainability of the process. Furthermore, continental brine concentration is intrinsically slow, taking 10–24 months, which means that this process is not responsive to short-term changes in demand. Consequently, production cannot be reduced if demand drops, for example, during the onset of the COVID-19 pandemic in March 2020. In addition, ramping up production takes approximately 4 years. The locations of suitable continental brines are also geographically restricted, with an estimated 50–85% of lithium-rich continental brine deposits located in the Lithium Triangle and with China as the next richest source9. Hard-rock ores are also geographically concentrated in Australia and China10,12,13.
Conversely, many more countries have access to less-concentrated lithium brine sources, such as geothermal brines and oilfield brines14,15,16,17, which have lower lithium concentrations (Supplementary Table 1). Unfortunately, evaporitic technology is not applicable to these more dilute brines18,19. The non-viability is due to the different chemistry, the much longer time frames that would be required for successful concentration and the fact that most of these deposits are not located in arid regions. Economically sound technologies to exploit these more dilute lithium resources are being explored with urgency to diversify lithium production. These new lithium extraction technologies, generically termed direct lithium extraction (DLE), could enable the processing of both continental and other more dilute brines without the need for evaporation ponds.
DLE encompasses a wide variety of technologies, including, for example, thermal and electrochemical processes. For the key process of lithium capture or concentration, very thorough chemical analysis and quantitative data are available. Unfortunately, quantitative data regarding native brine pre-processing, leading to the feasibility of the specific DLE process, are scarce or not available. Information on post-processing after the DLE is often also missing. However, all aspects of the multistep brine processing procedure should be comprehensively analysed for a full environmental impact analysis and costs estimation. As of 2022, several advanced DLE methods have been proposed that produce remarkable efficiencies. As such, it is now timely to review and compare traditional and new technologies by providing an analysis of the entire extraction process from brine pumping to packaging of the final lithium product, with the aim of identifying the required inputs and outputs for different technologies.
In this Review, we discuss the environmental impact of lithium mining from continental brines, outline the challenges in the nascent field of lithium mining from geothermal brines and assess the proposed DLE technologies in the framework of an overall mining and processing technology. The scope of the Review includes an assessment of DLE in terms of input of chemicals, production and fate of spent brines, energy and freshwater requirements and the potential for exploiting the by-products produced. These are factors that need to be considered in a discussion of the potential environmental impact of DLE. We also discuss the potential of DLE for scaling up to industrial levels.
Environmental impacts of current practice
Lithium is a fundamental element driving sustainable mobility and energy and its mining is therefore under scrutiny and will require social licensing20. A major open question regarding the sustainability of evaporitic technology is its intensive water usage, which is discussed subsequently.
Lithium mining from continental brines
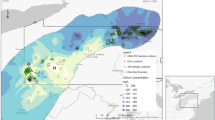
As of 2022, worldwide, there are eight full-scale active facilities that produce lithium compounds from continental brines9 and more are likely to become active before 2030 (Fig. 1a). The evaporitic technology (Fig. 1b) is currently in use at seven of those facilities18,19. Brines are pumped from underground reservoirs into open air ponds, in which over 90% of the original water content is lost through evaporation. Concentrated brines are then transferred to a refining plant for removal of impurities, followed by Li2CO3 precipitation via the addition of Na2CO3. Fresh water is needed at multiple steps of the process, including to dissolve CaO (needed to precipitate Mg2+) and Na2CO3, in the scrubbing of organic solvents (used for the removal of borates), for washing Li2CO3 crystals and for steam generation18,19. Over 90% of the salts other than LiCl in the original brines spontaneously crystallize in the ponds18 and are considered waste.
a, Active lithium mining from brine facilities and corresponding production capacities in 2022 for the following salars: Clayton Valley (USA); Lake Zabayu (or Zabuye), Dongtai Salt Lake and Xitai Salt Lake (China); Salar de Atacama 1 and 2 (Chile) and Salar del Hombre Muerto and Salar de Olaroz (Argentina). The inset zooms in on the Lithium Triangle in South America, which has the largest identified deposits in continental brines worldwide. Deposits that are mentioned in the Review but are not currently exploited are also shown: Salar de Uyuni (Bolivia) and geothermal fields in the Rhine region (France–Germany). LCE: Lithium carbonate equivalent. b, Schematic representation of evaporitic technology. The first step is brine pumping from underground reservoirs. Brines are poured into large shallow open air ponds, where over 90% of the original water content is lost via evaporation accelerated by solar radiation and wind. LiCl concentration increases gradually and salts from other cations crystallize in the ponds as saturation is reached. Concentrated brines then enter a refining plant for crystallization of the final product (usually lithium carbonate). Fresh water and chemicals are used at several steps of processing.
Starting in the early 2000s, environmental concerns have been raised about lithium mining from continental brines by local populations, non-governmental organizations and the press, first in Chile where operations started 10 years earlier and then in Argentina18,21,22,23,24,25,26. Communities close to these operations are all rural, and environmental conditions are very poorly documented26. Surprisingly, environmental life-cycle analysis of lithium brine mining has quantified energy consumption and carbon emissions, while disregarding the impacts on the water cycle or specific land uses27,28,29,30,31. Solid quantitative evidence of the negative environmental impacts of lithium mining was only reported from 2018. The data are still quite limited and relate only to Salar de Atacama, the first salar to be mined aside from Clayton Valley, which has a much smaller production capacity19.
In brine mining, two distinct aquifers are exploited, brine and fresh water18,32, which can potentially become physically connected. The question arises as to whether these water volumes should be considered when calculating the water footprint of the process24. Clearly, the freshwater volume should be included, whereas the brine volume that should be considered in the water footprint is less straightforward to estimate. Arguing that brine water is far from being suitable for either human consumption or agricultural use18,32, strong proponents of mining propose that brine should be completely disregarded in water footprint calculations. However, we suggest that brine must be considered, as the brine volume that is pumped will directly determine the amount of fresh water that naturally flows from outside the brine aquifer (Box 1), is mixed with brine and thus is no longer considered fresh water or can be used as such18,32,33,34,35,36. The volume of fresh water that flows or moves towards the salar is different during brine pumping or in the absence of mining. As both fresh water and brine are extracted from underground aquifers, salars are a hydrogeological case study (Box 1).
Determining whether excessive water extraction is occurring is difficult in the absence of hydrological data, and unfortunately, hydrological records in the Lithium Triangle are either unavailable or incomplete18,25,26,37. For example, a decrease in lagoon area or stream flow is a direct indication of water shortages. The surface area of lagoons on Salar de Atacama has decreased by half in the period 1985–2020 in winter but not in summer38. To the best of our knowledge, no other quantitative information is available on surface water trends for the Lithium Triangle. However, it is of the utmost importance to also consider the decrease in underground water reservoirs, both brine and fresh water. Satellite data reveal that the total water storage in Salar de Atacama decreased by –1.16 mm per year in the period 2010–201726 and soil moisture indexes decreased on average by –0.005 yearly in the period 1997–201737. Direct observation wells showed a radical reduction in the water table from pre-extraction to current time frames (1986–2015)33–35,39. In the region where brine wells are located, water table decreases of up to 9 m were recorded in 1990–2015. To date, these decreases are limited to the nucleus of the salar and do not seem to reach the salar borders or the regions where brine and fresh water mix33.
Another consideration in assessing underground water reservoirs is phreatic evaporation, a natural form of evapotranspiration that occurs in salars, even in the absence of brine pumping. Discordant results have been reported regarding evapotranspiration in Salar de Atacama. In two studies using satellite data, one study found no change in the phreatic evaporation rate in the period 2000–2017 for either the salar or the surrounding areas37, whereas the other found a 6% decrease in the period 1960–202038. A third study coupling field measurements with hydrogeological simulations estimated an average decrease in the evapotranspiration rate of approximately 15% over the whole salar surface since the start of brine mining in 199433. Evapotranspiration is strongly dependent on brine pumping volumes because of its dependence on the water table35,40,41. Thus, the decrease in evapotranspiration is a direct indication of a decreasing water table depth.
It has been hypothesized that changes in natural evaporation rates might serve, at least partially, as a compensation or damping mechanism to counteract brine pumping33,35. The idea is that while more water is lost through brine pumping, this is partially compensated for by a decrease in the amount of water that evaporates. Phreatic evaporation decreases exponentially with depth, so the damping capacity only works if water table levels do not drop below 2 m, being most efficient between 0 and 0.5 m (ref. 33). In the Salar de Atacama in particular, the damping capacity has already been exhausted in the region where the brine wells are located, since the water table depth is already below 2 m (ref. 35).
A reduction in water levels can also be inferred from changes in flora and fauna. Flamingos could be a strong indicator of environmental damage, owing to the landscape scale at which they use wetlands42. In the Salar de Atacama, a reduction of 10% and 12% has been reported in the populations of James and Andean flamingos, respectively, which is linked to the reduction of surface water, particularly in winter38. Reduced reproductive success in flamingos in 2017–2019 resulted in the population barely reaching the minimum number of 1,000 nestlings required for population size maintenance, below historical records43. Monitoring of the abundance of the brine shrimp Artemia could aid understanding of ecosystem dynamics owing to the role of this crustacean in trophic ecology. Unfortunately, no data on Artemia abundance or temporal evolution in the region has been reported, only a suggestion that it is an important taxa to be monitored43.
Three variables indicate a trend towards desertification. Satellite imaging data revealed a reduction in normalized difference vegetation index in the period 1997–2017, indicating an overall trend of more sparsely vegetated areas37,38. The same satellite data showed land surface temperature increases averaging 0.74% and 2.68% yearly in summer and winter, respectively. Finally, in one of the mining properties, a third of carob trees, a species known to be drought-tolerant, died in the period 2013–2017, strongly indicative of underground water shortages37.
Although segmented and not sufficient to generate an overall measure of sustainability26, publications from 2018 onwards strongly suggest that a negative impact is been produced, mostly regarding the water balance. Because of the complex nature of hydrogeological studies, it is difficult to quantify the extent of the impact.
Lithium mining from geothermal sources
Geothermal sources are deposits of interest for the production of lithium and other metals and for energy production. Mining from geothermal sources requires the development of new technologies adapted to specific operating conditions. Particularly in Europe, lithium deposits are found in the vicinity of densely populated areas, as opposed to the rural location of most salars and hard-rock ores deposits14,15.
A pilot scale DLE process, adapted from the one originally developed for brines in Argentina, successfully extracted lithium from geothermal brines located in north-eastern France that are used for energy production. After extracting the 180 °C geothermal fluid located at a depth of between 2,600 and 5,000 m, 90% of lithium was selectively extracted using patented ion exchange resins15,44. The lithium-depleted geothermal fluid is then re-injected underground. This type of lithium production from geothermal brines faces challenges, especially in the case of deep extraction because of the risk of micro-earthquakes45, as occurred during testing in late 2020 near Strasbourg, which halted drilling activities. If geothermal energy harvesting is stopped, lithium extraction would also be affected. However, it should be kept in mind that this area near Strasbourg is a seismic zone known to be potentially active. Furthermore, these risks can be reduced by using geothermal fluids located at a shallower depth46.
The high pressure and high temperature of geothermal fluids are additional difficulties when comparing the processing of geothermal and continental brines. After energy recovery, the temperature of the brine is close to 60–80 °C and the pressure is maintained at a minimum of 25 bars to avoid precipitation phenomena and excessive changes in the composition of the geothermal fluid to be re-injected47. Membrane technologies cannot be used at these temperatures without risking premature membrane ageing, whereas liquid–liquid extraction cannot be easily implemented at these pressures48,49. The best solution is probably to extract the lithium by high-pressure chromatography in columns filled with ion exchange resins, as well as using other technologies that are discussed in the following sections.
Direct lithium extraction
DLE technologies aim to tackle both the environmental and techno-economic shortcomings of evaporitic technology. Ideally, DLE should completely avoid open air evaporation ponds. Many different proposed technologies have been put forward, including ion exchange resins, thermally assisted processes, electrochemical methods, among others.
General working principles of DLE
DLE technologies can be classified into seven general categories50 (Fig. 2).
a, Ion exchange resins, also known as ion sieves or specific sorbents. b, Solvent or liquid–liquid extraction. c, Electromembrane processes with Li+-selective membranes (left) or permselective membranes (right) that are selective to anions or cations. d, Nanofiltration, NF. e, Electrochemical ion pumping, which is sometimes also termed electrochemical ion insertion or electrochemically switchable ion exchange. f, Selective precipitation of Li3PO4 via addition of Na3PO4. g, Thermal-assisted methods for brine concentration, other than open air evaporation. These methods include any type of evaporator, distillation device or membrane distillation. h, General scheme of DLE as part of an overall processing strategy. AEM, anion exchange membrane; CEM, cation exchange membrane.
Ionic exchange resins (Fig. 2a) are materials that have a high affinity for Li+ cations51,52,53, which are adsorbed onto small resin particles (often packed in columns) from brines, even when Li+ cations are at much lower concentrations than coexisting cations. Freshwater or acidic solutions are usually used to desorb Li+ cations from the resins to produce a fairly pure Li+ solution (usually LiCl).
Some organic solvents or solutions, such as tri-n-butylphosphate and FeCl3 (refs. 54,55), and ionic liquids, such as imidazole ionic liquids that serve as solvents in conjunction with tri-n-butylphosphate56,57, have a high affinity for Li+ (Fig. 2b). When brine comes into contact with these solvents, a large proportion of Li+ cations is transferred to the organic or ionic liquid phase. The Li+-loaded phase is then mixed with an aqueous phase to liberate the Li+ cations. pH changes are commonly needed to promote Li+ transfer between different phases.
Alternatively, membrane processes for selective lithium recovery can be driven by electrical fields (Fig. 2c) or mechanical forces (Fig. 2d). The use of electrical fields usually requires membranes selective for either anions or cations58,59 (Fig. 2c, right), with few membranes showing specific selectivities60 (Fig. 2c, left). Mechanical forces are used to drive brines across nanofiltration membranes, with multivalent species usually being retained61,62,63.
Electrochemical ion-pumping technology64,65 is based on materials that are highly specific for Li+ cations, similar to the case for ion exchange resins. However, in electrochemical ion pumping, Li+ is inserted in an electrode material subjected to a potential gradient (Fig. 2e, left), which undergoes an electrochemical reaction. No chemicals are needed and no species are concomitantly liberated to the brine. Subsequently, Li+ is de-inserted from the electrode material using recovery solutions requiring fresh water, producing a diluted LiCl solution (Fig. 2e, right). Electrochemical ion pumping is often coupled to ion-selective membranes66,67,68,69.
Selective precipitation (Fig. 2f) is based on the very low aqueous solubility of lithium phosphate (Li3PO4)70,71. A large proportion of Li+ from brines can often be recovered by the addition of different phosphates, provided that the brine has previously been depleted of multivalent species70,71,72.
A final DLE technology comprises processes in which the main objective is to concentrate native brines with concomitant water recovery73,74. Brine concentration is also the objective of open air evaporation ponds, except that in this case the evaporated water is lost to the atmosphere. Examples of these processes include membrane distillation and solar evaporators (Fig. 2g).
The sustainability and the potential scalability of the seven DLE working principles (Fig. 2a–g) for the capture or concentration of Li+ cations were assessed from compiled data (Supplementary Table 2). An analysis of the chemistry of the diverse materials (adsorbents, membranes, insertion electrodes, solvents) in these reports is beyond the scope of this Review; however, interested readers are referred to several previous reviews50,58,59,65,66,75,76,77,78. The capture or concentration of Li+ ions is a crucial processing step. However, native brine pre-processing (for example, heating or adjusting the pH of the brine) is often needed for the key DLE step to work. Furthermore, most proposed DLE technologies do not directly produce a pure lithium product but instead a purified solution that thus requires post-processing (Fig. 2h).
Testing of different lithium sources
Lithium brine processing involves the separation of a very diluted species, Li+, from a broth containing other much more concentrated species with similar chemical properties (Fig. 3 and Box 2). However, real brines were tested in only 30.4% of the analysed reports (Supplementary Fig. 1). Technology validation on simulated solutions is acceptable, provided that these solutions mimic reported ion concentration values for real brines. Unfortunately, this is often not the case, as 24.1% of the analysed reports work with either a single salt or binary mixtures (Supplementary Fig. 1). Matching ion concentrations to those of real systems is often achieved for Li+ and/or Mg2+ but not for other ions79,80,81,82. In addition, the effect of divalent cations is omitted83 or the effect of anions other than Cl− is not considered84. Beyond the specific chemistry of ions that are not included in these laboratory experiments, the activity coefficient of Li+ and the ionic strength of the solution are also modified in the absence of these ions. For example, Na+ and K+ have often been omitted or included at concentrations very similar to that of Li+ (refs. 85,86,87).
The concentrations of Li+, other ions, and total dissolved solids in brines. The data are provided in Supplementary Table 1. a, Li+, Ca2+ (left axis), and K+ and Mg2+ (right axis) concentrations. b, Major species. Na+ and Cl− (left axis) and total dissolved solids (TDS, right axis). Numbers on the bars correspond to concentrations that are larger than the corresponding Y-axis maximum. Li+ is usually the most diluted species and always 10 times more diluted than Na+.
In an analysis of the composition of the test solutions in 77 articles (Supplementary Table 2), only 41 test solutions contained Mg2+ and only 33 contained Na+, of which only 34 and 23 (respectively) report changes in the concentrations of these ions after DLE. The different DLE technologies result in reductions in the Mg2+/Li+ (Fig. 4a) and Na+/Li+ (Fig. 4b) concentration ratios after processing, but the magnitude of the change is markedly different. Moreover, many studies depart from solutions with Na+/Li+ molar ratios below 5. These results highlight how many reports work with solutions that do not mimic real brines (Box 2).
Full data compilation and data sources are provided in Supplementary Table 2. a, Mg+/Li+ concentration ratios before (0) and after (f) processing (in molar units). b, Na+/Li+ concentration ratios before and after processing (in molar units). c, Li+ recovery, defined as the percentage of the absolute amount of Li+ in the feed that is recovered in the output solution or solid. d, Varying freshwater requirements for direct lithium extraction (DLE) technologies. e, Varying final Li+ concentrations in the recovery solutions after DLE. For parts d and e, only articles on ion pumping, solvent extraction and ion exchange resins were analysed. f, Number of reported articles in which materials are cycled or used for a medium–long period of time. NF, nanofiltration; EM, electromembrane processes. S. precipitation, selective precipitation; L-L extraction, liquid–liquid extraction. DLE efficiency can be assessed by different performance indicators, and future research should focus on achieving competitiveness in several performance indicators simultaneously.
Two examples involving the use of Li+-selective membranes were analysed (Fig. 2c, left), which were able to increase the Li+/Na+ molar ratio by 2.84 (ref. 60) and showed an Li+/Na+ separation factor of 2.2 (ref. 87). These are very promising results, although it should be noted that in both examples the starting Li+/Na+ molar ratio was 1, which is quite different from native brine concentrations (Fig. 3). If the selectivity could be maintained for lower Li+/Na+ ratios, then the final solution would be enriched in Li+ but would still have a higher concentration of Na+ than Li+. Alternatively, the methodology might work as part of a more complex processing strategy that involves pre-processing and post-processing steps (Fig. 2h).
Electromembrane processes with commercial membranes are another interesting case example to illustrate the importance of using realistic concentrations. By varying temperature and applied voltage over a wide range, there were no experimental conditions in which Li+ could be selectively separated from either Na+ or K+ (refs. 85,86,88). Thus, attempting cation extraction through a cation-selective membrane will only concentrate a mixture of Li+, Na+ and K+. Despite this obvious limitation, research on electromembrane processes is still largely focused on separating Li+ from Mg2+ by testing binary solutions58. However, by using simple electrodialysis setups only, currently Li+ cannot be separated from the other monovalent cations in native brines89.
Because of the difficulty in comparing results obtained using solutions of different compositions, a proposal was put forward that the scientific community should agree to the use of a standard concentration of brine65. This is important from an academic perspective. However, reported data show that not all methods will be equally efficient for all concentrations. For example, Li+ recovery ratios increasing from 66% to 80% were obtained when four solutions with Li+ concentrations from 0.14 to 1.03 g l–1 and varying total salinity were tested on the same selective electrodialysis setup85. For a titanium oxide, the absorption capacity was tripled90 when increasing the Li+ concentration from 50 to 500 mg l–1. For the five technologies for which Li+ recovery data were compiled (Fig. 4c), large divergences are explained by different specific chemistries in each report, but also by concentrations in the tested solutions. An industrial-scale mining facility could therefore be implemented if a given methodology applied to a real native brine produces a high recovery rate, among other performance indicators. If the performance of this methodology is reduced for other Li+ concentrations, it should still be implemented in the first instance. Thus, we believe that it is still important to perform tests on different brine compositions, provided that these mimic the concentrations of some real deposits.
Fate of spent brines
In most of the analysed reports, with very few exceptions, a spent brine is produced, as most of the DLE technologies discussed here are focused on the selective capture of Li+ cations only (Fig. 2). This issue pertains to both geothermal and continental deposits but very often is not even mentioned18. Many researchers and technologists propose the re-injection of spent brines18,91,92,93, although from a technological perspective re-injection could dilute a valuable resource18,36. For example, in 80% of the cases in geothermal fields, re-injection wells show a rapid interference with production wells94.
Little practical knowledge is available regarding brine re-injection in salar basins, although re-injection could disrupt the layered stratigraphic structure of these basins18. Considering varying geological structures (Box 1), each deposit should be modelled and analysed individually to assess the risks and benefits of re-injection. Finally, spent brines are likely to contain chemical species that are exogenous to the salar, because of the leaching of active materials from DLE setups or altered pH. These changes might affect the surrounding ecosystems. On cycling, many adsorbents or electrodes slowly dissolve in brines (Supplementary Table 2), whereas solvent solubility in brines on the order of 200 ppm has been reported19.
An alternative to re-injection is the slow evaporation of spent brines in open air ponds, which overcomes the slowness of evaporitic technology in terms of production of lithium salts, but still results in evaporative loss of precious water and the production of waste. A second, higher cost option is the use of advanced technologies for desalination of hypersaline brines95,96,97,98,99,100,101,102,103.
Resources and inputs in DLE
In this section, we analyse aspects relating to fresh water, chemicals and energy consumption, including, as far as possible, inputs and outputs for an overall processing scheme (Fig. 2h) and the requirements for pre-processing and post-processing.
Freshwater usage
Freshwater consumption with current evaporative technology is 22.5 and 50 m3 per tonne Li2CO3 for Salar de Atacama and Salar de Olaroz, respectively104,105. These numbers should be considered a reference for comparison, as the freshwater consumption of current practice already raises environmental concerns.
Freshwater inputs are essential in some DLE technologies (Fig. 2). Ion exchange resins, solvent extraction, electromembrane processes and Li+ insertion electrodes all require fresh water for Li+ elution from a sorbent phase. We compiled data on the freshwater requirements of ion pumping, solvent extraction and ionic exchange resins from 57 articles in the period 2017–2022 (Fig. 4d and Supplementary Table 3). A quarter of these reports do not provide freshwater consumption data, 13 use lower amounts than current practice and 9 require similar amounts. A quarter of the analysed articles report freshwater requirements of over 500 m3 per tonne Li2CO3, over 10 times greater than that used in current practice. These volumes of fresh water are not available in the vicinity of salars and would even pose problems around less-arid geothermal resources. Although we acknowledge that the aim of many academic papers is to prove new concepts or materials and not ascertain the engineering details of working conditions, the data in Fig. 4d draw attention to a topic that is hardly ever studied.
One mining operation has been active at Salar del Hombre Muerto (Fig. 1a) since 1996, and this location is a full-scale example of a DLE facility in practice. Li+ is selectively captured in ion exchange resins and subsequently, a fairly pure LiCl solution is recovered by elution with fresh water19,106,107. Freshwater volumes required for elution have not been disclosed18,19. The overall water use of the entire facility is reported as 71 m3 per tonne Li2CO3 (ref. 107), 200% and 50% higher than the volume used at Salar de Atacama and Salar de Olaroz, respectively, highlighting that a given DLE might be more water-intensive than standard evaporitic technology.
Mining in Salar del Hombre Muerto testifies to another often neglected issue with many DLE technologies. The solution eluted from adsorption resins is about 10 g l−1 Li+ (ref. 19), which is not considered concentrated enough for crystallization of a pure lithium product. Consequently, the solution is thus sent to evaporation ponds for further concentration19,106 (Fig. 2), although for shorter intervals than in standard evaporitic technology. For any DLE technology that produces an effluent solution that is not concentrated enough, solutions will need to be concentrated by either time-consuming evaporation ponds, with consequent water loss, or ancillary complementary technologies that require high energy inputs. The latest sustainability report of the company operating in Salar del Hombre Muerto mentions an intention to install a mechanical evaporation unit107, which could be seen as an acknowledgement of the high freshwater consumption (mentioned earlier).
Data on the final Li+ concentration after elution are shown in Fig. 4e, using the same data compilation sources as Fig. 4d. Final concentrations are not reported in 20% of the articles, 50% of the articles report elution concentrations that are below the average continental brine concentration (0.5 g l−1) and only 10% report Li+ concentrations that could be considered sufficient for direct crystallization of Li2CO3. Most of those articles highlight the purity of the Li+ solution in comparison to the original solution. However, these pure but dilute Li+ solutions will require either solar evaporation or energy-consuming evaporators.
Evaporitic technology seeks to concentrate the brine to both crystallize other salts and produce a substantial increase in Li+ concentration. A change of perspective has been proposed by some researchers, with the aim of water recovery while the brine is being concentrated. Membrane distillation73,74,108,109,110 is a thermal method that has achieved freshwater recovery rates of 22.5 kg m−2 h−1 (ref. 108) and 3.5 kg m−2 h−1 (ref. 73) for lower and higher salinity feeds, respectively. These values are much higher than the evaporation rate at Salar de Atacama (0.37 kg m−2 h−1)19. Thus, not only is fresh water recovered but processing is also faster. A classical solar still at Salar de Olaroz achieved a lower performance, with a water recovery rate of 0.083 kg m−2 h−1 (ref. 111). Another approach for water recovery used graphite-promoted cyclopentane hydrate formation112. All three systems can be classified as assisted brine concentration (Fig. 2g).
Energy input
Energy requirements are yet to be provided for most DLE technologies. Cost analysis (Supplementary Table 2) and optimization processes are rarely reported85,113,114,115. Energy requirements are frequently reported only for the key DLE step, with a complete disregard for that of pre-processing and post-processing steps or ancillary equipment (Fig. 2h). For example, in an electrochemical ion-pumping process, the mechanical energy to pump solutions through the electrochemical cells was calculated to be 300-fold higher than the energy required to drive the electrochemical reactions83,84. However, often only the electrical work required for an electrochemical process is calculated65,79,85,114,116,117,118,119,120. The energy value of a single step is relevant but it is only a lower bound. Owing to this frequent partial reporting of energy requirements, it is not possible to make comparisons.
In addition, it is important to consider that pre-processing is a crucial DLE step that must be performed on very large brine volumes (on the order of 21 million litres per day). A thorough study of the efficiency of lithium manganese oxide as a Li+ absorbent found that heating the brine from 10 to 80 oC increased the Li+ adsorption efficiency from 15% to 70%, with a consequent increase in separation efficiency from other cations52. Other ion exchange resins also show improved absorption efficiency at higher brine temperature121,122,123,124,125. However, operational costs increase considerably if brine volumes on the order of 21,000 m3 daily (Box 2) need to be heated to approximately 80 °C. Brine heating is definitely an energy input that should be considered in the overall process analysis (Fig. 2h).
In the case of continental brines, it is important to recall that most deposits are located at high altitudes and at latitudes where the average annual horizontal global solar irradiation values are above 2000 kWh m−2 (ref. 126). These values are among the highest on Earth, making these ideal locations for solar energy harvesting. Installation of photovoltaic cells or solar-concentrating power capacity will increase the capital cost of a mining project but will strongly reduce the operational cost. For the current evaporitic technology, for which the precise energy requirements are known, parabolic trough solar collectors and linear Fresnel reflectors were analysed as options to provide thermal or electric energy in northwest Argentina, with and without thermal energy storage127. A parabolic trough plant for electric power generation coupled with energy storage provided a more substantial diversification of the energy matrix than other energy generation and storage configurations. Solar energy could reduce natural gas consumption over 51% and produce an annual CO2 mitigation of 403.3 gCO2 kg−1Li2CO3 (ref. 127).
Scaling-up requirements and potential
Published demonstrations of scaling up are scarce58,59,78,85,114,128,129, although it should be acknowledged that these efforts are rarely reported in academic journals130. Different technology readiness levels have been reported in the vast field of DLE. With one full-scale facility in operation since 199619, ion exchange technologies have an advantage. In addition, provided that efficient operational parameters are found, scaling up could be relatively straightforward for some technologies. For solvent extraction and some electromembrane and thermally assisted brine concentration processes, large-scale plants for the separation of other chemicals are already in place and equipment could be adapted fairly easily. The challenge with adjusting these processes is the application of a known methodology to a new, highly complex chemical system. Conversely, for other methodologies, notably, membrane distillation and ion pumping, no large-scale implementation has yet been developed.
Beyond successful proof-of-concept experiments of new ideas or materials, to increase the technology readiness level, the overall process must be analysed and both inputs and outputs quantified (Fig. 2h). We have already highlighted that energy and freshwater consumption need to be optimized to increase technology readiness levels. Other important aspects that need to be addressed are discussed subsequently.
Irrespective of the technology, long-term performance of the active materials responsible for the selective Li+ capture is key. Of 77 analysed reports, 22 do not show any cycling data (Fig. 4f). When data are reported, the average number of cycles is fewer than 10 (that is, <100 h)83,131,132,133,134,135. For the different LiMn2O4-based ion exchange systems, Li+ adsorption capacity is reduced by 17.2% after 50 cycles136, 2.5% after 5 cycles52 and 43% after 30 cycles137, pointing to the importance of medium-term to long-term stability tests. Active materials get inactivated138 or they are dissolved into either the spent brine or the recovery solution. Again, the dissolution rate for LiMn2O4 is 2.53–4.60% in 5 h of continuous operation139, whereas the reported dissolution rate for titanium is approximately 0.25–0.31% for every cycle121. These very preliminary numbers suggest that the active materials will need to be changed within months. Although this performance is not necessarily poor, it should be evaluated from the perspective of the environmental liability of waste disposal of spent active materials, their costs and the duration for which active material replacement would stop production.
Input of chemicals in DLE processes is another largely neglected topic. Aside from cost, another issue is the transportation of these chemicals to often remote locations18. In principle, electrochemical methods (Fig. 2c,e) do not require the addition of chemicals, as the driving force is the application of an electric field. However, ion exchange resins and solvent extraction often require that the pH of solutions be changed52,140,141. For example, LiMn2O4 seems to be more efficient when the brine pH has been adjusted to values around 10–11 (refs. 52,139,141). Large volumes of brine are processed daily (Box 2), so pH adjustment of 21,000 m3 of brine will require non-negligible acid or base amounts. Chemical inputs are also required for elution. For example, elution of Li+ with an LiFePO4-based ion exchange resin requires a 5 g l−1 Na2S2O8 solution (ref. 142). Selective precipitation of Li3PO4 requires phosphate salts or phosphoric acid70 (Fig. 2f). Some approaches, notably those that require phosphate salts, consider the possibility of recycling chemicals to minimize waste and to reduce the constant transport71,72. Unfortunately, regeneration is not always possible. For example, if the brine pH is modified to increase Li+ recovery, it is difficult to envision how to regenerate the acid or base.
Another aspect to be considered is final product purity71,75, as various species with water solubility similar to lithium products could co-precipitate during crystallization (Supplementary Table 4). The importance of Mg2+ cation removal has been extensively highlighted117,120,143. Removal of NaCl, KCl, Na2CO3 and K2CO3 will be straightforward by freshwater washing owing to their high-water solubilities144. However, there are few reports on the potential difficulties in removing borates, Ca2+ and \({\rm{S}}{{\rm{O}}}_{4}^{2-}\) products that could co-crystallize with lithium products144. Experiments with solutions that do not contain these species will likely produce misleading product purity values. Thus, tests on real brines are a necessary key step that should be undertaken for reliable assessment of final product purity77,89.
The circular economy approach
Evaporitic technology produces large amounts of waste. Essentially, all brine constituents other than Li+ cations end up as salt mixtures that accumulate in the vicinity of the brine deposits and pose a risk of slow leaching following infrequent rain. The precise amount of waste is estimated by considering brine composition and the recovery ratio. On average, waste production is 115 tonnes per tonne of Li2CO3 (ref. 18). For an annual production of 20,000 tonnes, after 10 years, 1.15 × 107 m3 of waste will have accumulated, which, if disposed of at the ground level (Fig. 1b) at a height of 1 m, will occupy an area of 11.5 km2 (ref. 18).
Sustainable raw material sourcing is interlinked with the idea of the circular economy. Recycling of lithium-ion batteries as a secondary source of raw materials30 is certainly important in the long term. However, in a scenario of continuous growing demand, relying on sourcing from recycling only will not be enough to satisfy this demand145,146,147. The amount of lithium from spent batteries in 2040 is estimated at 60 ktonnes per year, which corresponds to 5% of the total supply requirements7. From a circular economy perspective, research on resource recovery from mine tailings is becoming an active field148,149,150,151,152,153. In addition, processing of mineral resources should be designed for the simultaneous recovery of two or more products.
Brines are not only lithium resources but also potential sources of magnesium, potassium, calcium, sodium and boron products (Fig. 3 and Supplementary Table 1). None of these products has a market value comparable to pure lithium products, but they are still ubiquitous raw materials in many industrial processes. Variations in brine compositions lead to different amounts of recoverable by-products (Fig. 5), for the lithium deposits shown in Fig. 1a. Except for calcium, these amounts are larger than the equivalent amount of recoverable lithium carbonate.
a, Brine exploration, mining and processing could be tailored for the joint recovery of several minerals from lithium-rich brines. b, The volumes of fresh water and by-products that could be recovered while producing 1 tonne of Li2CO3 equivalent (LCE) were calculated on the basis of reported brine concentrations listed in Fig. 3 and Supplementary Table 1. A recovery efficiency of 100% and brine density of 1.2 g l−1 were assumed. If most salts are recovered from brines, fresh water could also be produced and used in the overall mineral processing and for irrigation.
Although not scarce, magnesium production is limited geographically154, with ~95% of magnesium chemicals worldwide currently sourced from China. The European Union has therefore listed both magnesium and magnesite as critical raw materials155. For Salar de Atacama (Fig. 5), MgCl2 production could potentially be as high as 17-fold the annual production of Li2CO3 (refs. 18,19). In turn, KCl is a fertilizer that could be widely used in the vast agricultural soils of South America. Evaporitic technology can be tailored for the joint recovery of Li2CO3 and KCl156,157. Indeed, the two mining facilities in the Salar de Atacama jointly recover these two products19,156,157, but this setup is not in place in any of the other six global active facilities we reviewed here.
Despite the favourable context, the large majority of DLE proposals only focus on the selective recovery of lithium products. Among very few examples, one scheme proposes using electromembrane processes for the concomitant production of MgCl2, MgSO4, NaCl, KCl and Li2CO3 (ref. 85), whereas another strategy produces fresh water and Mg(OH)2, Ca(OH)2, Na2CO3 and Li2CO3 (refs. 116,117,120,135,143). Finally, production of fresh water as a by-product during brine concentration73,108,111,112 (Fig. 5) as discussed previously should also be considered as contributing to the circular economy.
Summary and future perspectives
In this Review, we present the limitations of the current evaporitic technology for lithium carbonate production from aqueous sources. Water imbalances during mining25,26,33,35,37, in addition to the inherent slowness and its applicability to continental brines only, are major limitations of evaporitic technology. DLE technologies represent an alternative to evaporitic technology, and we classify DLE technologies according to seven general physico-chemical working principles.
Some proposed DLE approaches, such as ion pumping or Li+-selective membranes, are completely new and will require more ample engineering efforts to reach industrial scale. Conversely, other proposals, such as ion exchange, solvent extraction or electromembrane processes, have been studied for decades in related industrial separation chemical engineering processes, and the challenge here is to adapt these methodologies to the complexity of lithium-rich brines. Laboratory tests with real brines or solutions that closely mimic real compositions are paramount to increase the readiness level of these technologies. Another important point when aiming at scaling up from laboratory to industrial scale is the huge brine volumes to be processed daily. The analysis performed at laboratory scale in wide pH and temperature ranges makes for interesting academic research, but are less likely to contribute to technology implementation. Moreover, the remote location and the aridity of many deposits, particularly continental brines, create logistical issues and add extra costs in transporting chemicals, providing energy away from the grid and securing scarce freshwater sources.
DLE should be assessed as part of an overall mining and processing approach, from brine pumping to obtaining the final product. Although many advances have been produced regarding the specific operational unit for selective lithium capture or brine concentration, a lot of research remains to be undertaken regarding both earlier and later steps in the process. Ideally, DLE should completely avoid brine evaporation in ponds and thereby have a lower water footprint than evaporitic technology. Production of fairly pure LiCl or LiOH solutions instead of a solid lithium product is often reported, indicating that evaporation ponds might still be necessary to complement some DLE technologies.
The concentration of Li+ that can be achieved through different DLE approaches is another aspect that is seldom reported. For comparison, in evaporitic technology, precipitation is only attempted at lithium concentrations greater than 5–6 g l−1. Even at these concentrations, a large percentage of Li+ is not recovered and effluents are sent back to the ponds for further evaporation. DLE should produce solutions with Li+ concentrations greater than 25 g l−1, otherwise an additional concentration method will be necessary. Thermal methods for concentrating solutions are costly. Thus, if DLE produces LiCl-diluted solutions, open air evaporation is likely to be used, leading to the same issues as with evaporitic technology.
The inflating prices and high demand of lithium compounds have obscured the potential of both continental and geothermal brines as sources of other important raw materials. Following the circular economy concept, new processing methodologies should be developed that consider the simultaneous or sequential recovery of multiple by-products. Currently, some deposits in the Lithium Triangle are used for the production of borates, others for lithium carbonate and still others for sodium chloride. The simultaneous recovery of as many by-products as possible at a single deposit might require fewer mining facilities and produce lower amounts of waste. The harvesting of energy and the simultaneous recovery of lithium products at geothermal fields might bring similar benefits. A lot of knowledge could be transferred from related fields such as desalination of hypersaline brines100,102,158,159, resource recovery from brines produced in seawater desalination160,161 and mineral carbonation162.
The effects of the lithium mining industry on the environment can be assessed by examining the following strongly linked indicators: waterflows, soil composition and ecosystem biodiversity. Physico-chemical parameters and biota are also fundamental in biogeochemical cycles163,164. Ecosystems in the vicinity of lithium deposits are extremely fragile and linked in a food chain in which ecosystem services are crucial for livestock and rural populations38,43,165. Soil composition could be affected by decreasing water tables, but also leaching or drainage from accumulated waste, which could produce an increase in soil salinity166,167,168.
Records are necessary to assess waterflows, including reliable year-round precipitation data, river flows and a sufficient number of observation wells to follow in real time the water tables at different locations33. Precise hydrogeological modelling with accurate data regarding brine and freshwater well characteristics is a must, considering the exploitation of underground aquifers in lithium mining. Some researchers have suggested that a conceptual model for one deposit can be extrapolated to others, but our opinion is that each deposit requires a separate model owing to their unique hydrogeological characteristics. Numerical models attempting to quantify169 reduced evaporation rates are very strong qualitative indicators of the impact of lithium brine mining, but the quantitative figures obtained should be used with caution. Even researchers putting forward the concept of damping capacity acknowledge that a 0.5 m error in water table depths would modify the predicted evaporation rate by 60% (ref. 35).
Lithium extraction should be continuously monitored from the start of exploitation, as environmental impacts might only be observable over the long term43. Finally, in addition to monitoring by mine operators themselves, more measurements should be performed by independent experts or national authorities, a fundamental safeguard that is often not in place in South America170. Although these suggestions have been drafted with evaporitic technology in mind, they should hold for the application of any DLE technology, which will still consume brine and fresh water and produce residues. However, DLE will likely consume brine and fresh water in considerably less volumes than the evaporitic technology.
References
Trahey, L. et al. Energy storage emerging: a perspective from the Joint Center for Energy Storage Research. Proc. Natl Acad. Sci. USA 117, 12550–12557 (2020).
Koohi-Fayegh, S. & Rosen, M. A. A review of energy storage types, applications and recent developments. J. Energy Storage 27, 101047 (2020).
Dehghani-Sanij, A. R., Tharumalingam, E., Dusseault, M. B. & Fraser, R. Study of energy storage systems and environmental challenges of batteries. Renew. Sustain. Energy Rev. 104, 192–208 (2019).
Olivetti, E. A., Ceder, G., Gaustad, G. G. & Fu, X. Lithium-ion battery supply chain considerations: analysis of potential bottlenecks in critical metals. Joule 1, 229–243 (2017).
Global EV Outlook (IEA, 2021); https://www.iea.org/reports/global-ev-outlook-2021.
Tabelin, C. B. et al. Towards a low-carbon society: a review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 163, 106743 (2021).
The role of Critical World Energy Outlook Special Report Minerals in Clean Energy Transitions (IEA, 2022); https://iea.blob.core.windows.net/assets/ffd2a83b-8c30-4e9d-980a-52b6d9a86fdc/TheRoleofCriticalMineralsinCleanEnergyTransitions.pdf.
Xu, C. et al. Future material demand for automotive lithium-based batteries. Commun. Mater. 1, 99 (2020).
Mineral Commodity Summaries. LITHIUM (USGS, 2021); https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-lithium.pdf.
Kesler, S. E. et al. Global lithium resources: relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 48, 55–69 (2012).
Alessia, A., Alessandro, B., Maria, V. G., Carlos, V. A. & Francesca, B. Challenges for sustainable lithium supply: a critical review. J. Clean. Prod. 300, 126954 (2021).
Tadesse, B., Makuei, F., Albijanic, B. & Dyer, L. The beneficiation of lithium minerals from hard rock ores: a review. Miner. Eng. 131, 170–184 (2019).
Vikström, H., Davidsson, S. & Höök, M. Lithium availability and future production outlooks. Appl. Energy 110, 252–266 (2013).
Sanjuan, B. et al. Major geochemical characteristics of geothermal brines from the Upper Rhine Graben granitic basement with constraints on temperature and circulation. Chem. Geol. 428, 27–47 (2016).
Sanjuan, B. et al. Lithium-rich geothermal brines in Europe: an up-date about geochemical characteristics and implications for potential Li resources. Geothermics 101, 102385 (2022).
Stringfellow, W. T. & Dobson, P. F. Technology for the recovery of lithium from geothermal brines. Energies 14, 6805 (2021).
Dugamin, E. J. M. et al. Groundwater in sedimentary basins as potential lithium resource: a global prospective study. Sci. Rep. 11, 21091 (2021).
Flexer, V., Baspineiro, C. F. & Galli, C. I. Lithium recovery from brines: a vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total Environ. 639,, 1188–1204 (2018).
Garrett, D. E. Handbook of Lithium and Natural Calcium Chloride https://doi.org/10.1016/B978-0-12-276152-2.X5035-X (2004).
Mudd, G. M. Sustainable/responsible mining and ethical issues related to the Sustainable Development Goals. Geol. Soc. London, Spec. Publ. 508, 187 LP–199 (2021).
Jerez, B., Garcés, I. & Torres, R. Lithium extractivism and water injustices in the Salar de Atacama, Chile: the colonial shadow of green electromobility. Polit. Geogr. 87, 102382 (2021).
Alam, M. A. & Sepúlveda, R. Environmental degradation through mining for energy resources: the case of the shrinking Laguna Santa Rosa wetland in the Atacama Region of Chile. Energy Geosci. 3, 182–190 (2022).
Hailes, O. Lithium in international law: trade, investment, and the pursuit of supply chain justice. J. Int. Econ. Law 25, 148–170 (2022).
Bustos-Gallardo, B., Bridge, G. & Prieto, M. Harvesting lithium: water, brine and the industrial dynamics of production in the Salar de Atacama. Geoforum 119, 177–189 (2021).
Agusdinata, D. B., Liu, W., Eakin, H. & Romero, H. Socio-environmental impacts of lithium mineral extraction: towards a research agenda. Environ. Res. Lett. 13, 123001 (2018).
Liu, W. & Agusdinata, D. B. Interdependencies of lithium mining and communities sustainability in Salar de Atacama, Chile. J. Clean. Prod. 260, 120838 (2020).
Ellingsen, L. A. W., Singh, B. & Strømman, A. H. The size and range effect: lifecycle greenhouse gas emissions of electric vehicles. Environ. Res. Lett. 11, 054010 (2016).
Ambrose, H. & Kendall, A. Understanding the future of lithium: part 2, temporally and spatially resolved life-cycle assessment modeling. J. Ind. Ecol. 24, 90–100 (2020).
Porzio, J. & Scown, C. D. Life-cycle assessment considerations for batteries and battery materials. Adv. Energy Mater. 11, 2100771 (2021).
Pell, R. et al. Towards sustainable extraction of technology materials through integrated approaches. Nat. Rev. Earth Environ. 2, 665–679 (2021).
Stamp, A., Lang, D. J. & Wäger, P. A. Environmental impacts of a transition toward e-mobility: the present and future role of lithium carbonate production. J. Clean. Prod. 23, 104–112 (2012).
Ejeian, M., Grant, A., Shon, H. K. & Razmjou, A. Is lithium brine water? Desalination 518, 115169 (2021). Discussion of why brine water should be considered in environmental assessments.
Marazuela, M. A., Vázquez-Suñé, E., Ayora, C. & García-Gil, A. Towards more sustainable brine extraction in salt flats: learning from the Salar de Atacama. Sci. Total. Environ. 703, 135605 (2020). Conceptual hydrogeological modelling, calibrated with field data, proposing clever strategies to minimize water impacts during brine pumping.
Marazuela, M. A., Vázquez-Suñé, E., Ayora, C., García-Gil, A. & Palma, T. Hydrodynamics of salt flat basins: the Salar de Atacama example. Sci. Total. Environ. 651, 668–683 (2019).
Marazuela, M. A., Vázquez-Suñé, E., Ayora, C., García-Gil, A. & Palma, T. The effect of brine pumping on the natural hydrodynamics of the Salar de Atacama: the damping capacity of salt flats. Sci. Total. Environ. 654, 1118–1131 (2019).
Houston, J., Butcher, A., Ehren, P., Evans, K. & Godfrey, L. The evaluation of brine prospects and the requirement for modifications to filing standards. Econ. Geol. 106, 1125–1239 (2011). Conceptual hydrogeological modelling about brine pumping and freshwater recharge.
Liu, W., Agusdinata, D. B. & Myint, S. W. Spatiotemporal patterns of lithium mining and environmental degradation in the Atacama Salt Flat, Chile. Int. J. Appl. Earth Obs. Geoinf. 80, 145–156 (2019). Pioneering publication with solid data highlighting the environmental impacts related to lithium mining from continental brines.
Gutierrez, J. S. et al. Climate change and lithium mining influence flamingo abundance in the Lithium Triangle. Proc. R. Soc. B Biol. Sci. 289, 20212388 (2022).
Marazuela, M. A. et al. 3D mapping, hydrodynamics and modelling of the freshwater-brine mixing zone in salt flats similar to the Salar de Atacama (Chile). J. Hydrol. 561, 223–235 (2018).
Rosen, M. R. The importance of groundwater in playas: a review of playa classifications and the sedimentology and hydrology of playas. in Paleoclimate and Basin Evolution of Playa Systems Vol. 289 (ed. Rosen, M. R.) (Geological Society of America, 1994).
Currey, D. R. & Sack, D. Hemiarid Lake Basins: Hydrographic Patterns BT — Geomorphology of Desert Environments (eds Parsons, A. J. & Abrahams, A. D.) 471–487 (Springer Netherlands, 2009). https://doi.org/10.1007/978-1-4020-5719-9_15.
Marconi, P., Arengo, F. & Clark, A. The arid Andean plateau waterscapes and the Lithium Triangle: flamingos as flagships for conservation of high-altitude wetlands under pressure from mining development. Wetl. Ecol. Manag. https://doi.org/10.1007/s11273-022-09872-6 (2022).
Gajardo, G. & Redón, S. Andean hypersaline lakes in the Atacama Desert, northern Chile: between lithium exploitation and unique biodiversity conservation. Conserv. Sci. Pract. 1, e94 (2019).
Boualleg, M. & Burdet, F. A. P. Method of preparing an adsorbent material shaped in the absence of binder and method of extracting lithium from saline solutions using said material. US patent WO/097202 Al. (2015).
Chen, S., Zhang, Q., Andrews-Speed, P. & Mclellan, B. Quantitative assessment of the environmental risks of geothermal energy: a review. J. Environ. Manage. 276, 111287 (2020).
Megalooikonomou, K. G., Parolai, S. & Pittore, M. Toward performance-driven seismic risk monitoring for geothermal platforms: development of ad hoc fragility curves. Geotherm. Energy 6, 8 (2018).
Bosia, C., Mouchot, J., Ravier, G., Seibel, O. & Genter, A. Evolution of brine geochemical composition during operation of EGS geothermal plants (Alsace, France). In Proc. 46th Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, February 15–17, 2021 SGP-TR-218 (2021).
Tyszer, M., Tomaszewska, B. & Kabay, N. Desalination of geothermal wastewaters by membrane processes: strategies for environmentally friendly use of retentate streams. Desalination 520, 115330 (2021).
Chagnes, A. & Światowska, J. Lithium Process Chemistry: Resources, Extraction, Batteries and Recycling (Elsevier, 2015).
Khalil, A., Mohammed, S., Hashaikeh, R. & Hilal, N. Lithium recovery from brine: recent developments and challenges. Desalination 528, 115611 (2022).
Meng, Z. et al. Highly flexible interconnected Li+ ion-sieve porous hydrogels with self-regulating nanonetwork structure for marine lithium recovery. Chem. Eng. J. 445, 136780 (2022).
Marthi, R. & Smith, Y. R. Application and limitations of a H2TiO3 — diatomaceous earth composite synthesized from titania slag as a selective lithium adsorbent. Sep. Purif. Technol. 254, 117580 (2021).
Li, X. et al. Amorphous TiO2-derived large-capacity lithium ion sieve for lithium recovery. Chem. Eng. Technol. 43, 1784–1791 (2020).
Zhou, Z. et al. Recovery of lithium from salt-lake brines using solvent extraction with TBP as extractant and FeCl3 as co-extraction agent. Hydrometallurgy 191, 105244 (2020).
Song, J., Huang, T., Qiu, H., Li, X. M. & He, T. Recovery of lithium from salt lake brine of high Mg/Li ratio using Na[FeCl4 ∗ 2TBP] as extractant: thermodynamics, kinetics and processes. Hydrometallurgy 173, 63–70 (2017).
Li, R. et al. Novel ionic liquid as co-extractant for selective extraction of lithium ions from salt lake brines with high Mg/Li ratio. Sep. Purif. Technol. 277, 119471 (2021).
Shi, C. et al. Solvent extraction of lithium from aqueous solution using non-fluorinated functionalized ionic liquids as extraction agents. Sep. Purif. Technol. 172, 473–479 (2017).
Gmar, S. & Chagnes, A. Recent advances on electrodialysis for the recovery of lithium from primary and secondary resources. Hydrometallurgy 189, 105124 (2019).
Li, X. et al. Membrane-based technologies for lithium recovery from water lithium resources: a review. J. Memb. Sci. 591, 117317 (2019).
Zhao, Z., Liu, G., Jia, H. & He, L. Sandwiched liquid-membrane electrodialysis: lithium selective recovery from salt lake brines with high Mg/Li ratio. J. Memb. Sci. 596, 117685 (2020).
Li, Q. et al. Efficiently rejecting and concentrating Li+ by nanofiltration membrane under a reversed electric field. Desalination 535, 115825 (2022).
Li, Y., Zhao, Y. J., Wang, H. & Wang, M. The application of nanofiltration membrane for recovering lithium from salt lake brine. Desalination 468, 114081 (2019).
He, R. et al. Unprecedented Mg2+/Li+ separation using layer-by-layer based nanofiltration hollow fiber membranes. Desalination 525, 115492 (2022).
Calvo, E. J. Electrochemical methods for sustainable recovery of lithium from natural brines and battery recycling. Curr. Opin. Electrochem. 15, 102–108 (2019).
Battistel, A., Palagonia, M. S., Brogioli, D., La Mantia, F. & Trócoli, R. Electrochemical methods for lithium recovery: a comprehensive and critical review. Adv. Mater. 32, 1905440 (2020). Critical review about electrochemical ion pumping technologies, with suggestions about which experimental parameters need to be assessed. Not all electrochemical technologies are reviewed.
Calvo, E. J. Direct lithium recovery from aqueous electrolytes with electrochemical ion pumping and lithium intercalation. ACS Omega 6, 35213–35220 (2021).
He, L. et al. New insights into the application of lithium-ion battery materials: selective extraction of lithium from brines via a rocking-chair lithium-ion battery system. Glob. Chall. 2, 1700079 (2018).
Liu, D., Xu, W., Xiong, J., He, L. & Zhao, Z. Electrochemical system with LiMn2O4 porous electrode for lithium recovery and its kinetics. Sep. Purif. Technol. 270, 118809 (2021).
Liu, D., Zhao, Z., Xu, W., Xiong, J. & He, L. A closed-loop process for selective lithium recovery from brines via electrochemical and precipitation. Desalination 519, 115302 (2021).
Liu, D., Li, Z., He, L. & Zhao, Z. Facet engineered Li3PO4 for lithium recovery from brines. Desalination 514, 115186 (2021).
Lai, X., Xiong, P. & Zhong, H. Extraction of lithium from brines with high Mg/Li ratio by the crystallization–precipitation method. Hydrometallurgy 192, 105252 (2020).
Mendieta–George, D., Pérez–Garibay, R., Solís–Rodríguez, R., Fuentes–Aceituno, J. C. & Alvarado–Gómez, A. Study of the direct production of lithium phosphate with pure synthetic solutions and membrane electrolysis. Miner. Eng. 185, 107713 (2022).
Cerda, A. et al. Recovering water from lithium-rich brines by a fractionation process based on membrane distillation–crystallization. J. Water Process. Eng 41, 102063 (2021). Membrane distillation-based DLE proposal to recover freshwater during brine concentration from high-salinity brines.
Quist-Jensen, C. A., Ali, A., Mondal, S., Macedonio, F. & Drioli, E. A study of membrane distillation and crystallization for lithium recovery from high-concentrated aqueous solutions. J. Memb. Sci. 505, 167–173 (2016).
Zhou, G. et al. Progress in electrochemical lithium ion pumping for lithium recovery. J. Energy Chem. 59, 431–445 (2021).
Xu, P. et al. Materials for lithium recovery from salt lake brine. J. Mater. Sci. 56, 16–63 (2021).
Wang, J. et al. Electrochemical technologies for lithium recovery from liquid resources: a review. Renew. Sustain. Energy Rev. 154, 111813 (2022).
Sun, Y., Wang, Q., Wang, Y., Yun, R. & Xiang, X. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine. Sep. Purif. Technol. 256, 117807 (2021).
Nie, X.-Y., Sun, S.-Y., Sun, Z., Song, X. & Yu, J.-G. Ion-fractionation of lithium ions from magnesium ions by electrodialysis using monovalent selective ion-exchange membranes. Desalination 403, 128–135 (2017).
Ding, D., Yaroshchuk, A. & Bruening, M. L. Electrodialysis through nafion membranes coated with polyelectrolyte multilayers yields >99% pure monovalent ions at high recoveries. J. Memb. Sci. 647, 120294 (2022).
Li, Q. et al. Ultrahigh-efficient separation of Mg2+/Li+ using an in-situ reconstructed positively charged nanofiltration membrane under an electric field. J. Memb. Sci. 641, 119880 (2022).
Qiu, Y. et al. Integration of selectrodialysis and selectrodialysis with bipolar membrane to salt lake treatment for the production of lithium hydroxide. Desalination 465, 1–12 (2019).
Palagonia, M. S., Brogioli, D. & La Mantia, F. Lithium recovery from diluted brine by means of electrochemical ion exchange in a flow-through-electrodes cell. Desalination 475, 114192 (2020).
Palagonia, M. S., Brogioli, D. & Mantia, F. L. Effect of current density and mass loading on the performance of a flow-through electrodes cell for lithium recovery. J. Electrochem. Soc. 166, E286–E292 (2019).
Guo, Z.-Y. et al. Prefractionation of LiCl from concentrated seawater/salt lake brines by electrodialysis with monovalent selective ion exchange membranes. J. Clean. Prod. 193, 338–350 (2018).
Zhao, L.-M. et al. Separating and recovering lithium from brines using selective-electrodialysis: sensitivity to temperature. Chem. Eng. Res. Des. 140, 116–127 (2018).
Sharma, P. P. et al. Sulfonated poly (ether ether ketone) composite cation exchange membrane for selective recovery of lithium by electrodialysis. Desalination 496, 114755 (2020).
Chen, Q.-B. et al. Development of recovering lithium from brines by selective-electrodialysis: effect of coexisting cations on the migration of lithium. J. Memb. Sci. 548, 408–420 (2018).
Díaz Nieto, C. H. & Flexer, V. Is it possible to recover lithium compounds from complex brines employing electromembrane processes exclusively? Curr. Opin. Electrochem. 35, 101087 (2022).
Li, X. et al. Highly selective separation of lithium with hierarchical porous lithium-ion sieve microsphere derived from MXene. Desalination 537, 115847 (2022).
Parker SS, et al. Potential lithium extraction in the United States: environmental, economic, and policy implications (The Nature Conservancy, 2022); https://www.scienceforconservation.org/assets/downloads/Lithium_Report_FINAL.pdf.
Arkansas Smackover Project. Standard Lithium https://www.standardlithium.com/projects/arkansas-smackover (2022).
Grant, A. Re-injection enhanced production for direct lithium extraction (DLE) projects. https://www.jadecove.com/research/brinereinjection.
Horne, R. N. Geothermal reinjection experience in Japan. J. Pet. Technol. 34, 495–503 (1982).
Boo, C., Billinge, I. H., Chen, X., Shah, K. M. & Yin Yip, N. Zero liquid discharge of ultrahigh-salinity brines with temperature swing solvent extraction. Environ. Sci. Technol. 54, 9124–9131 (2020).
Deshmukh, A. et al. Thermodynamics of solvent-driven water extraction from hypersaline brines using dimethyl ether. Chem. Eng. J. 434, 134391 (2022).
Panagopoulos, A. Brine management (saline water & wastewater effluents): sustainable utilization and resource recovery strategy through minimal and zero liquid discharge (MLD & ZLD) desalination systems. Chem. Eng. Process. Process Intensif. 176, 108944 (2022).
Al-Ghouti, M. A., Al-Kaabi, M. A., Ashfaq, M. Y. & Da’na, D. A. Produced water characteristics, treatment and reuse: a review. J. Water Process Eng. 28, 222–239 (2019).
Samuel, O. et al. Oilfield-produced water treatment using conventional and membrane-based technologies for beneficial reuse: a critical review. J. Environ. Manag. 308, 114556 (2022).
Arena, J. T. et al. Management and dewatering of brines extracted from geologic carbon storage sites. Int. J. Greenh. Gas Control 63, 194–214 (2017).
Ogden, D. D. & Trembly, J. P. Desalination of hypersaline brines via Joule-heating: experimental investigations and comparison of results to existing models. Desalination 424, 149–158 (2017).
Kaplan, R., Mamrosh, D., Salih, H. H. & Dastgheib, S. A. Assessment of desalination technologies for treatment of a highly saline brine from a potential CO2 storage site. Desalination 404, 87–101 (2017).
Baspineiro, C. F., Franco, J. & Flexer, V. Potential water recovery during lithium mining from high salinity brines. Sci. Total Environ. 720, 137523 (2020).
Sustainable production. SQM https://www.sqmlithium.com/en/nosotros/produccion-sustentable/ (2022).
Sustainability Report (Orocobre, 2021); https://www.orocobre.com/wp/?mdocs-file=7259.
Grant, A. From Catamarca to Qinghai: the Commercial Scale Direct Lithium Extraction Operations. Jadecove https://www.jadecove.com/research/fromcatamarcatoqinghai (2020).
Sustainability report (Livent, 2021); https://livent.com/wp-content/uploads/2022/07/Livent_2021SustainabilityReport-English.pdf.
Park, S. H. et al. Lithium recovery from artificial brine using energy-efficient membrane distillation and nanofiltration. J. Memb. Sci. 598, 117683 (2020). Pioneering DLE proposal to recover freshwater during brine concentration via nanofiltration followed by membrane distillation.
Pramanik, B. K., Asif, M. B., Kentish, S., Nghiem, L. D. & Hai, F. I. Lithium enrichment from a simulated salt lake brine using an integrated nanofiltration-membrane distillation process. J. Environ. Chem. Eng. 7, 103395 (2019).
Ko, C.-C. et al. Performance of ceramic membrane in vacuum membrane distillation and in vacuum membrane crystallization. Desalination 440, 48–58 (2018).
Baspineiro, C. F., Franco, J. & Flexer, V. Performance of a double-slope solar still for the concentration of lithium rich brines with concomitant fresh water recovery. Sci. Total. Environ. 791, 148192 (2021).
Ling, Z. et al. Desalination and Li+ enrichment via formation of cyclopentane hydrate. Sep. Purif. Technol. 231, 115921 (2020).
Niu, J. et al. An electrically switched ion exchange system with self-electrical-energy recuperation for efficient and selective LiCl separation from brine lakes. Sep. Purif. Technol. 274, 118995 (2021).
Nie, X.-Y., Sun, S.-Y., Song, X. & Yu, J.-G. Further investigation into lithium recovery from salt lake brines with different feed characteristics by electrodialysis. J. Memb. Sci. 530, 185–191 (2017).
Jiang, C., Wang, Y., Wang, Q., Feng, H. & Xu, T. Production of lithium hydroxide from lake brines through electro-electrodialysis with bipolar membranes (EEDBM). Ind. Eng. Chem. Res. 53, 6103–6112 (2014).
Díaz Nieto, C. H., Rabaey, K. & Flexer, V. Membrane electrolysis for the removal of Na+ from brines for the subsequent recovery of lithium salts. Sep. Purif. Technol. 252, 117410 (2020).
Díaz Nieto, C. H. et al. Membrane electrolysis for the removal of Mg2+ and Ca2+ from lithium rich brines. Water Res. 154, 117–124 (2019).
Ji, P.-Y. et al. Effect of coexisting ions on recovering lithium from high Mg2+/Li+ ratio brines by selective-electrodialysis. Sep. Purif. Technol. 207, 1–11 (2018).
Lee, D.-H. et al. Selective lithium recovery from aqueous solution using a modified membrane capacitive deionization system. Hydrometallurgy 173, 283–288 (2017).
Díaz Nieto, C. H., Kortsarz, J. A., Vera, M. L. & Flexer, V. Effect of temperature, current density and mass transport during the electrolytic removal of magnesium ions from lithium rich brines. Desalination 529, 115652 (2022).
Li, X. et al. Taming wettability of lithium ion sieve via different TiO2 precursors for effective Li recovery from aqueous lithium resources. Chem. Eng. J. 392, 123731 (2020).
Sun, J. et al. Preparation of high hydrophilic H2TiO3 ion sieve for lithium recovery from liquid lithium resources. Chem. Eng. J. 453, 139485 (2023).
Qian, F. et al. Trace doping by fluoride and sulfur to enhance adsorption capacity of manganese oxides for lithium recovery. Mater. Des. 194, 108867 (2020).
Taghvaei, N., Taghvaei, E. & Askari, M. Synthesis of anodized TiO2 nanotube arrays as ion sieve for lithium extraction. ChemistrySelect 5, 10339–10345 (2020).
Qian, F. et al. Highly lithium adsorption capacities of H1.6Mn1.6O4 ion-sieve by ordered array structure. ChemistrySelect 4, 10157–10163 (2019).
Sarmiento, N. et al. A solar irradiation GIS as decision support tool for the Province of Salta, Argentina. Renew. Energy 132, 68–80 (2019).
Dellicompagni, P., Franco, J. & Flexer, V. CO2 emission reduction by integrating concentrating solar power into lithium mining. Energy Fuels 35, 15879–15893 (2021).
Zavahir, S. et al. A review on lithium recovery using electrochemical capturing systems. Desalination 500, 114883 (2021).
Joo, H. et al. Pilot-scale demonstration of an electrochemical system for lithium recovery from the desalination concentrate. Environ. Sci. Water Res. Technol. 6, 290–295 (2020). Pilot-scale demonstration of processing 6 tonnes of brine daily using electrochemical ion pumping.
Song, J. F., Nghiem, L. D., Li, X.-M. & He, T. Lithium extraction from Chinese salt-lake brines: opportunities, challenges, and future outlook. Environ. Sci. Water Res. Technol. 3, 593–597 (2017). Thorough review of pilot-scale projects for lithium mining from brines in China.
Yu, C. et al. Bio-inspired fabrication of ester-functionalized imprinted composite membrane for rapid and high-efficient recovery of lithium ion from seawater. J. Colloid Interf. Sci. 572, 340–353 (2020).
Lu, J. et al. Multilayered ion-imprinted membranes with high selectivity towards Li+ based on the synergistic effect of 12-crown-4 and polyether sulfone. Appl. Surf. Sci. 427, 931–941 (2018).
Ryu, T. et al. Lithium recovery system using electrostatic field assistance. Hydrometallurgy 151, 78–83 (2015).
Sun, Y., Wang, Y., Liu, Y. & Xiang, X. Highly efficient lithium extraction from brine with a high sodium content by adsorption-coupled electrochemical technology. ACS Sustain. Chem. Eng. 9, 11022–11031 (2021).
Torres, W. R., Díaz Nieto, C. H., Prévoteau, A., Rabaey, K. & Flexer, V. Lithium carbonate recovery from brines using membrane electrolysis. J. Memb. Sci. 615, 118416 (2020). A DLE methodology with three consecutive electromembrane processes for the sequential recovery of magnesium, calcium and sodium by-products, together with lithium carbonate and concomitant fresh-water production in a circular economy framework.
Du, X. et al. A novel electroactive λ-MnO2/PPy/PSS core–shell nanorod coated electrode for selective recovery of lithium ions at low concentration. J. Mater. Chem. A 4, 13989–13996 (2016).
Luo, G. et al. Electrochemical lithium ions pump for lithium recovery from brine by using a surface stability Al2O3–ZrO2 coated LiMn2O4 electrode. J. Energy Chem. 69, 244–252 (2022).
Oyarce, E., Roa, K., Boulett, A., Salazar-Marconi, P. & Sánchez, J. Removal of lithium ions from aqueous solutions by an ultrafiltration membrane coupled to soluble functional polymer. Sep. Purif. Technol. 288, 120715 (2022).
Han, H. J., Qu, W., Zhang, Y. L., Lu, H. D. & Zhang, C. L. Enhanced performance of Li+ adsorption for H1.6Mn1.6O4 ion-sieves modified by Co doping and micro array morphology. Ceram. Int. 47, 21777–21784 (2021).
Zhu, X. et al. Study on adsorption extraction process of lithium ion from West Taijinar brine by shaped titanium-based lithium ion sieves. Sep. Purif. Technol. 274, 119099 (2021).
Meng, Z. et al. Highly flexible interconnected Li+ion-sieve porous hydrogels with self-regulating nanonetwork structure for marine lithium recovery. Chem. Eng. J. 445, 136780 (2022).
Xiong, J., Zhao, Z., Liu, D. & He, L. Direct lithium extraction from raw brine by chemical redox method with LiFePO4/FePO4 materials. Sep. Purif. Technol. 290, 120789 (2022).
Vera, M. L. et al. A strategy to avoid solid formation within the reactor during magnesium and calcium electrolytic removal from lithium-rich brines. J. Solid State Electrochem. https://doi.org/10.1007/s10008-022-05219-6 (2022).
Lide, D. R. CRC Handbook of Chemistry and Physics (CRC Press. Boca Raton, 2005).
Neumann, J. et al. Recycling of lithium-ion batteries — current state of the art, circular economy, and next generation recycling. Adv. Energy Mater. 12, 2102917 (2022).
Amici, J. et al. A roadmap for transforming research to invent the batteries of the future designed within the European large scale research initiative BATTERY 2030+. Adv. Energy Mater. 12, 2102785 (2022).
Graedel, T. E. et al. What do we know about metal recycling rates? J. Ind. Ecol. 15, 355–366 (2011).
Kinnunen, P. H.-M. & Kaksonen, A. H. Towards circular economy in mining: opportunities and bottlenecks for tailings valorization. J. Clean. Prod. 228, 153–160 (2019).
Falagán, C., Grail, B. M. & Johnson, D. B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 106, 71–78 (2017).
Nwaila, G. T. et al. Valorisation of mine waste — part I: characteristics of, and sampling methodology for, consolidated mineralised tailings by using Witwatersrand gold mines (South Africa) as an example. J. Environ. Manage. 295, 113013 (2021).
Singh, S., Sukla, L. B. & Goyal, S. K. Mine waste & circular economy. Mater. Today Proc. 30, 332–339 (2020).
Purnell, P., Velenturf, A. P. M. & Marshall, R. New Governance for Circular Economy: Policy, Regulation and Market Contexts for Resource Recovery from Waste. (eds. Macaskie, L. E. et al.) Ch. 16 (Royal Society of Chemistry, 2019).
Velenturf, A. P. M. et al. Circular economy and the matter of integrated resources. Sci. Total Environ. 689, 963–969 (2019).
Yang, Y. et al. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloy. 9, 705–747 (2021).
Løvik, A. N., Hagelüken, C. & Wäger, P. Improving supply security of critical metals: current developments and research in the EU. Sustain. Mater. Technol. 15, 9–18 (2018).
Potash. Albemarle https://www.albemarle.com/businesses/lithium/products/pot-ash (2023).
Production processes. SQM https://www.sqm.com/en/acerca-de-sqm/recursos-naturales/proceso-de-produccion/ (2018).
Thiel, G. P. & Lienhard V, J. H. Treating produced water from hydraulic fracturing: composition effects on scale formation and desalination system selection. Desalination 346, 54–69 (2014).
Shaffer, D. L. et al. Desalination and reuse of high-salinity shale gas produced water: drivers, technologies, and future directions. Environ. Sci. Technol. 47, 9569–9583 (2013).
Ogunbiyi, O. et al. Sustainable brine management from the perspectives of water, energy and mineral recovery: a comprehensive review. Desalination 513, 115055 (2021).
Kumar, A. et al. Metals recovery from seawater desalination brines: technologies, opportunities, and challenges. ACS Sustain. Chem. Eng. 9, 7704–7712 (2021).
Snæbjörnsdóttir, S. Ó. et al. Carbon dioxide storage through mineral carbonation. Nat. Rev. Earth Environ. 1, 90–102 (2020).
Saccò, M. et al. Salt to conserve: a review on the ecology and preservation of hypersaline ecosystems. Biol. Rev. 96, 2828–2850 (2021).
Chiappero, M. F., Vaieretti, M. V. & Izquierdo, A. E. A baseline soil survey of two peatlands associated with a lithium-rich salt flat in the argentine puna: physico-chemical characteristics, carbon storage and biota. Mires Peat 27, 16 (2021).
Batanero, G. L. et al. Flamingos and drought as drivers of nutrients and microbial dynamics in a saline lake. Sci. Rep. 7, 12173 (2017).
Avila-Arias, H., Nies, L. F., Gray, M. B. & Turco, R. F. Impacts of molybdenum-, nickel-, and lithium-oxide nanomaterials on soil activity and microbial community structure. Sci. Total. Environ. 652, 202–211 (2019).
Robinson, B. H., Yalamanchali, R., Reiser, R. & Dickinson, N. M. Lithium as an emerging environmental contaminant: mobility in the soil–plant system. Chemosphere 197, 1–6 (2018).
Bolan, N. et al. From mine to mind and mobiles — lithium contamination and its risk management. Environ. Pollut. 290, 118067 (2021).
Shokri-Kuehni, S. M. S., Norouzi Rad, M., Webb, C. & Shokri, N. Impact of type of salt and ambient conditions on saline water evaporation from porous media. Adv. Water Resour. 105, 154–161 (2017).
Obaya, M., López, A. & Pascuini, P. Curb your enthusiasm. Challenges to the development of lithium-based linkages in Argentina. Resour. Policy 70, 101912 (2021).
Risacher, F., Alonso, H. & Salazar, C. The origin of brines and salts in Chilean salars: a hydrochemical review. Earth-Sci. Rev. 63, 249–293 (2003).
Corenthal, L. G., Boutt, D. F., Hynek, S. A. & Munk, L. A. Regional groundwater flow and accumulation of a massive evaporite deposit at the margin of the Chilean Altiplano. Geophys. Res. Lett. 43, 8017–8025 (2016).
Vásquez, C., Ortiz, C., Suárez, F. & Muñoz, J. F. Modeling flow and reactive transport to explain mineral zoning in the Atacama salt flat aquifer, Chile. J. Hydrol. 490, 114–125 (2013).
Acknowledgements
M.L.V. acknowledges a post-doctoral fellowship from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). W.R.T., C.I.G. and V.F. are CONICET permanent research fellows. This work was supported by Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (ANPCyT), AR (grant number PICT 2019–1939).
Author information
Authors and Affiliations
Contributions
M.L.V., W.R.T., A.C. and V.F. researched data for the article. M.L.V., W.R.T. and V.F. contributed substantially to discussion of the content. All authors wrote the article. V.F. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Earth & Environment thanks D. Alessi, W. Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Brine
-
Aqueous solutions of extremely high ionic strength, with total dissolved solids values of 100–400 g l−1, most solids are inorganic salts.
- Circular economy
-
A model of production and consumption. Following the European Union definition, it involves sharing, leasing, reusing, repairing, refurbishing and recycling existing materials and products as much as possible.
- Continental brines
-
Continental brines are found in underground reservoirs within salars, typically in locations with arid climates.
- Fresh water
-
Low salinity water, typically <3 g l−1 TDS, although this cut-off value varies.
- Life-cycle analysis
-
A quantitative methodology implemented to evaluate the environmental impact of a given product through its entire life cycle, from extraction and processing of raw materials, manufacturing, distribution, use, potential recycling and final disposal.
- Lithium Triangle
-
A region encompassing northwest Argentina, southwest Bolivia and northern Chile, where a large concentration of lithium-rich deposits is found.
- Native brines
-
Real brine samples, continental or geothermal brines as they are pumped from underground reservoirs, before undergoing any processing or chemical treatment.
- Oilfield brines
-
Brines that are found during deep rock penetration by drilling during oil and gas extraction and considered as waste by these industries.
- Phreatic evaporation
-
Refers to evaporation of shallow groundwater into the atmosphere directly from the soil through the porous ground surface.
- Salar
-
Salars (Spanish term for salt lake or salt flat) are endorheic sedimentary basins containing thick sequences of continental evaporites and clastic deposits.
- Salar de Atacama
-
The third largest salar in the Lithium Triangle, located in northern Chile; the two largest facilities for lithium mining from brines are located here.
- Spent brines
-
Brine that has undergone processing via some direct lithium extraction technology; with Li+ concentration largely depleted, but concentrations from other species similar to native brine.
- Total dissolved solids
-
(TDS). The sum, by mass, of all solids dissolved in an aqueous solution, irrespective of their chemical formulae.
- Water table
-
Surface below which water (or brine) fills any spaces between sediments or rocks. At the water table level, water and atmospheric pressure values are equal.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vera, M.L., Torres, W.R., Galli, C.I. et al. Environmental impact of direct lithium extraction from brines. Nat Rev Earth Environ 4, 149–165 (2023). https://doi.org/10.1038/s43017-022-00387-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43017-022-00387-5
This article is cited by
-
Precision ion separation via self-assembled channels
Nature Communications (2024)
-
Conversion of Lithium Chloride into Lithium Hydroxide Using a Two-Step Solvent Extraction Process in an Agitated Kühni Column
Journal of Sustainable Metallurgy (2024)
-
Quaternization-spiro design of chlorine-resistant and high-permeance lithium separation membranes
Nature Communications (2023)