Abstract
Ammonia has been long recognized as a metabolic waste product with well-known neurotoxic effects. However, little is known about the beneficial function of endogenous ammonia. Here, we show that gut ammonia links microbe nitrogen metabolism to host stress vulnerability by maintaining brain glutamine availability in male mice. Chronic stress decreases blood ammonia levels by altering gut urease-positive microbiota. A representative urease-producing strain, Streptococcus thermophilus, can reverse depression-like behaviours induced by gut microbiota that was altered by stress, whereas pharmacological inhibition of gut ammonia production increases stress vulnerability. Notably, abnormally low blood ammonia levels limit the brain’s availability of glutamine, a key metabolite produced by astrocytes that is required for presynaptic γ-aminobutyric acid (GABA) replenishment and confers stress vulnerability through cortical GABAergic dysfunction. Of therapeutic interest, ammonium chloride (NH4Cl), a commonly used expectorant in the clinic, can rescue behavioural abnormalities and GABAergic deficits in mouse models of depression. In sum, ammonia produced by the gut microbiome can help buffer stress in the host, providing a gut–brain signalling basis for emotional behaviour.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The 16S rRNA and metagenomic sequencing datasets are available from the NCBI SRA database with the accession numbers PRJNA919158 and PRJNA921725. Other data that support the findings of this study are available upon reasonable request from the corresponding author J.-G.C. Source data are provided with this paper.
References
Walker, V. Ammonia metabolism and hyperammonemic disorders. Adv. Clin. Chem. 67, 73–150 (2014).
Cagnon, L. & Braissant, O. Hyperammonemia-induced toxicity for the developing central nervous system. Brain Res. Rev. 56, 183–197 (2007).
Rangroo Thrane, V. et al. Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering. Nat. Med. 19, 1643–1648 (2013).
Romero-Gomez, M., Jover, M., Galan, J. J. & Ruiz, A. Gut ammonia production and its modulation. Metab. Brain Dis. 24, 147–157 (2009).
Mora, D. & Arioli, S. Microbial urease in health and disease. PLoS Pathog. 10, e1004472 (2014).
Mora, D. et al. Characterization of urease genes cluster of Streptococcus thermophilus. J. Appl. Microbiol. 96, 209–219 (2004).
Power, D. A., Burton, J. P., Chilcott, C. N., Dawes, P. J. & Tagg, J. R. Preliminary investigations of the colonisation of upper respiratory tract tissues of infants using a paediatric formulation of the oral probiotic Streptococcus salivarius K12. Eur. J. Clin. Microbiol. Infect. Dis. 27, 1261–1263 (2008).
Morais, L. H., Schreiber, H. L. T. & Mazmanian, S. K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255 (2021).
Hoyles, L. et al. Regulation of blood–brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome 9, 235 (2021).
Li, Z. et al. Multi-omics analyses of serum metabolome, gut microbiome and brain function reveal dysregulated microbiota–gut–brain axis in bipolar depression. Mol. Psychiatry 27, 4123–4135 (2022).
Li, D. et al. 3β-Hydroxysteroid dehydrogenase expressed by gut microbes degrades testosterone and is linked to depression in males. Cell Host Microbe 30, 329–339 (2022).
Needham, B. D. et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 602, 647–653 (2022).
Norenberg, M. D. & Martinez-Hernandez, A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 161, 303–310 (1979).
Bak, L. K., Schousboe, A. & Waagepetersen, H. S. The glutamate/GABA–glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 98, 641–653 (2006).
Andersen, J. V. et al. Deficient astrocyte metabolism impairs glutamine synthesis and neurotransmitter homeostasis in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 148, 105198 (2021).
Sandhu, M. R. S. et al. Astroglial glutamine synthetase and the pathogenesis of mesial temporal lobe epilepsy. Front. Neurol. 12, 665334 (2021).
Yang, C. et al. Brain-region specific metabolic abnormalities in Parkinson’s disease and levodopa-induced dyskinesia. Front. Aging Neurosci. 12, 75 (2020).
Lee, Y. et al. Glutamine deficiency in the prefrontal cortex increases depressive-like behaviours in male mice. J. Psychiatry Neurosci. 38, 183–191 (2013).
Winter, G., Hart, R. A., Charlesworth, R. P. G. & Sharpley, C. F. Gut microbiome and depression: what we know and what we need to know. Rev. Neurosci. 29, 629–643 (2018).
Yang, Z. et al. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol. Psychiatry 25, 2759–2772 (2020).
Zhu, X. et al. Dectin-1 signaling on colonic γδ T cells promotes psychosocial stress responses. Nat. Immunol. 24, 625–636 (2023).
Qu, Y. et al. Repeated use of 3,4-methylenedioxymethamphetamine is associated with the resilience in mice after chronic social defeat stress: a role of gut–microbiota–brain axis. Psychiatry Res. 320, 115020 (2023).
Zheng, P. et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796 (2016).
Millner, O. E. Jr. et al. Flurofamide: a potent inhibitor of bacterial urease with potential clinical utility in the treatment of infection induced urinary stones. J. Urol. 127, 346–350 (1982).
Herrmann, R. & Weber, F. L. Jr. Lactulose and ammonia. Lancet 2, 1333 (1987).
Price, R. B. et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol. Psychiatry 65, 792–800 (2009).
Veeraiah, P. et al. Dysfunctional glutamatergic and γ-aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression. Biol. Psychiatry 76, 231–238 (2014).
Waniewski, R. A. Physiological levels of ammonia regulate glutamine synthesis from extracellular glutamate in astrocyte cultures. J. Neurochem. 58, 167–174 (1992).
Qureshi, T. et al. Slc38a1 conveys astroglia-derived glutamine into GABAergic interneurons for neurotransmitter GABA synthesis. Cells 9, 1686 (2020).
Fogaca, M. V. & Duman, R. S. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front. Cell. Neurosci. 13, 87 (2019).
Zhao, Y. F., Wei, D. N. & Tang, Y. Gut microbiota regulate astrocytic functions in the brain: possible therapeutic consequences. Curr. Neuropharmacol. 19, 1354–1366 (2021).
Duan, Y., Wu, X., Liang, S. & Jin, F. Elevated blood ammonia level is a potential biological risk factor of behavioral disorders in prisoners. Behav. Neurol. 2015, 797862 (2015).
Rutherford, J. C. The emerging role of urease as a general microbial virulence factor. PLoS Pathog. 10, e1004062 (2014).
Ryvchin, R. et al. Alteration in urease-producing bacteria in the gut microbiomes of patients with inflammatory bowel diseases. J. Crohns Colitis 15, 2066–2077 (2021).
Hasler, G. et al. Reduced prefrontal glutamate/glutamine and γ-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 64, 193–200 (2007).
Li, C. X. et al. Cerebral metabolic changes in a depression-like rat model of chronic forced swimming studied by ex vivo high resolution 1H magnetic resonance spectroscopy. Neurochem. Res. 33, 2342–2349 (2008).
Croarkin, P. E., Levinson, A. J. & Daskalakis, Z. J. Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci. Biobehav. Rev. 35, 818–825 (2011).
Sanacora, G., Treccani, G. & Popoli, M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62, 63–77 (2012).
Tang, X. H. et al. A role of GABAA receptor α1 subunit in the hippocampus for rapid-acting antidepressant-like effects of ketamine. Neuropharmacology 225, 109383 (2023).
Wang, B. et al. Glutamine and intestinal barrier function. Amino Acids 47, 2143–2154 (2015).
Li, M. X. et al. Gene deficiency and pharmacological inhibition of caspase-1 confers resilience to chronic social defeat stress via regulating the stability of surface AMPARs. Mol. Psychiatry 23, 556–568 (2018).
He, J. G. et al. Transcription factor TWIST1 integrates dendritic remodeling and chronic stress to promote depressive-like behaviors. Biol. Psychiatry 89, 615–626 (2021).
Zhou, H. Y. et al. A-kinase anchoring protein 150 and protein kinase A complex in the basolateral amygdala contributes to depressive-like behaviors induced by chronic restraint stress. Biol. Psychiatry 86, 131–142 (2019).
Luo, H. et al. Angiotensin-converting enzyme inhibitor rapidly ameliorates depressive-type behaviors via bradykinin-dependent activation of mammalian target of rapamycin complex 1. Biol. Psychiatry 88, 415–425 (2020).
Wu, Z. et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 9, 184 (2021).
Chagwedera, D. N. et al. Nutrient sensing in CD11c cells alters the gut microbiota to regulate food intake and body mass. Cell Metab. 30, 364–373 (2019).
Xie, W. L. et al. Deficiency of glycosylated α-dystroglycan in ventral hippocampus bridges the destabilization of γ-aminobutyric acid type A receptors with the depressive-like behaviors of male mice. Biol. Psychiatry 91, 593–603 (2022).
Wu, P. F. et al. Erasing m6A-dependent transcription signature of stress-sensitive genes triggers antidepressant actions. Neurobiol. Stress 15, 100390 (2021).
Wang, J. et al. Microglia-dependent excessive synaptic pruning leads to cortical underconnectivity and behavioral abnormality following chronic social defeat stress in mice. Brain Behav. Immun. 109, 23–36 (2023).
Acknowledgements
This work was supported by the Foundation for the National Key R&D Program of China (nos. 2020YFA0803900 and 2021ZD0202900 to J.-G.C.), the National Natural Science Foundation of China (no. 82073834 to P.-F.W., no. 81971279 to F.W., no. 81973310 to J.-G.C.), the National Natural Science Foundation of China (grant no. 82130110 to J.-G.C. and grant no. U21A20363 to F.W.) and the Innovative Research Groups of the National Natural Science Foundation of China (grant no. 81721005 to J.-G.C. and F.W.).
Author information
Authors and Affiliations
Contributions
P.W. performed most animal experiments, stereotaxic surgery, electrophysiological recordings and molecular experiments. P.-F.W. conceived the study, helped with molecular detection and analysed sequencing data. H.-J.W. performed animal experiments and molecular experiments. F.L. contributed to animal experiments. P.W. and P.-F.W. wrote the paper and drew the figures. P.-F.W., F.W. and J.-G.C. supervised the project, designed experiments, revised the paper and supported funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Kenji Hashimoto and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Chronic stress induces depressive-like behaviors in mice.
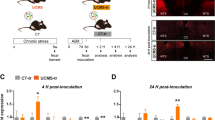
a, Heat maps showed the time distribution of control, susceptible or resilient mice during target and no target stage of social behavior test. b, CSDS-exposed mice were separated into susceptible or resilient groups according to the social interaction ratio (n = 14 mice, Control vs Susceptible, P < 0.0001). c, Time in the interaction zone in the absence or presence of social target (n = 14 mice, Control vs Susceptible, P < 0.0001). d, Sucrose preference of adult control, susceptible or resilient mice after CSDS (n = 14 mice, Control vs Susceptible, P < 0.0001). e, Body weight change ratio of control and CSDS-exposed mice before and after CSDS (n = 13 mice). f, The amount of food consumption during 2 hours and 12 hours after CSDS (n = 11, 17 mice). g, Schematic of CUMS procedure and behavioral tests. h, Depressive-like behaviors in CUMS mice, as measured by the sucrose preference in SPT (left) and the immobility time in FST (right), n = 9 mice. For SPT, P < 0.0001; For FST, P < 0.0001. i, Schematic of CRS procedure and behavioral tests. j, Depressive-like behaviors in CRS mice, as measured by the sucrose preference in SPT (left) and the immobility time in FST (right), n = 8, 8 mice. For SPT, P = 0.0004; For FST, P = 0.0051. k, The levels of blood ammonia in the mice exposed to CUMS (left) and CRS (right). For CUMS, n = 7, 8 mice, P = 0.0105; For CRS, n = 11, 12 mice, P = 0.0173. Data are the mean ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001. Statistical differences were determined by one-way ANOVA with Bonferroni’s multiple-comparisons test (b-d) and two-tailed unpaired Student’s t-test (e, f, h, j, k). The statistical details are provided in Supplementary Table 1.
Extended Data Fig. 2 Altered microbial communities are mainly responsible for gut ammonia production and depressive-like behaviors.
a, Timeline of oral broad-range antibiotics for 7 days and tissue collection. b, The levels of ammonia in the blood, feces and mPFC of vehicle and Abx-treated mice (n = 10 mice; Blood: P < 0.0001, Feces: P = 0.0023; mPFC: P = 0.0444). c, The levels of urease in the feces of vehicle and Abx-treated mice (n = 8 mice, P = 0.0008). d, Schematic of the FMT study: Abx-treated mice were colonized with fecal samples from control or CSDS donors followed by behavior tests. e, Behavioral tests of the recipient mice in the SIT, SPT, FST and TST. For SIT, n = 20, 18 mice, P = 0.0204; For SPT, n = 20, 18 mice, P = 0.0136; For FST n = 20, 17 mice, P = 0.0037; For TST, n = 10 mice, P = 0.0007. f, Behavioral tests of the recipient mice in the OFT (n = 10, 8 mice). Data are the mean ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001. Statistical differences were determined by two-tailed unpaired Student’s t-test. The statistical details are provided in Supplementary Table 1.
Extended Data Fig. 3 CSDS alters gut microbiota and urease-positive bacteria.
a, α-diversity index Shannon of gut microbiome of control and CSDS mice by two-sided Wilcoxon rank-sum test (n = 6 mice; whiskers indicate minimum to maximum values; the minima, first quartiles, median, third quartiles and maxima of control group are 7.671, 7.676, 7.763, 7.855, 7.938, CSDS group are 7.244, 7.278, 7.318, 7.430, 7.465; P = 0.026). b, Principal co-ordinates analysis (PCoA) plot of β-diversity (unweighted unifrac no label) in the feces (n = 6 mice). c, Taxonomic abundance of bacteria at phylum level of control and CSDS mice. Data were showed as relative abundance (%) of top 15 most abundant phylum in each group by Wilcoxon rank-sum test (n = 6 mice). d, Histogram of the LDA scores for differentially abundant KEGG pathway at level 1 and level 2 analyzed by LEfSe analysis (n = 3 mice, P < 0.05, LDA > 3). e, Urease-related pathways enriched from metagenomic pathway at level 3 (n = 3 mice). f, Relative abundance of urease in arginine biosynthesis pathway of control and CSDS-exposed mice (n = 3 mice, P = 0.0352). g, The levels of arginase in the feces and colon of control and CSDS-exposed mice (n = 8 mice). h, The bacterial taxa from taxonomic classification of urease genes as determined by sequence abundance. Data are presented as median with interquartile range (a) and mean ± s.e.m. (f, g), *P < 0.05. Statistical differences were determined by two-tailed unpaired Student’s t-test (f, g). The statistical details are provided in Supplementary Table 1.
Extended Data Fig. 4 Quantitative real time PCR analysis of fecal samples confirmed successful colonization of the S. thermophilus following oral gavage, related to Fig. 2.
a, CON-FMT + pasteurized S. thermophilus group mice (n = 4 mice); b, CON-FMT + live S. thermophilus group mice (n = 4 mice); c, CSDS-FMT + pasteurized S. thermophilus group mice (n = 4 mice); d, CSDS-FMT + live S. thermophilus group mice (n = 3 mice). e, Relative abundance of S. thermophilus in the fecal samples collected from the colon of CON-FMT + pasteurized S. thermophilus, CON-FMT + live S. thermophilus, CSDS-FMT + pasteurized S. thermophilus and CSDS-FMT + live S. thermophilus groups of mice (n = 4, 4, 4, 3 mice; CON-FMT + Pasteurized vs CON-FMT + Live, P = 0.0037; CSDS-FMT + Pasteurized vs CSDS-FMT + Live, P = 0.0176). Data are the mean ± s.e.m., *P < 0.05, **P < 0.01. Statistical differences were determined by two-tailed unpaired Student’s t-test. The statistical details are provided in Supplementary Table 1.
Extended Data Fig. 5 No differences in glutamine and ammonia levels are observed in some brain regions or peripheral tissues.
a, Levels of ammonia in the mPFC of control and LAC mice (n = 7, 8 mice). b, Levels of ammonia in the mPFC of control and CSDS mice (n = 7, 8 mice). c, Levels of ammonia in the ACC of control and CSDS mice (n = 9, 8 mice). d, Levels of ammonia in the Hip of control and CSDS mice (n = 9, 8 mice). e, Levels of ammonia in the VTA of control and CSDS mice (n = 9, 8 mice). f, Levels of ammonia in the NAc of control and CSDS mice (n = 9, 8 mice). g, Levels of Gln in the mPFC of mice subjected to CRS (n = 8 mice, P = 0.0106). h, Levels of Gln in the Hip of control and CSDS mice (n = 7 mice). i, Levels of Gln in the NAc of control and CSDS mice (n = 7 mice). j, Levels of Gln in the blood of control and CSDS mice (n = 10, 11 mice). k, Levels of Gln in the colon of control and CSDS mice (n = 10, 11 mice). Data are the mean ± s.e.m., *P < 0.05. Statistical differences were determined by two-tailed unpaired Student’s t-test. The statistical details are provided in Supplementary Table 1.
Extended Data Fig. 6 No change in astrocyte or GABAergic neuron population in the mPFC of C57BL/6 mice after CSDS.
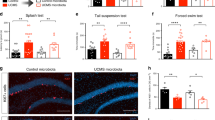
a, Glutamine synthetase (GS) is particularly expressed in astrocytes. Double immunofluorescence labeling of GS (green) and GFAP (red), Iba1(red) and NeuN (red) in mPFC. Scale bar, 50 μm. b, The immunofluorescence labeling of slc38a1 in the mPFC of mice. Top, representative mPFC image for slc38a1 (green) and GAD67 (red). Scale bar, 50μm. Bottom, GABA expression (red) and co-labeled with slc38a1 (green) in the mPFC. Scale bar, 50 μm. For a-b, experiments were independently performed at least three times and similar results were observed. Each independent experiment was performed with at least three biological replicates. c, Representative confocal microscopy images of GFAP immunoreactivity (left) and statistical analysis showing the density of GFAP immunoreactivity (right) in the mPFC of control and CSDS-exposed mice (n = 6 slices from 3 mice). Scale bar, 50 μm. d, Representative confocal microscopy images of GAD67 immunoreactivity (left) and statistical analysis showing the density of GAD67 immunoreactivity (right) in the mPFC of control and CSDS-exposed mice (n = 6 slices from 3 mice). Scale bar, 50 μm. Data are the mean ± s.e.m. (c, d). Statistical differences were determined by two-tailed unpaired Student’s t-test. The statistical details are provided in Supplementary Table 1.
Extended Data Fig. 7 No change in Gln metabolism-related protein expression in the mPFC of C57BL/6 mice after CSDS.
a-d, Western blot image (top) and quantification (bottom) of GS levels in mPFC (a), ACC (b), Hip (c) and BLA (d). Protein expression was normalized by the control level. For a, n = 6, 7 mice; For b, n = 4, 5 mice; For c, n = 6 mice; For d, n = 6, 7 mice. e, Western blot image (top) and quantification (bottom) of GAD67 levels in the mPFC of control and CSDS-exposed mice (n = 4, 5 mice). f, Western blot image (top) and quantification (bottom) of GAD65 levels in the mPFC of control and CSDS-exposed mice (n = 4, 5 mice). g, Western blot image (top) and quantification (bottom) of GLS levels in the mPFC of control and CSDS-exposed mice (n = 4, 5 mice). h, Western blot image (top) and quantification (bottom) of GLDH1 levels in the mPFC of control and CSDS-exposed mice (n = 4, 5 mice). i, Western blot image (top) and quantification (bottom) of GLDH2 levels in the mPFC of control and CSDS-exposed mice (n = 4 mice). j-m, Representative western blot analysis for slc38a1 in mPFC (j), ACC (k), Hip (l) and BLA (m) of control and CSDS mice as indicated. Protein expression was normalized by the control level. For j, n = 6, 7 mice, P = 0.0110; For k, n = 10, 11 mice; For l, n = 6 mice; For m, n = 6, 7 mice. Data are the mean ± s.e.m., *P < 0.05. Statistical differences were determined by two-tailed unpaired Student’s t-test. The statistical details are provided in Supplementary Table 1.
Extended Data Fig. 8 NH4Cl at high dosage significantly increased stress susceptibility.
a, The effect of NH4Cl on immobility time in FST (n = 10, 9, 11 mice). b, The effect of NH4Cl on immobility time in TST (n = 7, 7, 8 mice). c, Sucrose preference was measured in mice subjected to SSDS after NH4Cl injection (125 and 250 mg/kg, intraperitoneally, n = 9 mice). d, Social interaction ratio was measured in mice subjected to SSDS after NH4Cl injection (n = 9, 8, 9 mice, P = 0.0303). e, The level of Gln in the mPFC of mice (n = 8, 7, 8, 8 mice; Sal + Veh vs Sal + NH4Cl, P = 0.0264; Sal + Veh vs MSO + Veh, P = 0.0009). f, The level of ammonia in the mPFC of mice (n = 8, 7, 8, 8 mice, MSO + Veh vs MSO + NH4Cl, P = 0.0293). g, The urease activity in feces of NH4Cl-treated mice (n = 9, 10 mice). h, Behavioral tests in the SIT (e) and SPT (f) of control and CSDS mice 1 hour following NH4Cl injection (SIT: n = 8, 6, 6, 7 mice; SPT: n = 8, 7, 7, 8 mice). i-m, Western blot image (upper) and quantification (bottom) of the protein expression of GABAARα1 (i), GABAARα2 (j), GABAARα3 (k), GABAARα5 (l), and GABAARβ2 (m) in the mPFC. For i, n = 6, 7 mice; For j, n = 6, 7 mice; For k, n = 6 mice; For l, n = 6, 7 mice; For m, n = 6, 7 mice. Data are the mean ± s.e.m., *P < 0.05, ***P < 0.001. Statistical differences were determined by one-way ANOVA (a-d), two-way ANOVA with Bonferroni’s multiple-comparisons test (e, f, h) and two-tailed unpaired Student’s t-test (g, i-m). The statistical details are provided in Supplementary Table 1.
Extended Data Fig. 9 No significant damage in colonic epithelial barrier is detected in CSDS and lactulose exposed mice compared with control.
a, Gene expression levels of CLDN1 (left), CLDN5 (middle) and OCLN (right) in the colon of control and CSDS group mice. For CLDN1, n = 16 mice; For CLDN5, n = 16 mice; For OCLN, n = 15, 16 mice. b, Gene expression levels of CLDN1 (left), CLDN5 (middle) and OCLN (right) in the colon of control and LAC group mice (n = 6 mice). Data are the mean ± s.e.m. Statistical differences were determined by two-tailed unpaired Student’s t-test. The statistical details are provided in Supplementary Table 1.
Supplementary information
Supplementary Information
Supplementary Table 1
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, P., Wu, PF., Wang, HJ. et al. Gut microbiome-derived ammonia modulates stress vulnerability in the host. Nat Metab 5, 1986–2001 (2023). https://doi.org/10.1038/s42255-023-00909-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00909-5
This article is cited by
-
Keep calm with ammonia-producing microbiota
Nature Reviews Microbiology (2024)