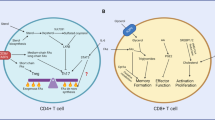
Abstract
The broad effectiveness of T cell-based therapy for treating solid tumour cancers remains limited. This is partly due to the growing appreciation that immune cells must inhabit and traverse a metabolically demanding tumour environment. Accordingly, recent efforts have centred on using genome-editing technologies to augment T cell-mediated cytotoxicity by manipulating specific metabolic genes. However, solid tumours exhibit numerous characteristics restricting immune cell-mediated cytotoxicity, implying a need for metabolic engineering at the pathway level rather than single gene targets. This emerging concept has yet to be put into clinical practice as many questions concerning the complex interplay between metabolic networks and T cell function remain unsolved. This Perspective will highlight key foundational studies that examine the relevant metabolic pathways required for effective T cell cytotoxicity and persistence in the human tumour microenvironment, feasible strategies for metabolic engineering to increase the efficiency of chimeric antigen receptor T cell-based approaches, and the challenges lying ahead for clinical implementation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ovarian Tumor Tissue Analysis (OTTA) Consortium. et al. Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol. 3, e173290 (2017).
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44, 380–390 (2016).
Chang, C.-H. & Pearce, E. L. Emerging concepts of T cell metabolism as a target of immunotherapy. Nat. Immunol. 17, 364–368 (2016).
Renauer, P. et al. Immunogenetic metabolomics reveals key enzymes that modulate CAR T-cell metabolism and function. Cancer Immunol. Res. 11, 1068–1084 (2023).
Harada, S. et al. Intercellular mitochondrial transfer enhances metabolic fitness and anti-tumor effects of CAR T cells. Blood 140, 2356–2357 (2022).
Kishton, R. J., Sukumar, M. & Restifo, N. P. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 26, 94–109 (2017).
Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021).
Priceman, S. J. et al. Regional delivery of chimeric antigen receptor–engineered T cells effectively targets HER2+ breast cancer metastasis to the brain. Clin. Cancer Res. 24, 95–105 (2018).
Rangel Rivera, G. O. et al. Fundamentals of T cell metabolism and strategies to enhance cancer immunotherapy. Front. Immunol. 12, 645242 (2021).
Wofford, J. A., Wieman, H. L., Jacobs, S. R., Zhao, Y. & Rathmell, J. C. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 111, 2101–2111 (2008).
Bental, M. & Deutsch, C. Metabolic changes in activated T cells: an NMR study of human peripheral blood lymphocytes. Magn. Reson. Med. 29, 317–326 (1993).
Krishna, S. et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science 370, 1328–1334 (2020).
Gubser, P. M. et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat. Immunol. 14, 1064–1072 (2013).
Chang, C. -H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Wellen, K. E. et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009).
Yaqoob, P. & Calder, P. C. Glutamine requirement of proliferating T lymphocytes. Nutrition 13, 646–651 (1997).
Madden, M. Z. et al. Differential effects of glutamine inhibition strategies on antitumor CD8 T cells. J. Immunol. 211, 563–575 (2023).
Ma, E. H. et al. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 25, 345–357 (2017).
Sinclair, L. V. et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 14, 500–508 (2013).
Leone, R. D. et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366, 1013–1021 (2019).
Buck, M. D. et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell 166, 63–76 (2016).
Previte, D. M. et al. Reactive oxygen species are required for driving efficient and sustained aerobic glycolysis during CD4+ T cell activation. PLoS ONE 12, e0175549 (2017).
Vardhana, S. A. et al. Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat. Immunol. 21, 1022–1033 (2020).
López-Cantillo, G., Urueña, C., Camacho, B. A. & Ramírez-Segura, C. CAR-T cell performance: how to improve their persistence? Front. Immunol. 13, 878209 (2022).
Klebanoff, C. A. et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl Acad. Sci. USA 102, 9571–9576 (2005).
O’Sullivan, D. et al. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41, 75–88 (2014).
van der Windt, G. J. W. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
van der Windt, G. J. W. & Pearce, E. L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol. Rev. 249, 27–42 (2012).
Cao, K. et al. Mitochondrial dynamics regulate genome stability via control of caspase-dependent DNA damage. Dev. Cell 57, 1211–1225 (2022).
Ganapathy, V., Thangaraju, M. & Prasad, P. D. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 121, 29–40 (2009).
Shi, L. Z. et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 (2011).
Cham, C. M., Driessens, G., O’Keefe, J. P. & Gajewski, T. F. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur. J. Immunol. 38, 2438–2450 (2008).
Rodriguez, P. C. et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64, 5839–5849 (2004).
Geiger, R. et al. l-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842 (2016).
Johnson, M. O. et al. Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell 175, 1780–1795 (2018).
Ye, L. et al. A genome-scale gain-of-function CRISPR screen in CD8 T cells identifies proline metabolism as a means to enhance CAR-T therapy. Cell Metab. 34, 595–614 (2022). Ye et al. demonstrated that overexpression of PRODH2, a critical gene in the proline synthesis pathway, in CAR-T cells enhances their antitumor immunity in a leukaemia murine model.
Li, P. & Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 50, 29–38 (2018).
Wu, G. & Morris, S. M. Jr Arginine metabolism: nitric oxide and beyond. Biochem. J. 336, 1–17 (1998).
Young, A. et al. Co-inhibition of CD73 and A2AR adenosine signaling improves anti-tumor immune responses. Cancer Cell 30, 391–403 (2016).
Munn, D. H. et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 189, 1363–1372 (1999).
Rattigan, Y. I. et al. Lactate is a mediator of metabolic cooperation between stromal carcinoma associated fibroblasts and glycolytic tumor cells in the tumor microenvironment. Exp. Cell. Res. 318, 326–335 (2012).
Kilgour, M. K. et al. 1-Methylnicotinamide is an immune regulatory metabolite in human ovarian cancer. Sci. Adv. 7, eabe1174 (2021). Kilgour et al. showed that an abundance of MNA, a previously unappreciated immunosuppressive metabolite, in human ovarian cancer could suppress CAR-T cell function.
Baumann, T. et al. Regulatory myeloid cells paralyze T cells through cell–cell transfer of the metabolite methylglyoxal. Nat. Immunol. 21, 555–566 (2020).
Bunse, L. et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat. Med. 24, 1192–1203 (2018).
Ohta, A. et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl Acad. Sci. USA 103, 13132–13137 (2006).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
Campesato, L. F. et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by l-kynurenine. Nat. Commun. 11, 4011 (2020).
Liu, Y. et al. Tumor-repopulating cells induce PD-1 expression in CD8+ T cells by transferring kynurenine and AhR activation. Cancer Cell 33, 480–494 (2018).
Fischer, K. et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109, 3812–3819 (2007).
Zhang, Y. et al. Lactate: the mediator of metabolism and immunosuppression. Front. Endocrinol. 13, 901495 (2022).
Sadelain, M., Rivière, I. & Riddell, S. Therapeutic T cell engineering. Nature 545, 423–431 (2017).
Kotowski, M. & Sharma, S. CRISPR-based editing techniques for genetic manipulation of primary T cells. Methods Protoc. 3, 79 (2020).
Rezalotfi, A., Fritz, L., Förster, R. & Bošnjak, B. Challenges of CRISPR-based gene editing in primary T cells. Int. J. Mol. Sci. 23, 1689 (2022).
Nagahama, Y. et al. Regnase-1 controls colon epithelial regeneration via regulation of mTOR and purine metabolism. Proc. Natl Acad. Sci. USA 115, 11036–11041 (2018).
Wei, J. et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature 576, 471–476 (2019).
Mao, R. et al. Regnase-1, a rapid response ribonuclease regulating inflammation and stress responses. Cell. Mol. Immunol. 14, 412–422 (2017).
Mai, D. et al. Combined disruption of T cell inflammatory regulators Regnase-1 and Roquin-1 enhances antitumor activity of engineered human T cells. Proc. Natl Acad. Sci. USA 120, e2218632120 (2023).
Giuffrida, L. et al. CRISPR/Cas9 mediated deletion of the adenosine A2A receptor enhances CAR T cell efficacy. Nat. Commun. 12, 3236 (2021). Giuffrida et al. showed that CRISPR–Cas9-mediated deletion of A2AR, encoding the gene that encodes a human adenosine receptor, in CAR T cells causes resistance to adenosine-mediated transcriptional changes, resulting in enhanced CAR-T cell antitumor immunity.
Decking, S.-M. et al. LDHB overexpression can partially overcome T cell inhibition by lactic acid. Int. J. Mol. Sci. 23, 5970 (2022).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020).
Fultang, L. et al. Metabolic engineering against the arginine microenvironment enhances CAR-T cell proliferation and therapeutic activity. Blood 136, 1155–1160 (2020). Fultang et al. created CAR-T cells resistant to the arginine deplete tumor microenvironment by overexpressing two critical genes in the arginine synthesis pathway.
Hoogstrate, Y. et al. Transcriptome analysis reveals tumor microenvironment changes in glioblastoma. Cancer Cell 41, 678–692 (2023).
Kim, H. et al. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res. 25, 316–327 (2015).
Wang, Q. et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 32, 42–56 (2017).
Somerville, R. P. T. & Dudley, M. E. Bioreactors get personal. Oncoimmunology 1, 1435–1437 (2012).
Weller, M., Cloughesy, T., Perry, J. R. & Wick, W. Standards of care for treatment of recurrent glioblastoma—are we there yet? Neuro. Oncol. 15, 4–27 (2013).
Chamie, K. et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer 119, 3219–3227 (2013).
Giornelli, G. H. Management of relapsed ovarian cancer: a review. Springerplus 5, 1197 (2016).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022).
Halvorson, T., Tuomela, K. & Levings, M. K. Targeting regulatory T cell metabolism in disease: novel therapeutic opportunities. Eur. J. Immunol. 53, e2250002 (2023).
Reina-Campos, M., Scharping, N. E. & Goldrath, A. W. CD8+ T cell metabolism in infection and cancer. Nat. Rev. Immunol. 21, 718–738 (2021).
Crompton, J. G. et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 75, 296–305 (2015).
Wenes, M. et al. The mitochondrial pyruvate carrier regulates memory T cell differentiation and antitumor function. Cell Metab. 34, 731–746 (2022).
Benedetti, E. et al. A multimodal atlas of tumor metabolism reveals the architecture of gene-metabolite co-regulation. Nat. Metab. https://doi.org/10.1101/2022.11.23.517549 (2022).
Hicks, K. G. et al. Protein–metabolite interactomics of carbohydrate metabolism reveal regulation of lactate dehydrogenase. Science 379, 996–1003 (2023). Hicks et al. developed a high-throughput method for profiling protein–metabolite interactions, opening avenues for systematically mapping the intricate metabolic landscape of human cells.
Tang, C. et al. Immunometabolic coevolution defines unique microenvironmental niches in ccRCC. Cell Metab. 35, 1424–1440 (2023). Tang et al. used computational analysis to correlate metabolome identity and abundance to immune cell infiltration, thereby advancing understanding of how metabolites influence immune cell function.
Ma, E. H. et al. Metabolic profiling using stable isotope tracing reveals distinct patterns of glucose utilization by physiologically activated CD8+ T cells. Immunity 51, 856–870 (2019).
MacPherson, S. et al. Clinically relevant T cell expansion media activate distinct metabolic programs uncoupled from cellular function. Mol. Ther. Methods Clin. Dev. 24, 380–393 (2022). MacPherson et al. showed that T cell expansion in different media formulation dictates metabolism and function, underscoring the importance of for-purpose media selection to expand T cells for adoptive therapy.
Klein Geltink, R. I. et al. Metabolic conditioning of CD8+ effector T cells for adoptive cell therapy. Nat. Metab. 2, 703–716 (2020). Klein Geltink et al. demonstrated that glucose restriction before adoptive transfer enhances T cell cytoxicity in vivo.
Bishop, E. L., Gudgeon, N. & Dimeloe, S. Control of T cell metabolism by cytokines and hormones. Front. Immunol. 12, 653605 (2021).
Alizadeh, D. et al. IL15 enhances CAR-T cell antitumor activity by reducing mTORC1 activity and preserving their stem cell memory phenotype. Cancer Immunol. Res. 7, 759–772 (2019).
Ghassemi, S. et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol. Res. 6, 1100–1109 (2018).
Ghassemi, S. et al. Rapid manufacturing of non-activated potent CAR T cells. Nat. Biomed. Eng. 6, 118–128 (2022).
Cheng, H. et al. Extracellular acidosis restricts one-carbon metabolism and preserves T cell stemness. Nat. Metab. 5, 314–330 (2023).
Van Bruggen, J. A. C. et al. Chronic lymphocytic leukemia cells impair mitochondrial fitness in CD8+ T cells and impede CAR T cell efficacy. Blood 134, 44–58 (2018).
Lontos, K. et al. Metabolic reprogramming via an engineered PGC-1α improves human chimeric antigen receptor T-cell therapy against solid tumors. J. Immunother. Cancer 11, e006522 (2023).
Sukumar, M. et al. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab. 23, 63–76 (2016).
Ron-Harel, N. et al. Mitochondrial biogenesis and proteome remodeling promotes one carbon metabolism for T cell activation. Cell Metab. 24, 104–117 (2016).
Guillaumond, F. et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc. Natl Acad. Sci. USA 110, 3919–3924 (2013).
Li, Z., Sun, C. & Qin, Z. Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics 11, 8322–8336 (2021).
M. de-Brito, N. et al. Aerobic glycolysis is a metabolic requirement to maintain the M2-like polarization of tumor-associated macrophages. Biochim. Biophys. Acta Mol. Cell Res. 1867, 118604 (2020).
Jian, S.-L. et al. Glycolysis regulates the expansion of myeloid-derived suppressor cells in tumor-bearing hosts through prevention of ROS-mediated apoptosis. Cell Death Dis. 8, e2779 (2017).
Edwards, D. N. et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J. Clin. Invest. 131, e140100 (2021).
Li, X. et al. Role of glutamine and its metabolite ammonia in crosstalk of cancer-associated fibroblasts and cancer cells. Cancer Cell Int. 21, 479 (2021).
Oh, M. -H. et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J. Clin. Invest. 130, 3865–3884 (2020).
Jin, J., Byun, J. -K., Choi, Y. -K. & Park, K. -G. Targeting glutamine metabolism as a therapeutic strategy for cancer. Exp. Mol. Med. 55, 706–715 (2023).
Cheng, P. N.-M. et al. Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res. 67, 309–317 (2007).
Bronte, V. & Zanovello, P. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 5, 641–654 (2005).
Giatromanolaki, A., Harris, A. L. & Koukourakis, M. I. The prognostic and therapeutic implications of distinct patterns of argininosuccinate synthase 1 (ASS1) and arginase-2 (ARG2) expression by cancer cells and tumor stroma in non-small-cell lung cancer. Cancer Metab. 9, 28 (2021).
Tu, S. et al. Crosstalk between tumor-associated microglia/macrophages and CD8-positive T cells plays a key role in glioblastoma. Front. Immunol. 12, 650105 (2021).
Peyraud, F. et al. Targeting tryptophan catabolism in cancer immunotherapy era: challenges and perspectives. Front Immunol. 13, 807271 (2022).
Yu, J. et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J. Immunol. 190, 3783–3797 (2013).
Chen, L. et al. Cancer associated fibroblasts promote renal cancer progression through a TDO/Kyn/AhR dependent signaling pathway. Front. Oncol. 11, 628821 (2021).
Lee, G. K. et al. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 107, 452–460 (2002).
Fallarino, F. et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naive T cells. J. Immunol. 176, 6752–6761 (2006).
San-Millán, I. & Brooks, G. A. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 38, 119–133 (2017).
Sazeides, C. & Le, A. Metabolic relationship between cancer-associated fibroblasts and cancer cells. in The Heterogeneity of Cancer Metabolism [Internet]. 2nd edition https://doi.org/10.1007/978-3-030-65768-0_14 (Springer, 2021).
Ohta, A. A metabolic immune checkpoint: adenosine in tumor microenvironment. Front. Immunol. 7, 109 (2016).
Morello, S., Pinto, A., Blandizzi, C. & Antonioli, L. Myeloid cells in the tumor microenvironment: role of adenosine. Oncoimmunology 5, e1108515 (2015).
Vigano, S. et al. Targeting adenosine in cancer immunotherapy to enhance T-cell function. Front. Immunol. 10, 925 (2019).
Jennings, M. R., Munn, D. & Blazeck, J. Immunosuppressive metabolites in tumoral immune evasion: redundancies, clinical efforts, and pathways forward. J. Immunother. Cancer 9, e003013 (2021).
Routy, J. -P., Routy, B., Graziani, G. M. & Mehraj, V. The kynurenine pathway is a double-edged sword in immune-privileged sites and in cancer: implications for immunotherapy. Int. J. Tryptophan Res. 9, 67–77 (2016).
Nokin, M.-J. et al. Methylglyoxal, a glycolysis metabolite, triggers metastasis through MEK/ERK/SMAD1 pathway activation in breast cancer. Breast Cancer Res. 21, 11 (2019).
Cheng, J. et al. Cancer-cell-derived fumarate suppresses the anti-tumor capacity of CD8+ T cells in the tumor microenvironment. Cell Metab. 35, 961–978 (2023).
Zhao, H. et al. Myeloid-derived itaconate suppresses cytotoxic CD8+ T cells and promotes tumour growth. Nat. Metab. 4, 1660–1673 (2022).
Gudgeon, N. et al. Succinate uptake by T cells suppresses their effector function via inhibition of mitochondrial glucose oxidation. Cell Rep. 40, 111193 (2022).
Leney-Greene, M. A., Boddapati, A. K., Su, H. C., Cantor, J. R. & Lenardo, M. J. Human plasma-like medium improves T lymphocyte activation. iScience 23, 100759 (2020).
Ghassemi, S. et al. Enhancing chimeric antigen receptor T cell anti-tumor function through advanced media design. Mol. Ther. Methods Clin. Dev. 18, 595–606 (2020).
Chang, C.-H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015).
Singh, N., Shi, J., June, C. H. & Ruella, M. Genome-editing technologies in adoptive T cell immunotherapy for cancer. Curr. Hematol. Malig. Rep. 12, 522–529 (2017).
Atsavapranee, E. S., Billingsley, M. M. & Mitchell, M. J. Delivery technologies for T cell gene editing: applications in cancer immunotherapy. eBioMedicine 67, 103354 (2021).
Mailankody, S. et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results. Nat. Med. 29, 422–429 (2023).
Zhang, J. et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature 609, 369–374 (2022).
Roth, T. L. et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405–409 (2018).
Shy, B. R. et al. High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails. Nat. Biotechnol. 41, 521–531 (2023).
Titov, A. et al. Adoptive immunotherapy beyond CAR T-cells. Cancers 13, 743 (2021).
Song, H. W., Somerville, R. P., Stroncek, D. F. & Highfill, S. L. Scaling up and scaling out: advances and challenges in manufacturing engineered T cell therapies. Int. Rev. Immunol. 41, 638–648 (2022).
Acknowledgements
S.J.M. is supported by a Graduate Award from the University of Victoria and G.A.C. is supported by a Vanier Canada Graduate Scholarship. This manuscript was supported by grants from the Canadian Institutes of Health Research (PJT407274; to J.J.L.), the Terry Fox Research Institute New Frontiers Program Project (to J.J.L.) and a US Department of Defence Ovarian Cancer Research Program Teal Expansion Award (OC210019; to J.J.L.). The authors apologize to researchers in the field whose work has been inadvertently overlooked or could not be cited due to space restrictions. The laboratory of J.J.L. is situated on the traditional territory of the lək̓ʷəŋən People, specifically the Songhees and Esquimalt First Nations. We acknowledge and respect our traditional hosts and thank them for allowing us to conduct research while on their lands.
Author information
Authors and Affiliations
Contributions
J.J.L. and S.M. conceived the idea. J.J.L. and S.M. designed the scope of the article and conducted the literature review. S.M. compiled the reference list, drafted the initial manuscript and created the figures and tables. All authors contributed to reviewing, editing and revising the article, figures and tables for intellectual content and clarity.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Junyoung Park and Yi Fan for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McPhedran, S.J., Carleton, G.A. & Lum, J.J. Metabolic engineering for optimized CAR-T cell therapy. Nat Metab 6, 396–408 (2024). https://doi.org/10.1038/s42255-024-00976-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-024-00976-2