Abstract
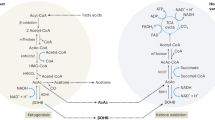
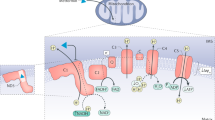
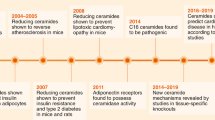
Prospective molecular targets and therapeutic applications for ketone body metabolism have increased exponentially in the past decade. Initially considered to be restricted in scope as liver-derived alternative fuel sources during periods of carbohydrate restriction or as toxic mediators during diabetic ketotic states, ketogenesis and ketone bodies modulate cellular homeostasis in multiple physiological states through a diversity of mechanisms. Selective signalling functions also complement the metabolic fates of the ketone bodies acetoacetate and d-β-hydroxybutyrate. Here we discuss recent discoveries revealing the pleiotropic roles of ketone bodies, their endogenous sourcing, signalling mechanisms and impact on target organs, and considerations for when they are either stimulated for endogenous production by diets or pharmacological agents or administered as exogenous wellness-promoting agents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rosenbloom, J. The acetone bodies in diabetes mellitus: influence of low and high protein intake on the excretion of acetone, diacetic acid and β-oxybutyric acid. J. Am. Med. Assoc. LXV, 1715–1717 (1915).
Tollens, B. Diabetic urine. Ann. Chem. 209, 30–38 (1881).
Ewing, J. Acidosis and associated conditions. Arch. Intern. Med. II, 330–354 (1908).
Cahill, G. F. Jr Fuel metabolism in starvation. Annu. Rev. Nutr. 26, 1–22 (2006).
Wilder, R. M. The effect of ketonemia on the course of epilepsy. Clin. Bull. 2 307 (1921).
Owen, O. E. et al. Brain metabolism during fasting. J. Clin. Invest. 6, 1589–1595 (1967).
Puchalska, P. & Crawford, P. A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 25, 262–284 (2017).
Puchalska, P. & Crawford, P. A. Metabolic and signaling roles of ketone bodies in health and disease. Annu. Rev. Nutr. 41, 49–77 (2021).
McGarry, J., Wright, P. H. & Foster, D. Hormonal control of ketogenesis. Rapid activation of hepatic ketogenic capacity in fed rats by anti-insulin serum and glucagon. J. Clin. Invest. https://doi.org/10.1172/JCI108038 (1975).
Williamson, D., Lund, P. & Krebs, H. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem. J. https://doi.org/10.1042/bj1030514 (1967).
Williamson, D., Bates, M. W. & Krebs, H. Activity and intracellular distribution of enzymes of ketone-body metabolism in rat liver. Biochem. J. https://doi.org/10.1042/bj1080353 (1968).
Page, M., Krebs, H. A. & Williamson, D. Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem. J. https://doi.org/10.1042/bj1210049 (1971).
Quant, P., Robin, D., Robin, P., Girard, J. & Brand, M. Control of acetoacetate production from exogenous palmitoyl-CoA in isolated rat liver mitochondria. Biochem. Soc. Trans. https://doi.org/10.1042/bst0171089 (1989).
Wakeman, A. J. D. H. D. On the decomposition of β-oxybutyric acid and aceto-acetic acid by enzymes of the liver. J. Biol. Chem. 6, 373–389 (1909).
Lehninger, A., Sudduth, H. C. & Wise, J. D β-hydroxybutyric dehydrogenase of muitochondria. J. Biol. Chem. https://doi.org/10.1016/S0021-9258(18)64641-1 (1960).
Stagg D.B. et al. Diminished ketone interconversion, hepatic TCA cycle flux, and glucose production in D-β-hydroxybutyrate dehydrogenase hepatocyte-deficient mice. Mol. Metab. https://doi.org/10.1016/j.molmet.2021.101269 (2021).
Stern, J., Coon, M. J., Del Campillo, A. & Schneider, M. Enzymes of fatty acid metabolism. IV. Preparation and properties of coenzyme A transferase. J. Biol. Chem. https://doi.org/10.1016/S0021-9258(18)65225-1 (1956).
Kashiwaya, Y. et al. Control of glucose utilization in working perfused rat heart. J. Biol. Chem. https://doi.org/10.1016/S0021-9258(18)47278-X (1994).
Taegtmeyer, H., Hems, R. & Krebs, H. Utilization of energy-providing substrates in the isolated working rat heart. Biochem. J. https://doi.org/10.1042/bj1860701 (1980).
Randle, P., Garland, P. B., Hales, C. N. & Newsholme, E. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet https://doi.org/10.1016/s0140-6736(63)91500-9 (1963).
Stern, J. A role of acetoacetyl-CoA synthetase in acetoacetate utilization by rat liver cell fractions. Biochem. Biophys. Res. Commun. https://doi.org/10.1016/0006-291x(71)90811-4 (1971).
Endemann, G., Goetz, P. G., Edmond, J. & Brunengraber, H. Lipogenesis from ketone bodies in the isolated perfused rat liver. Evidence for the cytosolic activation of acetoacetate. J. Biol. Chem. https://doi.org/10.1016/S0021-9258(18)34796-3 (1982).
Kang, H. et al. Metabolic rewiring by oncogenic BRAF V600E links ketogenesis pathway to BRAF-MEK1 signaling. Mol. Cell https://doi.org/10.1016/j.molcel.2015.05.037 (2015).
Henning, S. & Hird, F. Ketogenesis from butyrate and acetate by the caecum and the colon of rabbits. Biochem. J. https://doi.org/10.1042/bj1300785 (1972).
Cheng, C. et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell https://doi.org/10.1016/j.cell.2019.07.048 (2019).
Silva, B. et al. Glia fuel neurons with locally synthesized ketone bodies to sustain memory under starvation. Nat. Metab. https://doi.org/10.1038/s42255-022-00528-6 (2022).
Adijanto, J. et al. The retinal pigment epithelium utilizes fatty acids for ketogenesis. J. Biol. Chem. https://doi.org/10.1074/jbc.M114.565457 (2014).
Venable, A. et al. Fasting-induced HMGCS2 expression in the kidney does not contribute to circulating ketones. Am. J. Physiol. Renal Physiol. https://doi.org/10.1152/ajprenal.00447.2021 (2022).
Taggart, A. K. et al. D-β-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. https://doi.org/10.1074/jbc.C500213200 (2005).
Cahill, G. et al. Hormone-fuel interrelationships during fasting. J. Clin. Invest. https://doi.org/10.1172/JCI105481 (1966).
Miyamoto, J. et al. Ketone body receptor GPR43 regulates lipid metabolism under ketogenic conditions. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1912573116 (2019).
Kimura, I. et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl Acad. Sci. USA 108, 8030–8035 (2011).
Han, Y. et al. β-Hydroxybutyrate prevents vascular senescence through hnRNP A1-mediated upregulation of Oct4. Mol. Cell https://doi.org/10.1016/j.molcel.2018.07.036 (2018).
Shimazu, T. et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013).
Xie, Z. et al. Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol. Cell 62, 194–206 (2016).
Youm, Y.-H. et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 21, 263–269 (2015).
Tsusaka, T. et al. Non-specific recognition of histone modifications by H3K9bhb antibody. iScience https://doi.org/10.1016/j.isci.2023.107235 (2023).
Puchalska, P., Nelson, A. B., Stagg, D. B. & Crawford, P. A. Determination of ketone bodies in biological samples via rapid UPLC-MS/MS. Talanta 225, 122048 (2021).
Webber, R. & J, Edmond. Utilization of L(+)-3-hydroxybutyrate, D(-)-3-hydroxybutyrate, acetoacetate, and glucose for respiration and lipid synthesis in the 18-day-old rat. J. Biol. Chem. https://doi.org/10.1016/S0021-9258(19)63335-1 (1977).
Hsu, W.-Y. et al. Enantioselective determination of 3-hydroxybutyrate in the tissues of normal and streptozotocin-induced diabetic rats of different ages. J. Chromatogr. B 879, 3331–3336 (2011).
Salomón, T. et al. Ketone body acetoacetate buffers methylglyoxal via a non-enzymatic conversion during diabetic and dietary ketosis. Cell Chem. Biol. https://doi.org/10.1016/j.chembiol.2017.07.012 (2017).
Parry-Strong, A. et al. Very low carbohydrate (ketogenic) diets in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 24, 2431–2442 (2022).
Hall, K. D. et al. Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat. Med. 27, 344–353 (2021).
Saslow, L. R. et al. Comparing very low-carbohydrate vs DASH diets for overweight or obese adults with hypertension and prediabetes or type 2 diabetes: a randomized trial. Ann. Fam. Med. 21, 256–263 (2023).
Deemer, S. E. et al. Exogenous dietary ketone ester decreases body weight and adiposity in mice housed at thermoneutrality. Obesity 28, 1447–1455 (2020).
Moore, M. P. et al. A dietary ketone ester mitigates histological outcomes of NAFLD and markers of fibrosis in high-fat diet fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 320, G564–G572 (2021).
Buga, A. et al. The effects of a 6-week controlled, hypocaloric ketogenic diet, with and without exogenous ketone salts, on body composition responses. Front Nutr. 8, 6185200 (2021).
Katsuya, S., Kawata, Y., Goto, T. & Tsubota, J. Daily intake of D-β-hydroxybutyric acid (D-BHB) reduces body fat in Japanese adult participants: a randomized, double-blind, placebo-controlled study. J. Nutr. Sci. Vitaminol. 69, 121–128 (2023).
Stubbs, B. J. et al. A ketone ester drink lowers human ghrelin and appetite. Obesity 26, 269–273 (2018).
Vestergaard, E. T. et al. Acute ketosis inhibits appetite and decreases plasma concentrations of acyl ghrelin in healthy young men. Diabetes Obes. Metab. 23, 1834–1842 (2021).
Liu, Y., Bharmal, S. H., Kimita, W. & Petrov, M. S. Effect of d-β-hydroxybutyrate-(R)-1,3 butanediol on appetite regulation in people with prediabetes. Mol. Nutr. Food Res 67, e2200615 (2023).
Falkenhain, K., Daraei, A. & Little, J. P. The effect of novel exogenous ketone supplements on blood β-hydroxybutyrate and glucose. J. Diet Suppl. https://doi.org/10.1080/19390211.2023.2179152 (2023).
Satapati, S. et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J. Clin. Invest. https://doi.org/10.1172/JCI82204 (2015).
Cunha, G. M. et al. Efficacy of a 2-month very low-calorie ketogenic diet (VLCKD) compared to a standard low-calorie diet in reducing visceral and liver fat accumulation in patients with obesity. Front. Endocrinol. 11, 607 (2020).
Browning, J. D., Davis, J., Saboorian, M. H. & Burgess, S. C. A low-carbohydrate diet rapidly and dramatically reduces intrahepatic triglyceride content. Hepatology 44, 487–488 (2006).
Garbow, J. R. et al. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G956–967 (2011).
Luukkonen, P. K. et al. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc. Natl Acad. Sci. USA 117, 7347–7354 (2020).
Long, F. et al. A low-carbohydrate diet induces hepatic insulin resistance and metabolic associated fatty liver disease in mice. Mol. Metab. 69, 101675 (2023).
Cotter, D. G. et al. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J. Clin. Invest. 124, 5175–5190 (2014).
Asif, S. et al. Hmgcs2-mediated ketogenesis modulates high-fat diet-induced hepatosteatosis. Mol. Metab. 61, 101494 (2022).
Fletcher, J. A. et al. Impaired ketogenesis and increased acetyl-CoA oxidation promote hyperglycemia in human fatty liver. JCI Insight https://doi.org/10.1172/jci.insight.127737 (2019).
Puchalska, P. et al. Hepatocyte-macrophage acetoacetate shuttle protects against tissue fibrosis. Cell Metab. 29, 383–398 (2019).
Johnson, R., Walton, J. L., Krebs, H. A. & Williamson, D. Post-exercise ketosis. Lancet https://doi.org/10.1016/s0140-6736(69)90931-3 (1969).
Féry, F. & Balasse, E. Ketone body turnover during and after exercise in overnight-fasted and starved humans. Am. J. Physiol. https://doi.org/10.1152/ajpendo.1983.245.4.E318 (1983).
Noakes, T. D., Prins, P. J., Volek, J. S., D’Agostino, D. P. & Koutnik, A. P. Low carbohydrate high fat ketogenic diets on the exercise crossover point and glucose homeostasis. Front. Physiol. 14, 1150265 (2023).
Chiarello, N. et al. Effect of a four-week isocaloric ketogenic diet on physical performance at very high-altitude: a pilot study. BMC Sports Sci. Med. Rehabil. 15, 37 (2023).
Burke, L. M. et al. Adaptation to a low carbohydrate high fat diet is rapid but impairs endurance exercise metabolism and performance despite enhanced glycogen availability. J. Physiol. 599, 771–790 (2021).
Whitfield, J. et al. Acute ketogenic diet and ketone ester supplementation impairs race walk performance. Med. Sci. Sports Exerc. 53, 776–784 (2021).
McCarthy, D. G. et al. Effect of acute ketone monoester ingestion on cardiorespiratory responses to exercise and the influence of blood acidosis. Med Sci. Sports Exerc. https://doi.org/10.1249/mss.0000000000003141 (2023).
Poffé, C., Robberechts, R., Van Thienen, R. & Hespel, P. Exogenous ketosis elevates circulating erythropoietin and stimulates muscular angiogenesis during endurance training overload. J. Physiol. https://doi.org/10.1113/jp284346 (2023).
Cox, P. J. et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 24, 256–268, (2016).
Torres, J. A. et al. Ketosis ameliorates renal cyst growth in polycystic kidney disease. Cell Metab. 30, 1007–1023 (2019).
Strubl, S. et al. Ketogenic dietary interventions in autosomal dominant polycystic kidney disease-a retrospective case series study: first insights into feasibility, safety and effects. Clin. Kidney J. 15, 1079–1092 (2022).
Chakraborty, S. et al. Salt-responsive metabolite, β-hydroxybutyrate, attenuates hypertension. Cell Rep. 25, 677–689 (2018).
Tomita, I. et al. SGLT2 inhibition mediates protection from diabetic kidney disease by promoting ketone body-induced mTORC1 inhibition. Cell Metab. 32, 404–419 (2020).
Ang, Q. et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell https://doi.org/10.1016/j.cell.2020.04.027 (2020).
Dmitrieva-Posocco, O. et al. β-Hydroxybutyrate suppresses colorectal cancer. Nature https://doi.org/10.1038/s41586-022-04649-6 (2022).
Suzuki, R. et al. The novel sustained 3-hydroxybutyrate donor poly-D-3-hydroxybutyric acid prevents inflammatory bowel disease through upregulation of regulatory T-cells. FASEB J. https://doi.org/10.1096/fj.202200919R (2023).
Devi, N., Madaan, P., Kandoth, N., Bansal, D. & Sahu, J. K. Efficacy and safety of dietary therapies for childhood drug-resistant epilepsy: a systematic review and network meta-analysis. JAMA Pediatr. 177, 258–266 (2023).
Sondhi, V. et al. Efficacy of ketogenic diet, modified atkins diet, and low glycemic index therapy diet among children with drug-resistant epilepsy: a randomized clinical trial. JAMA Pediatr. https://doi.org/10.1001/jamapediatrics.2020.2282 (2020).
Hemingway, C., Freeman, J. M., Pillas, D. J. & Pyzik, P. L. The ketogenic diet: a 3- to 6-year follow-up of 150 children enrolled prospectively. Pediatrics 108, 898–905 (2001).
Rho, J. M. How does the ketogenic diet induce anti-seizure effects? Neurosci. Lett. 637, 4–10 (2017).
Murugan, M. & Boison, D. Ketogenic diet, neuroprotection, and antiepileptogenesis. Epilepsy Res. 167, 106444 (2020).
McNally, M. A. & Hartman, A. L. Ketone bodies in epilepsy. J. Neurochem. 121, 28–35 (2012).
Mu, C. et al. Targeted gut microbiota manipulation attenuates seizures in a model of infantile spasms syndrome. JCI Insight https://doi.org/10.1172/jci.insight.158521 (2022).
Kraeuter, A. K., Guest, P. C. & Sarnyai, Z. The Therapeutic potential of ketogenic diet throughout life: focus on metabolic, neurodevelopmental and neurodegenerative disorders. Adv. Exp. Med Biol. 1178, 77–101 (2019).
Morris, J. K. et al. Cognitively impaired elderly exhibit insulin resistance and no memory improvement with infused insulin. Neurobiol. Aging 39, 19–24 (2016).
Baker, L. D. et al. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch. Neurol. 68, 51–57 (2011).
Shippy, D. C., Wilhelm, C., Viharkumar, P. A., Raife, T. J. & Ulland, T. K. β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J. Neuroinflammation 17, 280 (2020).
Fortier, M. et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. 15, 625–634 (2019).
Taylor, M. K., Sullivan, D. K., Mahnken, J. D., Burns, J. M. & Swerdlow, R. H. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimers Dement. https://doi.org/10.1016/j.trci.2017.11.002 (2017).
Newman, J. C. et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 26, 547–557 (2017).
Yin, J. X. et al. Ketones block amyloid entry and improve cognition in an Alzheimer’s model. Neurobiol. Aging 39, 25–37 (2016).
Mujica-Parodi, L. R. et al. Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc. Natl Acad. Sci. USA 117, 6170–6177 (2020).
Tieu, K. et al. D-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Invest. 112, 892–901 (2003).
Yu, X. et al. Ketone body β-hydroxybutyric acid ameliorates dopaminergic neuron injury through modulating Zinc finger protein 36/acyl-CoA synthetase long-chain family member 4 signaling axis-mediated ferroptosis. Neuroscience https://doi.org/10.1016/j.neuroscience.2022.11.018 (2022).
Kuter, K. Z., Olech, Ł., Głowacka, U. & Paleczna, M. Increased β-hydroxybutyrate level is not sufficient for the neuroprotective effect of long-term ketogenic diet in an animal model of early Parkinson’s disease. Exploration of brain and liver energy metabolism markers. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22147556 (2021).
Har-Even, M. et al. Ketogenic diet as a potential treatment for traumatic brain injury in mice. Sci. Rep. 11, 23559 (2021).
Seira, O. et al. Ketogenesis controls mitochondrial gene expression and rescues mitochondrial bioenergetics after cervical spinal cord injury in rats. Sci. Rep. 11, 16359 (2021).
Lin, J. et al. Neuroprotective effect of ketone metabolism on inhibiting inflammatory response by regulating macrophage polarization after acute cervical spinal cord injury in rats. Front. Neurosci. https://doi.org/10.3389/fnins.2020.583611 (2020).
Enders, J. et al. Ketolysis is required for the proper development and function of the somatosensory nervous system. Exp. Neurol. https://doi.org/10.1016/j.expneurol.2023.114428 (2023).
Nishiguchi, T. et al. Stress increases blood β-hydroxybutyrate levels and prefrontal cortex NLRP3 activity jointly in a rodent model. Neuropsychopharmacol. Rep. 41, 159–167 (2021).
Kajitani, N. et al. Prefrontal cortex infusion of β-hydroxybutyrate, an endogenous NLRP3 inflammasome inhibitor, produces antidepressant-like effects in a rodent model of depression. Neuropsychopharmacol. Rep. 40, 157–165 (2020).
Chen, L., Miao, Z. & Xu, X. β-hydroxybutyrate alleviates depressive behaviors in mice possibly by increasing the histone3-lysine9-β-hydroxybutyrylation. Biochem. Biophys. Res. Commun. 490, 117–122 (2017).
Sleiman, S. F. et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β- hydroxybutyrate. eLife https://doi.org/10.7554/eLife.15092 (2016).
Marosi, K. et al. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 139, 769–781 (2016).
Li, H. et al. β-hydroxybutyrate reduces reinstatement of cocaine conditioned place preference through hippocampal CaMKII-α β-hydroxybutyrylation. Cell Rep. 41, 111724 (2022).
Bing, R. The metabolism of the heart. Harvey Lect. 50, 27–70 (1954).
Lopaschuk, G. D., Karwi, Q. G., Tian, R., Wende, A. R. & Abel, E. D. Cardiac energy metabolism in heart failure. Circ. Res. 128, 1487–1513 (2021).
Lommi, J. et al. Blood ketone bodies in congestive heart failure. J. Am. Coll. Cardiol. https://doi.org/10.1016/0735-1097(96)00214-8 (1996).
Bedi, K. et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation https://doi.org/10.1161/circulationaha.115.017545 (2016).
Aubert, G. et al. The failing heart relies on ketone bodies as a fuel. Circulation https://doi.org/10.1161/circulationaha.115.017355 (2016).
Matsuura, T. R., Puchalska, P., Crawford, P. A. & Kelly, D. P. Ketones and the heart: metabolic principles and therapeutic implications. Circ. Res. 132, 882–898 (2023).
Schugar, R. C. et al. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol. Metab. 3, 754–769 (2014).
Horton, J. L. et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight https://doi.org/10.1172/jci.insight.124079 (2019).
Uchihashi, M. et al. Cardiac-specific Bdh1 overexpression ameliorates oxidative stress and cardiac remodeling in pressure overload-induced heart failure. Circ. Heart Fail. https://doi.org/10.1161/circheartfailure.117.004417 (2017).
Yu, Y. et al. Treatment with D-β-hydroxybutyrate protects heart from ischemia/reperfusion injury in mice. Eur. J. Pharmacol. 829, 121–128 (2018).
Yurista, S. R. et al. Therapeutic potential of ketone bodies for patients with cardiovascular disease: JACC focus seminar. J. Am. Coll. Cardiol. https://doi.org/10.1016/j.jacc.2020.12.065 (2021).
Gormsen, L. C. et al. Ketone body infusion with 3-hydroxybutyrate reduces myocardial glucose uptake and increases blood flow in humans: a positron emission tomography study. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.116.005066 (2017).
Nielsen, R. et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation 139, 2129–2141 (2019).
Selvaraj, S., Kelly, D. P. & Margulies, K. B. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation 141, 1800–1812 (2020).
Wentz, A. et al. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J. Biol. Chem. https://doi.org/10.1074/jbc.M110.100651 (2010).
Zhang, Y. et al. Mitochondrial pyruvate carriers are required for myocardial stress adaptation. Nat. Metab. https://doi.org/10.1038/s42255-020-00288-1 (2020).
McCommis, K. et al. Nutritional modulation of heart failure in mitochondrial pyruvate carrier-deficient mice. Nat. Metab. https://doi.org/10.1038/s42255-020-00296-1 (2020).
Krebs, P. et al. Lethal mitochondrial cardiomyopathy in a hypomorphic Med30 mouse mutant is ameliorated by ketogenic diet. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1117835108 (2011).
Berg-Hansen, K. et al. Beneficial effects of ketone ester in patients with cardiogenic shock: a randomized, controlled, double-blind trial. JACC Heart Fail. https://doi.org/10.1016/j.jchf.2023.05.029 (2023).
Weis, E. M. et al. Ketone body oxidation increases cardiac endothelial cell proliferation. EMBO Mol. Med. https://doi.org/10.15252/emmm.202114753 (2022).
Garcia-Caballero, M. et al. Role and therapeutic potential of dietary ketone bodies in lymph vessel growth. Nat. Metab. 1, 666–675 (2019).
Nakamura, M. & Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 15, 387–407 (2018).
McCarthy, C. G. et al. Ketone body β-hydroxybutyrate is an autophagy-dependent vasodilator. JCI Insight https://doi.org/10.1172/jci.insight.149037 (2021).
McCarthy, C. G. et al. Low-dose 1,3-butanediol reverses age-associated vascular dysfunction independent of ketone body β-hydroxybutyrate. Am. J. Physiol. Heart Circ. Physiol. 322, H466–H473 (2022).
Deng, Y. et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ. Res. 128, 232–245 (2021).
Gopalasingam, N. et al. Stimulation of the hydroxycarboxylic acid receptor 2 with the ketone body 3-hydroxybutyrate and niacin in patients with chronic heart failure: hemodynamic and metabolic effects. J. Am. Heart Assoc. 12, e029849 (2023).
Goldberg, E. L. et al. β-Hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. 18, 2077–2087 (2017).
Kim, S. R. et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 11, 2127 (2020).
Luo, S. et al. β-Hydroxybutyrate against cisplatin-induced acute kidney injury via inhibiting NLRP3 inflammasome and oxidative stress. Int. Immunopharmacol. 111, 109101 (2022).
Fu, S. P. et al. BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-κB activation. Mediators Inflamm. 2014, 983401 (2014).
Ferrere, G. et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight https://doi.org/10.1172/jci.insight.145207 (2021).
Thio, C. L., Lai, A. C., Ting, Y. T., Chi, P. Y. & Chang, Y. J. The ketone body β-hydroxybutyrate mitigates ILC2-driven airway inflammation by regulating mast cell function. Cell Rep. 40, 111437 (2022).
Zhang, L. et al. Ketogenesis acts as an endogenous protective programme to restrain inflammatory macrophage activation during acute pancreatitis. eBioMedicine 78, 103959 (2022).
Zhang, H. et al. Ketogenesis-generated β-hydroxybutyrate is an epigenetic regulator of CD8(+) T-cell memory development. Nat. Cell Biol. 22, 18–25 (2020).
Arima Y. et al. Murine neonatal ketogenesis preserves mitochondrial energetics by preventing protein hyperacetylation. Nat. Metab. https://doi.org/10.1038/s42255-021-00342-6 (2021).
Luda, K. et al. Ketolysis drives CD8+ T cell effector function through effects on histone acetylation. Immunity https://doi.org/10.1016/j.immuni.2023.07.002 (2023).
Adam, C. et al. Acetoacetate protects macrophages from lactic acidosis-induced mitochondrial dysfunction by metabolic reprograming. Nat. Commun. 12, 7115 (2021).
Kaymak, I. et al. Carbon source availability drives nutrient utilization in CD8(+) T cells. Cell Metab. 34, 1298–1311 (2022).
Goldberg, E. L. et al. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat. Metab. 2, 50–61 (2020).
Beylot, M., Guiraud, M., Grau, G. & Bouletreau, P. Regulation of ketone body flux in septic patients. Am. J. Physiol. 257, E665–674 (1989).
Wannemacher, R. W. Jr et al. Role of the liver in regulation of ketone body production during sepsis. J. Clin. Invest. 64, 1565–1572 (1979).
Paumelle, R. et al. Hepatic PPARα is critical in the metabolic adaptation to sepsis. J. Hepatol. 70, 963–973 (2019).
Beylot, M. et al. Metabolic effects of a D-β-hydroxybutyrate infusion in septic patients: inhibition of lipolysis and glucose production but not leucine oxidation. Crit. Care Med. 22, 1091–10987 (1994).
Goossens, C. et al. Altered cholesterol homeostasis in critical illness-induced muscle weakness: effect of exogenous 3-hydroxybutyrate. Crit. Care 25, 252 (2021).
Bruzzone, C. et al. SARS-CoV-2 infection dysregulates the metabolomic and lipidomic profiles of serum. iScience 23, 101645 (2020).
Hirschberger, S. et al. Ketone bodies improve human CD8(+) cytotoxic t-cell immune response during COVID-19 infection. Front. Med. 9, 923502, (2022).
Karagiannis, F. et al. Impaired ketogenesis ties metabolism to T cell dysfunction in COVID-19. Nature 609, 801–807 (2022).
Murashige, D. et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science https://doi.org/10.1126/science.abc8861 (2020).
Balasse, E. Kinetics of ketone body metabolism in fasting humans. Metab. Clin. Exp. https://doi.org/10.1016/0026-0495(79)90166-5 (1979).
Kies, C., Tobin, R. B., Fox, H. M. & Mehlman, M. A. Utilization of 1,3-butanediol and nonspecific nitrogen in human adults. J. Nutr. https://doi.org/10.1093/jn/103.8.1155 (1973).
Clarke, K. et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. https://doi.org/10.1016/j.yrtph.2012.04.008 (2012).
Veech, R. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids https://doi.org/10.1016/j.plefa.2003.09.007 (2004).
Poff, A. M., Koutnik, A. P. & Egan, B. Nutritional ketosis with ketogenic diets or exogenous ketones: features, convergence, and divergence. Curr. Sports Med. Rep. https://doi.org/10.1249/JSR.0000000000000732 (2020).
Kennedy, A. R. et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am. J. Physiol. Endocrinol. Metab. 292, E1724–E1739 (2007).
de Cabo, R. & Mattson, M. P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 381, 2541–2551 (2019).
Caffa, I. et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 583, 620–624 (2020).
Saris, C. G. J. & Timmers, S. Ketogenic diets and ketone suplementation: a strategy for therapeutic intervention. Front. Nutr. 9, 947567 (2022).
Belany, P. et al. Effects of hypocaloric low-fat, ketogenic and ketogenic & ketone supplement diets on aldosterone and renin. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgad009 (2023).
Retterstol, K., Svendsen, M., Narverud, I. & Holven, K. B. Effect of low carbohydrate high fat diet on LDL cholesterol and gene expression in normal-weight, young adults: a randomized controlled study. Atherosclerosis 279, 52–61 (2018).
Athinarayanan, S. et al. Impact of a 2-year trial of nutritional ketosis on indices of cardiovascular disease risk in patients with type 2 diabetes. Cardiovasc. Diabetol. https://doi.org/10.1186/s12933-020-01178-2 (2020).
Hyde, P. N. et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight https://doi.org/10.1172/jci.insight.128308 (2019).
Schugar, R. C., Huang, X., Moll, A. R., Brunt, E. M. & Crawford, P. A. Role of choline deficiency in the fatty liver phenotype of mice fed a low protein, very low carbohydrate ketogenic diet. PLOS ONE 8, e74806 (2013).
Cai, Q. Y. et al. Safety and tolerability of the ketogenic diet used for the treatment of refractory childhood epilepsy: a systematic review of published prospective studies. World J. Pediatr. 13, 528–536 (2017).
Stubbs, B. et al. On the metabolism of exogenous ketones in humans. Front. Physiol. https://doi.org/10.3389/fphys.2017.00848 (2017).
Ferrannini, E. et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 65, 1190–1195 (2016).
Zinman, B. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa1504720 (2015).
Abdelgani, S. et al. Distinct mechanisms responsible for the increase in glucose production and ketone formation caused by empagliflozin in T2DM patients. Diabetes Care https://doi.org/10.2337/dc22-0885 (2023).
Saucedo-Orozco, H., Voorrips, S. N., Yurista, S. R., de Boer, R. A. & Westenbrink, B. D. SGLT2 inhibitors and ketone metabolism in heart failure. J. Lipid Atheroscler. 11, 1–19 (2022).
Lupsa, B. C. Kibbey, R. G. & Inzucchi, S. E. Ketones: the double-edged sword of SGLT2 inhibitors? Diabetologia https://doi.org/10.1007/s00125-022-05815-1 (2023).
Falkenhain, K., Daraei, A., Forbes, S. C. & Little, J. P. Effects of exogenous ketone supplementation on blood glucose: a systematic review and meta-analysis. Adv. Nutr. 13, 1697–1714 (2022).
Soni, S. et al. Exogenous ketone ester administration attenuates systemic inflammation and reduces organ damage in a lipopolysaccharide model of sepsis. Biochim. Biophys. Acta Mol. Basis Dis. 1868, 166507 (2022).
Koutnik, A. P. et al. Ketone bodies attenuate wasting in models of atrophy. J. Cachexia Sarcopenia Muscle 11, 973–996, https://doi.org/10.1002/jcsm.12554 (2020).
Acknowledgements
The authors are grateful for support from the National Institutes of Health (grants DK091538, AG069781, DK007203 and HL166142).
Author information
Authors and Affiliations
Contributions
A.B.N., E.D.Q., P.P. and P.A.C. generated the manuscript’s outline and drafted and revised the manuscript. E.D.Q. and P.P. generated the figures.
Corresponding authors
Ethics declarations
Competing interests
P.A.C. has served as an external consultant for Pfizer, Abbott Laboratories, Janssen Research & Development and Selah Therapeutics. All other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Andrew Murray and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nelson, A.B., Queathem, E.D., Puchalska, P. et al. Metabolic Messengers: ketone bodies. Nat Metab 5, 2062–2074 (2023). https://doi.org/10.1038/s42255-023-00935-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00935-3