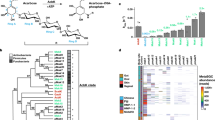
Abstract
Drugs can be modified or degraded by the gut microbiota, which needs to be considered in personalized therapy. The clinical efficacy of the antidiabetic drug acarbose, an inhibitor of α-glucosidase, varies greatly among individuals for reasons that are largely unknown. Here we identify in the human gut acarbose-degrading bacteria, termed Klebsiella grimontii TD1, whose presence is associated with acarbose resistance in patients. Metagenomic analyses reveal that the abundance of K. grimontii TD1 is higher in patients with a weak response to acarbose and increases over time with acarbose treatment. In male diabetic mice, co-administration of K. grimontii TD1 reduces the hypoglycaemic effect of acarbose. Using induced transcriptome and protein profiling, we further identify an acarbose preferred glucosidase, Apg, in K. grimontii TD1, which can degrade acarbose into small molecules with loss of inhibitor function and is widely distributed in human intestinal microorganisms, especially in Klebsiella. Our results suggest that a comparatively large group of individuals could be at risk of acarbose resistance due to its degradation by intestinal bacteria, which may represent a clinically relevant example of non-antibiotic drug resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Data supporting the findings of this research are available within the paper and its Supplementary Information files. The genome sequencing data have been deposited in the Sequence Read Archive (SRA) under accession code PRJNA801205. The RNA-seq and metagenomics data are available in NCBI under the SRA with Bioproject No. PRJNA801096 and SRP418540, respectively. Source data are provided with this paper.
Code availability
The generated source code is available at https://github.com/lovingstudy/Apg_gut.
References
Tseng, T. S. et al. Sugar intake from sweetened beverages and diabetes: a narrative review. World J. Diabetes 12, 1530–1538 (2021).
Weng, J. et al. Standards of care for type 2 diabetes in China. Diabetes Metab. Res. Rev. 32, 442–458 (2016).
Jia, W. et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab. Res. Rev. 35, e3158 (2019).
Balaich, J. et al. The human microbiome encodes resistance to the antidiabetic drug acarbose. Nature 600, 110–115 (2021).
Breuer, H. W. Review of acarbose therapeutic strategies in the long-term treatment and in the prevention of type 2 diabetes. Int. J. Clin. Pharmacol. Ther. 41, 421–440 (2003).
Chiasson, J. L. et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 359, 2072–2077 (2002).
Clissold, S. P. & Edwards, C. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs 35, 214–243 (1988).
Martin, A. E. & Montgomery, P. A. Acarbose: an α-glucosidase inhibitor. Am. J. Health Syst. Pharm. 53, 2277–2290 (1996).
Gu, Y. et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat. Commun. 8, 1785–1785 (2017).
Baxter, N. T., Lesniak, N. A., Sinani, H., Schloss, P. D. & Koropatkin, N. M. The glucoamylase inhibitor acarbose has a diet-dependent and reversible effect on the murine gut microbiome. mSphere 4, e00528–00518 (2019).
Zhang, L. et al. Alpha-glucosidase inhibitors alter gut microbiota and ameliorate collagen induced arthritis. Front. Pharmacol. 10, 1684–1684 (2020).
Maini Rekdal, V., Bess, E. N., Bisanz, J. E., Turnbaugh, P. J. & Balskus, E. P. Discovery and inhibition of an interspecies gut bacterial pathway for levodopa metabolism. Science 364, eaau6323 (2019).
Klünemann, M. et al. Bioaccumulation of therapeutic drugs by human gut bacteria. Nature 597, 533–538 (2021).
Scott, T. A. et al. Host–microbe co-metabolism dictates cancer drug efficacy in C. elegans. Cell 169, 442–456 (2017).
Javdan, B. et al. Personalized mapping of drug metabolism by the human gut microbiome. Cell 181, 1661–1679 (2020).
Dai, D. et al. GMrepo v2: a curated human gut microbiome database with special focus on disease markers and cross-dataset comparison. Nucleic Acids Res. 50, D777–D784 (2022).
Pastor, A. et al. Role of N-terminal region of Escherichia coli maltodextrin glucosidase in folding and function of the protein. Biochim. Biophys. Acta 1864, 1138–1151 (2016).
Santilli, A. D., Dawson, E. M., Whitehead, K. J. & Whitehead, D. C. Nonmicrobicidal small molecule inhibition of polysaccharide metabolism in human gut microbes: a potential therapeutic avenue. ACS Chem. Biol. 13, 1165–1172 (2018).
Kastl, A. J. Jr. et al. The structure and function of the human small intestinal microbiota: current understanding and future directions. Cell Mol. Gastroenterol. Hepatol. 9, 33–45 (2020).
Breitmeier, D., Günther, S. & Heymann, H. Acarbose and 1-deoxynojirimycin inhibit maltose and maltooligosaccharide hydrolysis of human small intestinal glucoamylase–maltase in two different substrate-induced modes. Arch. Biochem. Biophys. 346, 7–14 (1997).
Zhao, B. et al. Protective effects of acarbose against insulitis in multiple low-dose streptozotocin-induced diabetic mice. Life Sci. 263, 118490 (2020).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Zhai, K. et al. NLRs guard metabolism to coordinate pattern- and effector-triggered immunity. Nature 601, 245–251 (2022).
Liu, H. L. et al. Acylated aminooligosaccharides with inhibitory effects against α-amylase from Streptomyces sp. HO1518. Mar. Drugs 16, 403 (2018).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 38, 3022–3027 (2021).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, D., Liu, C. M., Luo, R., Sadakane, K. & Lam, T. W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015).
Noguchi, H., Park, J. & Takagi, T. MetaGene: prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 34, 5623–5630 (2006).
Fu, L., Niu, B., Zhu, Z., Wu, S. & Li, W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28, 3150–3152 (2012).
Li, R., Li, Y., Kristiansen, K. & Wang, J. SOAP: short oligonucleotide alignment program. Bioinformatics 24, 713–714 (2008).
Buchfink, B., Xie, C. & Huson, D. H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60 (2015).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (no. 31921006 to W.J. and no. 82170839 to G.Q.) and the Shanghai Super Post-doctoral Excellence Program (no. 2020458 to J.T.). We are grateful to Y. Wang, J. Xu, X. Xu and Y. Liu for HPLC and LC–MS/MS analysis. We thank Y. Zhang and H. Zhang for FPLC analysis. We also thank Z. Zhang for field emission scanning electron microscopy analysis.
Author information
Authors and Affiliations
Contributions
J.T., W.J., G.Q., Y.G., Q.S. and G.Z. conceived and initiated the project; J.T. and Z.D. performed the biochemical experiments; C.L., Y.X., F.X., L.W., Y.M., X.G. and G.Q. recruited volunteers and collected the sample; Y.Y., P.Y. and Q.S. performed the animal experiments; J.X. performed the docking experiments and prepared graphical illustrations; W.J., Y.G., J.T., Z.D., J.X., Y.Y., C.L. and Y.X. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Sofia Forslund, Michael Zimmerman and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary handling editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Identification of the isolates capable of metabolizing acarbose from human stool samples.
a, Gradually increased acarbose consumption of intestinal microbiota by enrichment using acarbose as the stress. The concentrations of acarbose used in the enrichment experiment were 0.01, 0.1, 1, 10 and 20 mg/ml for passage 1, 2, 3, 4 and 5, respectively. Data are represented as mean ± SD (n = 3). Error bars show SDs. b, Identification of the isolates capable of consuming acarbose. The isolates for testing were cultivated anaerobically in the mGAM medium supplemented with acarbose (1.5 mg/ml) for 24 h. Among the 76 tested isolates, 18 were found to efficiently metabolize acarbose and then identified as K. grimontii strains. c, d, The colony and cell morphology of K. grimontii TD1. The micro-structure of the K. grimontii TD1 cells was observed by scanning electron microscope. e, In vivo degradation of acarbose by K. grimonii TD1 in T2DM mice. A total of 12 T2DM mice were separated into two groups: the group I (n = 5) was gavaged with 50 mg/kg acarbose while the group II (n = 7) was gavaged with both acarbose (50 mg/kg) and K. grimontii TD1 (1011 cfu per mouse). The fecal samples were collected at 3, 6, 18 and 24 h. The residual acarbose contained in feces was assayed by HPLC analysis. Data are presented as mean ± SEM. The statistical significance between two groups was analysed using a two-tailed Student’s t-test.
Extended Data Fig. 2 Separation and purification of enzymes responsible for acarbose degradation in K. grimontii TD1.
a, Separation of total proteins of the K. grimontii TD1 cell lysate using HitrapTM Q HP by FPLC. The fractions 16, 17 and 18 showed catalytic activity towards acarbose. b, Separation of the protein mixture of the above three fractions (16–18) using HitrapTM SP HP by FPLC. The resulting fractions 27‒31 showed catalytic activity towards acarbose. c, A further separation of the protein mixture of the above fractions 27‒31 using Hiload 16/600 Superdex 200. The resulting fractions 28 and 29 showed catalytic activity towards acarbose. Each fraction was incubated with acarbose at 37 °C for 6 h and the concentrations of residual acarbose in the reaction mixture were assayed by HPLC. d, SDS-PAGE analysis of protein fractions from K. grimontii TD1 for testing acarbose catalytic activity. The fractions 27‒32 from size exclusion chromatography (c) were analysed by SDS-PAGE. Among them, the factions 28 and 29 exhibited obvious activity towards acarbose degradation. The red asterisks indicated the protein components with specific high content in these two factions. The marked band in fraction 28 was then used for the proteomic assay. e, Confirmation of the in vivo activity of Apg in E. coli. The E. coli strain that expressed apg was cultivated at 37 °C for 4 h with the supplementation of 1.8 mg/ml of acarbose. The initial (0 h) and residual (4 h) acarbose concentration were determined. Data are represented as the mean ± s. d. (n = 3). Error bars show s. d. Statistical analysis was performed by a two-tailed Student’s t-tes.
Extended Data Fig. 3 A full maximum likelihood phylogenetic tree of Apg homologues across different species and genera.
The numbers on the branches indicate bootstrap supports. The BLASTp search identified 5,000 putative Apg-like enzymes (amino acid identity ≥ 70%, coverage ≥ 80%, e-value ≤ 1e − 10) from 11 genera (Cedecea, Citrobacter, Enterobacter, Klebsiella, Leclercia, Lelliottia, Raoultella, Salmonella, Scandinavium, Shigella and Trabulsiella) as reference.
Extended Data Fig. 4 Abundance analysis of apg+ bacteria in human intestinal microbiota.
Prevalence and abundance of apg+ bacteria in the intestinal microbiota of healthy human (blue, from GMrepo, n = 5,372) and T2DM patients (pink, from GMrepo, n = 247). Embraced numbers indicate the sample size of the categories. All the T2DM patients were treated with acarbose.
Extended Data Fig. 5 The prevalence and abundance of apg+ bacteria among healthy human populations of different gender, country, body mass and age groups.
a, The prevalence of apg+ bacteria in male and female samples. Two-tailed Fisher exact test was performed between the two gender groups. b, The abundance of apg+ bacteria in positive samples of male and female. c, The prevalence of apg+ bacteria among different countries. Two-tailed Fisher exact tests were performed between a specific country and the general ratio derived from all samples. d, The abundance of apg+ bacteria in positive samples from different countries. e, The prevalence of apg+ bacteria among groups of different body mass. Two-tailed Fisher exact tests were performed between the normal group and each abnormal group. f, The abundance of apg+ bacteria in positive samples with different grades of body mass. The body mass categories are defined by the BMI as suggested by the Centers for Disease Control and Prevention of the United States. Underweight: BMI < 18.5, normal: BMI of 18.5~24.9, overweight: BMI of 25.0~29.9, and obesity: BMI ≥ 30. g, The prevalence of apg+ bacteria among different age groups. Two-tailed Fisher exact tests were performed between a specific age group and the general ratio derived from all samples. h, The abundance of apg+ bacteria in positive samples from different age groups. In a, c, e, and g, apg+ is defined as the abundance of apg homolog containing bacteria greater than 0.01%. Embraced numbers indicate the sample size of the categories. P-values are labelled in the scientific notion (for example, 4.58E − 05 means 4.58 times ten raised to the power of −5). In the enhanced box plots of b, d, f, and h, the vertical line in the middle of each distribution indicates the median value. The diamond dots indicate outliers, which are 0.07% outside of a fitted normal distribution. The number of samples in each group is indicated in brackets.
Extended Data Fig. 6 The prevalence of the apg homologs in the intestinal microbiota of T2DM patients.
a, The prevalence of the apg homologs in the intestinal microbiota of 393 enrolled T2DM patients included in the metagenomic analyses. LR: low drug responders; HR: high drug responders. b, The prevalence of the apg homologs in the intestinal microbiota of 22 randomly selected T2DM patients with high or low response to the drug (11 from each group). The intestinal microorganisms in fecal samples of the patients were tested in acarbose metabolism using an ex vivo system. The parameters used to assess the metagenomic abundance of apg homologs in T2DM patients are as follows: identity ≥ 80%, e-value ≤1e − 10 and percent coverage of query and target alignment ≥ 80%.
Supplementary information
Supplementary Data 1
Information of patients with T2DM.
Supplementary Data 2
Raw data of RNA-seq.
Supplementary Data 3
Distribution of Apg-like enzymes in microbial communities.
Supplementary Data 4
Comparison of Apg with homologous proteins for key residues.
Supplementary Tables 1–6
Supplementary Tables 1–6.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, J., Li, C., Dong, Z. et al. Inactivation of the antidiabetic drug acarbose by human intestinal microbial-mediated degradation. Nat Metab 5, 896–909 (2023). https://doi.org/10.1038/s42255-023-00796-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00796-w
This article is cited by
-
Novel Techniques and Models for Studying the Role of the Gut Microbiota in Drug Metabolism
European Journal of Drug Metabolism and Pharmacokinetics (2024)
-
Geroprotector drugs and exercise: friends or foes on healthy longevity?
BMC Biology (2023)
-
Gut bacteria thwart the blood sugar-lowering effect of acarbose
Nature Metabolism (2023)
-
Gut microbiota influences effectiveness of anti-diabetic drug
Nature Reviews Endocrinology (2023)