Abstract
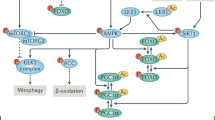
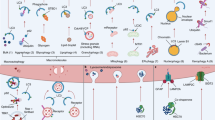
Maintaining optimal mitochondrial function is a feature of health. Mitophagy removes and recycles damaged mitochondria and regulates the biogenesis of new, fully functional ones preserving healthy mitochondrial functions and activities. Preclinical and clinical studies have shown that impaired mitophagy negatively affects cellular health and contributes to age-related chronic diseases. Strategies to boost mitophagy have been successfully tested in model organisms, and, recently, some have been translated into clinics. In this Review, we describe the basic mechanisms of mitophagy and how mitophagy can be assessed in human blood, the immune system and tissues, including muscle, brain and liver. We outline mitophagy’s role in specific diseases and describe mitophagy-activating approaches successfully tested in humans, including exercise and nutritional and pharmacological interventions. We describe how mitophagy is connected to other features of ageing through general mechanisms such as inflammation and oxidative stress and forecast how strengthening research on mitophagy and mitophagy interventions may strongly support human health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Palikaras, K., Lionaki, E. & Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 20, 1013–1022 (2018).
Palikaras, K., Lionaki, E. & Tavernarakis, N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 521, 525–528 (2015).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
López-Otín, C. & Kroemer, G. Hallmarks of health. Cell 184, 33–63 (2021).
Esteban-Martínez, L. et al. Programmed mitophagy is essential for the glycolytic switch during cell differentiation. EMBO J. 36, 1688–1706 (2017).
Sandoval, H. et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature 454, 232–235 (2008).
McWilliams, T. G. et al. mito-QC illuminates mitophagy and mitochondrial architecture in vivo. J. Cell Biol. 214, 333–345 (2016).
Sun, N. et al. Measuring in vivo mitophagy. Mol. Cell 60, 685–696 (2015).
Sekine, S. & Youle, R. J. PINK1 import regulation; a fine system to convey mitochondrial stress to the cytosol. BMC Biol. 16, 2 (2018).
Terešak, P. et al. Regulation of PRKN-independent mitophagy. Autophagy 18, 24–39 (2022).
Lazarou, M. et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309–314 (2015).
Zhang, T. et al. BNIP3 protein suppresses PINK1 kinase proteolytic cleavage to promote mitophagy. J. Biol. Chem. 291, 21616–21629 (2016).
Lee, Y., Lee, H.-Y., Hanna, R. A. & Gustafsson, Å. B. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of parkin in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 301, H1924–H1931 (2011).
Lou, G. et al. Mitophagy and neuroprotection. Trends Mol. Med. 26, 8–20 (2020).
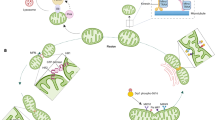
Soubannier, V., Rippstein, P., Kaufman, B. A., Shoubridge, E. A. & McBride, H. M. Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo. PloS ONE 7, e52830 (2012).
Roberts, R. F., Tang, M. Y., Fon, E. A. & Durcan, T. M. Defending the mitochondria: the pathways of mitophagy and mitochondrial-derived vesicles. Int. J. Biochem. Cell Biol. 79, 427–436 (2016).
Soubannier, V. et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr. Biol. 22, 135–141 (2012).
McLelland, G.-L., Soubannier, V., Chen, C. X., McBride, H. M. & Fon, E. A. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 33, 282–295 (2014).
Nicolás-Ávila, J. A. et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183, 94–109 (2020).
Liang, W. et al. Mitochondria are secreted in extracellular vesicles when lysosomal function is impaired. Nat. Commun. 14, 5031 (2023).
Rosina, M. et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 34, 533–548 (2022).
Davis, C. O. et al. Transcellular degradation of axonal mitochondria. Proc. Natl Acad. Sci. USA 111, 9633–9638 (2014).
Melentijevic, I. et al. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 542, 367–371 (2017).
Hao, T. et al. Hypoxia-reprogramed megamitochondrion contacts and engulfs lysosome to mediate mitochondrial self-digestion. Nat. Commun. 14, 4105 (2023).
Wu, W. et al. FUNDC1 regulates mitochondrial dynamics at the ER–mitochondrial contact site under hypoxic conditions. EMBO J. 35, 1368–1384 (2016).
Pryde, K. R., Smith, H. L., Chau, K.-Y. & Schapira, A. H. V. PINK1 disables the anti-fission machinery to segregate damaged mitochondria for mitophagy. J. Cell Biol. 213, 163–171 (2016).
Oshima, Y. et al. Parkin-independent mitophagy via Drp1-mediated outer membrane severing and inner membrane ubiquitination. J. Cell Biol. 220, e202006043 (2021).
Munson, M. J. et al. GAK and PRKCD are positive regulators of PRKN-independent mitophagy. Nat. Commun. 12, 6101 (2021).
Gegg, M. E. et al. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum. Mol. Genet. 19, 4861–4870 (2010).
Ziviani, E. & Whitworth, A. J. How could parkin-mediated ubiquitination of mitofusin promote mitophagy? Autophagy 6, 660–662 (2010).
Palikaras, K. & Tavernarakis, N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp. Gerontol. 56, 182–188 (2014).
Cantó, C. & Auwerx, J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 20, 98–105 (2009).
Malik, N. et al. Induction of lysosomal and mitochondrial biogenesis by AMPK phosphorylation of FNIP1. Science 380, eabj5559 (2023).
Lionaki, E., Markaki, M., Palikaras, K. & Tavernarakis, N. Mitochondria, autophagy and age-associated neurodegenerative diseases: new insights into a complex interplay. Biochim. Biophys. Acta 1847, 1412–1423 (2015).
Laker, R. C. et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 8, 548 (2017).
Iorio, R., Celenza, G. & Petricca, S. Mitophagy: molecular mechanisms, new concepts on parkin activation and the emerging role of AMPK/ULK1 axis. Cells 11, 30 (2021).
D’Amico, D. et al. The RNA-binding protein PUM2 impairs mitochondrial dynamics and mitophagy during aging. Mol. Cell 73, 775–787 (2019).
Shin, H. J. et al. Pink1-mediated chondrocytic mitophagy contributes to cartilage degeneration in osteoarthritis. J. Clin. Med. 8, 1849 (2019).
Kuroda, Y. et al. Parkin enhances mitochondrial biogenesis in proliferating cells. Hum. Mol. Genet. 15, 883–895 (2006).
Egan, B. & Zierath, J. R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 17, 162–184 (2013).
Gaitanos, G. C., Williams, C., Boobis, L. H. & Brooks, S. Human muscle metabolism during intermittent maximal exercise. J. Appl. Physiol. 75, 712–719 (1993).
Sin, J. et al. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy 12, 369–380 (2016).
Hong, X. et al. Mitochondrial dynamics maintain muscle stem cell regenerative competence throughout adult life by regulating metabolism and mitophagy. Cell Stem Cell 29, 1298–1314 (2022).
Leduc-Gaudet, J.-P. et al. Parkin overexpression attenuates sepsis-induced muscle wasting. Cells 9, 1454 (2020).
Leduc-Gaudet, J.-P. et al. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 6, 17923–17937 (2015).
García-Prat, L. et al. Autophagy maintains stemness by preventing senescence. Nature 529, 37–42 (2016).
Luan, P. et al. Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy. Sci. Transl. Med. 13, eabb0319 (2021).
Ryu, D. et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 22, 879–888 (2016).
Fang, E. F. et al. Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway. Sci. Rep. 7, 46208 (2017).
D’Amico, D. et al. Urolithin A improves mitochondrial health, reduces cartilage degeneration, and alleviates pain in osteoarthritis. Aging Cell 21, e13662 (2022).
Choi, S. et al. 31P magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the Baltimore Longitudinal Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 71, 1638–1645 (2016).
Zane, A. C. et al. Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell 16, 461–468 (2017).
Tian, Q. et al. Muscle mitochondrial energetics predicts mobility decline in well-functioning older adults: the Baltimore Longitudinal Study of Aging. Aging Cell 21, e13552 (2022).
Gouspillou, G. et al. Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J. 28, 1621–1633 (2014).
Balan, E. et al. Regular endurance exercise promotes fission, mitophagy, and oxidative phosphorylation in human skeletal muscle independently of age. Front. Physiol. 10, 1088 (2019).
Crane, J. D., Devries, M. C., Safdar, A., Hamadeh, M. J. & Tarnopolsky, M. A. The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J. Gerontol. A Biol. Sci. Med. Sci. 65, 119–128 (2010).
Ploumi, C., Daskalaki, I. & Tavernarakis, N. Mitochondrial biogenesis and clearance: a balancing act. FEBS J. 284, 183–195 (2017).
Liu, L. et al. Mitophagy receptor FUNDC1 is regulated by PGC-1α/NRF1 to fine tune mitochondrial homeostasis. EMBO Rep. 22, e50629 (2021).
Drummond, M. J. et al. Downregulation of E3 ubiquitin ligases and mitophagy-related genes in skeletal muscle of physically inactive, frail older women: a cross-sectional comparison. J. Gerontol. A Biol. Sci. Med. Sci. 69, 1040–1048 (2014).
Picca, A. et al. Relationship between mitochondrial quality control markers, lower extremity tissue composition, and physical performance in physically inactive older adults. Cells 12, 183 (2023).
Askanas, V., Engel, W. K. & Nogalska, A. Sporadic inclusion-body myositis: a degenerative muscle disease associated with aging, impaired muscle protein homeostasis and abnormal mitophagy. Biochim. Biophys. Acta 1852, 633–643 (2015).
Rygiel, K. A. et al. Mitochondrial and inflammatory changes in sporadic inclusion body myositis. Neuropathol. Appl. Neurobiol. 41, 288–303 (2015).
Mangner, N. et al. Molecular mechanisms of diaphragm myopathy in humans with severe heart failure. Circ. Res. 128, 706–719 (2021).
Ferrucci, L. et al. Transcriptomic and proteomic of gastrocnemius muscle in peripheral artery disease. Circ. Res. 132, 1428–1443 (2023).
Murphy, E. et al. Mitochondrial function, biology, and role in disease: a scientific statement from the American Heart Association. Circ. Res. 118, 1960–1991 (2016).
Kubli, D. A. et al. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J. Biol. Chem. 288, 915–926 (2013).
Zhang, W., Siraj, S., Zhang, R. & Chen, Q. Mitophagy receptor FUNDC1 regulates mitochondrial homeostasis and protects the heart from I/R injury. Autophagy 13, 1080–1081 (2017).
Soh, J. E. C. et al. RhoA rescues cardiac senescence by regulating Parkin-mediated mitophagy. J. Biol. Chem. 299, 102993 (2023).
Sweet, M. E. et al. Transcriptome analysis of human heart failure reveals dysregulated cell adhesion in dilated cardiomyopathy and activated immune pathways in ischemic heart failure. BMC Genomics 19, 812 (2018).
Billia, F. et al. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc. Natl Acad. Sci. USA 108, 9572–9577 (2011).
Wang, B. et al. AMPKα2 protects against the development of heart failure by enhancing mitophagy via PINK1 phosphorylation. Circ. Res. 122, 712–729 (2018).
Rugarli, E. I. & Langer, T. Mitochondrial quality control: a matter of life and death for neurons. EMBO J. 31, 1336–1349 (2012).
Faitg, J. et al. 3D neuronal mitochondrial morphology in axons, dendrites, and somata of the aging mouse hippocampus. Cell Rep. 36, 109509 (2021).
Fiesel, F. C. et al. (Patho-)physiological relevance of PINK1-dependent ubiquitin phosphorylation. EMBO Rep. 16, 1114–1130 (2015).
Deng, Z., Sheehan, P., Chen, S. & Yue, Z. Is amyotrophic lateral sclerosis/frontotemporal dementia an autophagy disease? Mol. Neurodegener. 12, 90 (2017).
Castellazzi, M. et al. Autophagy and mitophagy biomarkers are reduced in sera of patients with Alzheimer’s disease and mild cognitive impairment. Sci. Rep. 9, 20009 (2019).
Fang, E. F. et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 22, 401–412 (2019).
Du, F. et al. PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer’s disease. Brain 140, 3233–3251 (2017).
Kerr, J. S. et al. Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci. 40, 151–166 (2017).
Fleming, A. et al. The different autophagy degradation pathways and neurodegeneration. Neuron 110, 935–966 (2022).
Lautrup, S., Sinclair, D. A., Mattson, M. P. & Fang, E. F. NAD+ in brain aging and neurodegenerative disorders. Cell Metab. 30, 630–655 (2019).
Hou, X. et al. Mitophagy alterations in Alzheimer’s disease are associated with granulovacuolar degeneration and early tau pathology. Alzheimers Dement. 17, 417–430 (2020).
Xie, C. et al. Amelioration of Alzheimer’s disease pathology by mitophagy inducers identified via machine learning and a cross-species workflow. Nat. Biomed. Eng. 6, 76–93 (2022).
Sorrentino, V. et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 552, 187–193 (2017).
Mizuno, Y., Hattori, N., Mori, H., Suzuki, T. & Tanaka, K. Parkin and Parkinson’s disease. Curr. Opin. Neurol. 14, 477–482 (2001).
West, A. B. & Maidment, N. T. Genetics of parkin-linked disease. Hum. Genet. 114, 327–336 (2004).
Kahle, P. J., Waak, J. & Gasser, T. DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic. Biol. Med. 47, 1354–1361 (2009).
Hague, S. et al. Early-onset Parkinson’s disease caused by a compound heterozygous DJ-1 mutation. Ann. Neurol. 54, 271–274 (2003).
Newman, L. E. & Shadel, G. S. Pink1/parkin link inflammation, mitochondrial stress, and neurodegeneration. J. Cell Biol. 217, 3327–3329 (2018).
Liu, J., Liu, W., Li, R. & Yang, H. Mitophagy in Parkinson’s disease: from pathogenesis to treatment. Cells 8, 712 (2019).
Watzlawik, J. O. et al. Sensitive ELISA-based detection method for the mitophagy marker p-S65-Ub in human cells, autopsy brain, and blood samples. Autophagy 17, 2613–2628 (2021).
Hsieh, C.-H. et al. Miro1 marks Parkinson’s disease subset and Miro1 reducer rescues neuron loss in Parkinson’s models. Cell Metab. 30, 1131–1140 (2019).
Markovinovic, A. et al. Optineurin in amyotrophic lateral sclerosis: multifunctional adaptor protein at the crossroads of different neuroprotective mechanisms. Prog. Neurobiol. 154, 1–20 (2017).
Evans, C. S. & Holzbaur, E. L. F. Autophagy and mitophagy in ALS. Neurobiol. Dis. 122, 35–40 (2019).
Liu, X. et al. TBK1 variants in Chinese patients with amyotrophic lateral sclerosis. Neurobiol. Aging 97, 149.e9–149.e15 (2021).
Harding, O. et al. ALS- and FTD-associated missense mutations in TBK1 differentially disrupt mitophagy. Proc. Natl Acad. Sci. USA 118, e2025053118 (2021).
Foster, A. D. & Rea, S. L. The role of sequestosome 1/p62 protein in amyotrophic lateral sclerosis and frontotemporal dementia pathogenesis. Neural Regen. Res. 15, 2186–2194 (2020).
Soto-Heredero, G., Gómez de Las Heras, M. M., Gabandé-Rodríguez, E., Oller, J. & Mittelbrunn, M. Glycolysis—a key player in the inflammatory response. FEBS J. 287, 3350–3369 (2020).
Sun, L. et al. Lack of PINK1 alters glia innate immune responses and enhances inflammation-induced, nitric oxide-mediated neuron death. Sci. Rep. 8, 383 (2018).
Fülöp, T., Dupuis, G., Witkowski, J. M. & Larbi, A. The role of immunosenescence in the development of age-related diseases. Rev. Invest. Clin. 68, 84–91 (2016).
Franceschi, C. et al. Immunobiography and the heterogeneity of immune responses in the elderly: a focus on inflammaging and trained immunity. Front. Immunol. 8, 982 (2017).
Phadwal, K. et al. A novel method for autophagy detection in primary cells: impaired levels of macroautophagy in immunosenescent T cells. Autophagy 8, 677–689 (2012).
Gerland, L.-M. et al. Autolysosomes accumulate during in vitro CD8+ T-lymphocyte aging and may participate in induced death sensitization of senescent cells. Exp. Gerontol. 39, 789–800 (2004).
Bektas, A. et al. Age-associated changes in human CD4+ T cells point to mitochondrial dysfunction consequent to impaired autophagy. Aging 11, 9234–9263 (2019).
Raz, Y. et al. Activation-induced autophagy is preserved in CD4+ T-cells in familial longevity. J. Gerontol. A Biol. Sci. Med. Sci. 72, 1201–1206 (2017).
McCormick, J. J. et al. The effect of aging on the autophagic and heat shock response in human peripheral blood mononuclear cells. Physiol. Int. 105, 247–256 (2018).
Bensalem, J. et al. Basal autophagic flux measured in blood correlates positively with age in adults at increased risk of type 2 diabetes. GeroScience https://doi.org/10.1007/s11357-023-00884-5 (2023).
Martino, S. et al. Deficient mitophagy pathways in sickle cell disease. Br. J. Haematol. 193, 988–993 (2021).
Apostolova, N., Vezza, T., Muntane, J., Rocha, M. & Víctor, V. M. Mitochondrial dysfunction and mitophagy in type 2 diabetes: pathophysiology and therapeutic targets. Antioxid. Redox Signal. 39, 278–320 (2023).
Yamada, T. et al. Mitochondrial stasis reveals p62-mediated ubiquitination in parkin-independent mitophagy and mitigates nonalcoholic fatty liver disease. Cell Metab. 28, 588–604 (2018).
Liu, L. et al. Mitophagy and its contribution to metabolic and aging-associated disorders. Antioxid. Redox Signal. 32, 906–927 (2020).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J. Hepatol. 73, 202–209 (2020).
Czaja, M. J. Function of autophagy in nonalcoholic fatty liver disease. Dig. Dis. Sci. 61, 1304–1313 (2016).
Undamatla, R. et al. Reduced mitophagy is an early feature of NAFLD and liver-specific PARKIN knockout hastens the onset of steatosis, inflammation and fibrosis. Sci. Rep. 13, 7575 (2023).
Hidvegi, T. et al. An autophagy-enhancing drug promotes degradation of mutant α1-antitrypsin Z and reduces hepatic fibrosis. Science 329, 229–232 (2010).
Pastore, N. et al. Gene transfer of master autophagy regulator TFEB results in clearance of toxic protein and correction of hepatic disease in α-1-anti-trypsin deficiency. EMBO Mol. Med. 5, 397–412 (2013).
Qiang, X. et al. Demethyleneberberine attenuates non-alcoholic fatty liver disease with activation of AMPK and inhibition of oxidative stress. Biochem. Biophys. Res. Commun. 472, 603–609 (2016).
Drake, J. C. et al. Mitochondria-localized AMPK responds to local energetics and contributes to exercise and energetic stress-induced mitophagy. Proc. Natl Acad. Sci. USA 118, e2025932118 (2021).
Koliaki, C. et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 21, 739–746 (2015).
Ma, X., McKeen, T., Zhang, J. & Ding, W.-X. Role and mechanisms of mitophagy in liver diseases. Cells 9, 837 (2020).
Gancheva, S., Jelenik, T., Álvarez-Hernández, E. & Roden, M. Interorgan metabolic crosstalk in human insulin resistance. Physiol. Rev. 98, 1371–1415 (2018).
Liu, H.-Y. et al. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J. Biol. Chem. 284, 31484–31492 (2009).
Sidarala, V. et al. Mitophagy protects β cells from inflammatory damage in diabetes. JCI Insight 5, e141138 (2020).
Tong, M. et al. Alternative mitophagy protects the heart against obesity-associated cardiomyopathy. Circ. Res. 129, 1105–1121 (2021).
Scheele, C. et al. Altered regulation of the PINK1 locus: a link between type 2 diabetes and neurodegeneration? FASEB J. 21, 3653–3665 (2007).
Czajka, A. et al. Altered mitochondrial function, mitochondrial DNA and reduced metabolic flexibility in patients with diabetic nephropathy. EBioMedicine 2, 499–512 (2015).
Fabbri, E. et al. Insulin resistance is associated with reduced mitochondrial oxidative capacity measured by 31P-magnetic resonance spectroscopy in participants without diabetes from the Baltimore Longitudinal Study of Aging. Diabetes 66, 170–176 (2017).
Guan, Y. et al. Mitophagy in carcinogenesis, drug resistance and anticancer therapeutics. Cancer Cell Int. 21, 350 (2021).
Cesari, R. et al. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25–q27. Proc. Natl Acad. Sci. USA 100, 5956–5961 (2003).
Letessier, A. et al. Correlated break at PARK2/FRA6E and loss of AF-6/Afadin protein expression are associated with poor outcome in breast cancer. Oncogene 26, 298–307 (2007).
Poulogiannis, G. et al. PARK2 deletions occur frequently in sporadic colorectal cancer and accelerate adenoma development in Apc mutant mice. Proc. Natl Acad. Sci. USA 107, 15145–15150 (2010).
Mustafa, M. F. et al. Expression of autophagy and mitophagy markers in breast cancer tissues. Front. Oncol. 11, 612009 (2021).
Giatromanolaki, A., Koukourakis, M. I., Gatter, K. C., Harris, A. L. & Sivridis, E. BNIP3 expression in endometrial cancer relates to active hypoxia inducible factor 1α pathway and prognosis. J. Clin. Pathol. 61, 217–220 (2008).
Rosenfeldt, M. T. et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature 504, 296–300 (2013).
Poillet-Perez, L. & White, E. MDVs to the rescue: how autophagy-deficient cancer cells adapt to defective mitophagy. Dev. Cell 56, 2010–2012 (2021).
Anderson, R. et al. Phase II trial of cytarabine and mitoxantrone with devimistat in acute myeloid leukemia. Nat. Commun. 13, 1673 (2022).
Tang, Y. et al. Targeting mitophagy to promote apoptosis is a potential therapeutic strategy for cancer. Autophagy 19, 1031–1033 (2023).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
Deretic, V. & Levine, B. Autophagy balances inflammation in innate immunity. Autophagy 14, 243–251 (2018).
Galluzzi, L. & Vanpouille-Box, C. BAX and BAK at the gates of innate immunity. Trends Cell Biol. 28, 343–345 (2018).
Kepp, O., Galluzzi, L. & Kroemer, G. Mitochondrial control of the NLRP3 inflammasome. Nat. Immunol. 12, 199–200 (2011).
Kim, J., Kim, H.-S. & Chung, J. H. Molecular mechanisms of mitochondrial DNA release and activation of the cGAS–STING pathway. Exp. Mol. Med. 55, 510–519 (2023).
Chu, C. T. et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell Biol. 15, 1197–1205 (2013).
Li, M. et al. Surface-binding to cardiolipin nanodomains triggers cytochrome c pro-apoptotic peroxidase activity via localized dynamics. Structure 27, 806–815 (2019).
Pizzuto, M. & Pelegrin, P. Cardiolipin in immune signaling and cell death. Trends Cell Biol. 30, 892–903 (2020).
Bueno, M. et al. PINK1 attenuates mtDNA release in alveolar epithelial cells and TLR9 mediated profibrotic responses. PloS ONE 14, e0218003 (2019).
Sliter, D. A. et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature 561, 258–262 (2018).
Zhong, W. et al. Defective mitophagy in aged macrophages promotes mitochondrial DNA cytosolic leakage to activate STING signaling during liver sterile inflammation. Aging Cell 21, e13622 (2022).
Picca, A. et al. Circulating mitochondrial DNA at the crossroads of mitochondrial dysfunction and inflammation during aging and muscle wasting disorders. Rejuvenation Res. 21, 350–359 (2018).
Babbar, M., Basu, S., Yang, B., Croteau, D. L. & Bohr, V. A. Mitophagy and DNA damage signaling in human aging. Mech. Ageing Dev. 186, 111207 (2020).
Valentin-Vega, Y. A. et al. Mitochondrial dysfunction in ataxia–telangiectasia. Blood 119, 1490–1500 (2012).
Cleaver, J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature 218, 652–656 (1968).
Fang, E. F. et al. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD+/SIRT1 reduction. Cell 157, 882–896 (2014).
Lautrup, S. et al. Studying Werner syndrome to elucidate mechanisms and therapeutics of human aging and age-related diseases. Biogerontology 20, 255–269 (2019).
Fang, E. F. et al. NAD+ replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metab. 24, 566–581 (2016).
Fang, E. F. et al. NAD+ augmentation restores mitophagy and limits accelerated aging in Werner syndrome. Nat. Commun. 10, 5284 (2019).
Tarpey, M. D. et al. Skeletal muscle autophagy and mitophagy in endurance-trained runners before and after a high-fat meal. Mol. Metab. 6, 1597–1609 (2017).
Borges, I. B. P. et al. Exercise training attenuates ubiquitin–proteasome pathway and increases the genes related to autophagy on the skeletal muscle of patients with inflammatory myopathies. J. Clin. Rheumatol. 27, S224–S231 (2021).
Schwalm, C., Deldicque, L. & Francaux, M. Lack of activation of mitophagy during endurance exercise in human. Med. Sci. Sports Exerc. 49, 1552–1561 (2017).
Mesquita, P. H. C. et al. Acute and chronic effects of resistance training on skeletal muscle markers of mitochondrial remodeling in older adults. Physiol. Rep. 8, e14526 (2020).
Estébanez, B. et al. Effects of a resistance-training programme on endoplasmic reticulum unfolded protein response and mitochondrial functions in PBMCs from elderly subjects. Eur. J. Sport Sci. 19, 931–940 (2019).
Pileggi, C. A. et al. Minimal adaptation of the molecular regulators of mitochondrial dynamics in response to unilateral limb immobilisation and retraining in middle-aged men. Eur. J. Appl. Physiol. 123, 249–260 (2023).
Chung, K. W. & Chung, H. Y. The effects of calorie restriction on autophagy: role on aging intervention. Nutrients 11, 2923 (2019).
Gutiérrez-Casado, E. et al. The impact of aging, calorie restriction and dietary fat on autophagy markers and mitochondrial ultrastructure and dynamics in mouse skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 74, 760–769 (2019).
Civitarese, A. E. et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 4, e76 (2007).
Menshikova, E. V. et al. Calorie restriction-induced weight loss and exercise have differential effects on skeletal muscle mitochondria despite similar effects on insulin sensitivity. J. Gerontol. A Biol. Sci. Med. Sci. 73, 81–87 (2018).
Shirakabe, A. et al. Evaluating mitochondrial autophagy in the mouse heart. J. Mol. Cell. Cardiol. 92, 134–139 (2016).
Islam, H. et al. Increasing whole-body energetic stress does not augment fasting-induced changes in human skeletal muscle. Pflugers Arch. 473, 241–252 (2021).
D’Amico, D. et al. Impact of the natural compound urolithin A on health, disease, and aging. Trends Mol. Med. 27, 687–699 (2021).
Singh, A. et al. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep. Med. 3, 100633 (2022).
Denk, D. et al. Expansion of T memory stem cells with superior anti-tumor immunity by urolithin A-induced mitophagy. Immunity 55, 2059–2073 (2022).
Vannini, N. et al. The NAD-booster nicotinamide riboside potently stimulates hematopoiesis through increased mitochondrial clearance. Cell Stem Cell 24, 405–418 (2019).
Dollerup, O. L. et al. Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men. J. Physiol. 598, 731–754 (2020).
Remie, C. M. E. et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am. J. Clin. Nutr. 112, 413–426 (2020).
Lapatto, H. A. K. et al. Nicotinamide riboside improves muscle mitochondrial biogenesis, satellite cell differentiation, and gut microbiota in a twin study. Sci. Adv. 9, eadd5163 (2023).
Schroeder, S. et al. Dietary spermidine improves cognitive function. Cell Rep. 35, 108985 (2021).
Makarov, M. & Korkotian, E. Differential role of active compounds in mitophagy and related neurodegenerative diseases. Toxins 15, 202 (2023).
Nieman, D. C. et al. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 42, 338–345 (2010).
Nieman, D. C. et al. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med. Sci. Sports Exerc. 41, 1467–1475 (2009).
Chin, R. M. et al. Pharmacological PINK1 activation ameliorates pathology in Parkinson’s disease models. Preprint at bioRxiv https://doi.org/10.1101/2023.02.14.528378 (2023).
Sadovnikova, I. S. et al. Nrf2/ARE activators improve memory in aged mice via maintaining of mitochondrial quality control of brain and the modulation of gut microbiome. Pharmaceuticals 14, 607 (2021).
Georgakopoulos, N. D. et al. Reversible Keap1 inhibitors are preferential pharmacological tools to modulate cellular mitophagy. Sci. Rep. 7, 10303 (2017).
Yao, Z. et al. A novel small-molecule activator of Sirtuin-1 induces autophagic cell death/mitophagy as a potential therapeutic strategy in glioblastoma. Cell Death Dis. 9, 767 (2018).
Seabright, A. P. et al. AMPK activation induces mitophagy and promotes mitochondrial fission while activating TBK1 in a PINK1–parkin independent manner. FASEB J. 34, 6284–6301 (2020).
Bhansali, S., Bhansali, A., Dutta, P., Walia, R. & Dhawan, V. Metformin upregulates mitophagy in patients with T2DM: a randomized placebo-controlled study. J. Cell. Mol. Med. 24, 2832–2846 (2020).
Tufi, R. et al. High-content phenotypic screen to identify small molecule enhancers of parkin-dependent ubiquitination and mitophagy. SLAS Discov. 28, 73–87 (2023).
Mottis, A., Herzig, S. & Auwerx, J. Mitocellular communication: shaping health and disease. Science 366, 827–832 (2019).
Monzel, A. S., Enríquez, J. A. & Picard, M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat. Metab. 5, 546–562 (2023).
Mahapatra, G. et al. Blood-based bioenergetic profiling reveals differences in mitochondrial function associated with cognitive performance and Alzheimer’s disease. Alzheimers Dement. 19, 1466–1478 (2023).
Picca, A. et al. Extracellular vesicles and damage-associated molecular patterns: a Pandora’s box in health and disease. Front. Immunol. 11, 601740 (2020).
Acknowledgements
We thank the president, C. Rinsch, and the CMO, A. Singh, of Amazentis for their helpful comments on the manuscript. We apologize for the omission of many relevant studies due to space constraints. The work was supported by the École Polytechnique Fédérale de Lausanne and grants from the European Research Council (ERC-AdG-787702) and the Swiss National Science Foundation (SNSF 31003A_179435). This work was supported in part by the Intramural Research Program of the National Institute on Aging, NIH, Baltimore, MD, USA.
Author information
Authors and Affiliations
Contributions
A.P. contributed to writing and revising the manuscript. J.F. contributed to writing and revising the manuscript. J.A. read and revised the manuscript. L.F. contributed to writing and revising the manuscript. D.D’A. contributed to writing and revising the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.F. and D.D’A. are employees of Amazentis. J.A. is a founder and/or consultant of MitoBridge–Astellas, MetroBiotech, Amazentis, Vandria, OrsoBio and NOV Metapharma. A.P. and L.F. declare no conflict of interest.
Peer review
Peer review information
Nature Metabolism thanks Jonathan Brestoff, Evandro Fang and Alvaro Elorza for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Picca, A., Faitg, J., Auwerx, J. et al. Mitophagy in human health, ageing and disease. Nat Metab 5, 2047–2061 (2023). https://doi.org/10.1038/s42255-023-00930-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00930-8