Abstract
Iron is essential to many fundamental biological processes, but its cellular compartmentalization and concentration must be tightly controlled. Although iron overload can contribute to obesity-associated metabolic deterioration, the subcellular localization and accumulation of iron in adipose tissue macrophages is largely unknown. Here, we show that macrophage mitochondrial iron levels control systemic metabolism in male mice by altering adipocyte iron concentrations. Using various transgenic mouse models to manipulate the macrophage mitochondrial matrix iron content in an inducible fashion, we demonstrate that lowering macrophage mitochondrial matrix iron increases numbers of M2-like macrophages in adipose tissue, lowers iron levels in adipocytes, attenuates inflammation and protects from high-fat-diet-induced metabolic deterioration. Conversely, elevating macrophage mitochondrial matrix iron increases M1-like macrophages and iron levels in adipocytes, exacerbates inflammation and worsens high-fat-diet-induced metabolic dysfunction. These phenotypes are robustly reproduced by transplantation of a small amount of fat from transgenic to wild-type mice. Taken together, we identify macrophage mitochondrial iron levels as a crucial determinant of systemic metabolic homeostasis in mice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated or analysed during this study are available with this paper. Source data are provided with this paper.
References
Gustafson, B., Hedjazifar, S., Gogg, S., Hammarstedt, A. & Smith, U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. 26, 193–200 (2015).
Kloting, N. & Bluher, M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 15, 277–287 (2014).
Sun, K., Kusminski, C. M. & Scherer, P. E. Adipose tissue remodeling and obesity. J. Clin. Invest. 121, 2094–2101 (2011).
Sun, K., Tordjman, J., Clement, K. & Scherer, P. E. Fibrosis and adipose tissue dysfunction. Cell Metab. 18, 470–477 (2013).
Chaurasia, B. & Summers, S. A. Ceramides—lipotoxic inducers of metabolic disorders: (Trends in Endocrinology and Metabolism 26, 538–550; 2015). Trends Endocrinol. Metab. 29, 66–67 (2018).
Perry, R. J., Samuel, V. T., Petersen, K. F. & Shulman, G. I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510, 84–91 (2014).
Kusminski, C. M., Shetty, S., Orci, L., Unger, R. H. & Scherer, P. E. Diabetes and apoptosis: lipotoxicity. Apoptosis 14, 1484–1495 (2009).
Winer, S. & Winer, D. A. The adaptive immune system as a fundamental regulator of adipose tissue inflammation and insulin resistance. Immunol. Cell Biol. 90, 755–762 (2012).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Orr, J. S. et al. Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes 63, 421–432 (2014).
Hubler, M. J., Erikson, K. M., Kennedy, A. J. & Hasty, A. H. MFehi adipose tissue macrophages compensate for tissue iron perturbations in mice. Am. J. Physiol. Cell Physiol. 315, C319–C329 (2018).
Joffin, N. et al. Mitochondrial metabolism is a key regulator of the fibro-inflammatory and adipogenic stromal subpopulations in white adipose tissue. Cell Stem Cell https://doi.org/10.1016/j.stem.2021.01.002 (2021).
Zhang, Z. et al. Adipocyte iron levels impinge on a fat-gut cross-talk to regulate intestinal lipid absorption and mediate protection from obesity. Cell Metab. 33, 1624–1639 (2021).
Ameka, M. K. & Hasty, A. H. Fat and iron don’t mix. Immunometabolism https://doi.org/10.20900/immunometab20200034 (2020).
Gabrielsen, J. S. et al. Adipocyte iron regulates adiponectin and insulin sensitivity. J. Clin. Invest. 122, 3529–3540 (2012).
Zheng, J., Chen, M., Liu, G., Xu, E. & Chen, H. Ablation of hephaestin and ceruloplasmin results in iron accumulation in adipocytes and type 2 diabetes. FEBS Lett. 592, 394–401 (2018).
Mills, E. L. & O’Neill, L. A. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur. J. Immunol. 46, 13–21 (2016).
Van den Bossche, J. et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 17, 684–696 (2016).
Aerbajinai, W. et al. Glia maturation factor-gamma regulates murine macrophage iron metabolism and M2 polarization through mitochondrial ROS. Blood Adv. 3, 1211–1225 (2019).
Drapier, J. C. & Hibbs, J. B. Jr. Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in l-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J. Immunol. 140, 2829–2838 (1988).
Soares, M. P. & Hamza, I. Macrophages and iron metabolism. Immunity 44, 492–504 (2016).
Tong, W. H. et al. TLR-activated repression of Fe–S cluster biogenesis drives a metabolic shift and alters histone and tubulin acetylation. Blood Adv. 2, 1146–1156 (2018).
Barros, M. H., Hauck, F., Dreyer, J. H., Kempkes, B. & Niedobitek, G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE 8, e80908 (2013).
Moreno-Navarrete, J. M. et al. HMOX1 as a marker of iron excess-induced adipose tissue dysfunction, affecting glucose uptake and respiratory capacity in human adipocytes. Diabetologia 60, 915–926 (2017).
Lee, W., Yun, S., Choi, G. H. & Jung, T. W. Fibronectin type III domain containing 4 attenuates hyperlipidemia-induced insulin resistance via suppression of inflammation and ER stress through HO-1 expression in adipocytes. Biochem. Biophys. Res. Commun. 502, 129–136 (2018).
Kusminski, C. M. et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 18, 1539–1549 (2012).
Kusminski, C. M., Park, J. & Scherer, P. E. MitoNEET-mediated effects on browning of white adipose tissue. Nat. Commun. 5, 3962 (2014).
Corsi, B. et al. Human mitochondrial ferritin expressed in HeLa cells incorporates iron and affects cellular iron metabolism. J. Biol. Chem. 277, 22430–22437 (2002).
Kusminski, C. M. et al. A novel model of diabetic complications: adipocyte mitochondrial dysfunction triggers massive beta-cell hyperplasia. Diabetes 69, 313–330 (2020).
Zhu, Q. et al. Suppressing adipocyte inflammation promotes insulin resistance in mice. Mol. Metab. 39, 101010 (2020).
Fernandez-Real, J. M., Lopez-Bermejo, A. & Ricart, W. Cross-talk between iron metabolism and diabetes. Diabetes 51, 2348–2354 (2002).
Ford, E. S. & Cogswell, M. E. Diabetes and serum ferritin concentration among US adults. Diabetes Care 22, 1978–1983 (1999).
Jiang, R. et al. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 291, 711–717 (2004).
Forouhi, N. G. et al. Elevated serum ferritin levels predict new-onset type 2 diabetes: results from the EPIC-Norfolk prospective study. Diabetologia 50, 949–956 (2007).
Corna, G. et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica 95, 1814–1822 (2010).
Recalcati, S. et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur. J. Immunol. 40, 824–835 (2010).
Hubler, M. J., Peterson, K. R. & Hasty, A. H. Iron homeostasis: a new job for macrophages in adipose tissue? Trends Endocrinol. Metab. 26, 101–109 (2015).
de Mello, A. H., Costa, A. B., Engel, J. D. G. & Rezin, G. T. Mitochondrial dysfunction in obesity. Life Sci. 192, 26–32 (2018).
Caslin, H. L., Bhanot, M., Bolus, W. R. & Hasty, A. H. Adipose tissue macrophages: unique polarization and bioenergetics in obesity. Immunol. Rev. 295, 101–113 (2020).
Rodriguez-Prados, J. C. et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol. 185, 605–614 (2010).
Pereira, M. et al. Acute iron deprivation reprograms human macrophage metabolism and reduces inflammation in vivo. Cell Rep. 28, 498–511 (2019).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Jha, A. K. et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42, 419–430 (2015).
El Kasmi, K. C. & Stenmark, K. R. Contribution of metabolic reprogramming to macrophage plasticity and function. Semin. Immunol. 27, 267–275 (2015).
Galvan-Pena, S. & O’Neill, L. A. Metabolic reprograming in macrophage polarization. Front Immunol. 5, 420 (2014).
Crooks, D. R. et al. Acute loss of iron-sulfur clusters results in metabolic reprogramming and generation of lipid droplets in mammalian cells. J. Biol. Chem. 293, 8297–8311 (2018).
Behmoaras, J. The versatile biochemistry of iron in macrophage effector functions. FEBS J. 288, 6972–6989 (2021).
Kelly, B. & O’Neill, L. A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 25, 771–784 (2015).
Palsson-McDermott, E. M. et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 21, 65–80 (2015).
Mounier, R. et al. AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 18, 251–264 (2013).
Shapiro, H., Lutaty, A. & Ariel, A. Macrophages, meta-inflammation, and immuno-metabolism. ScientificWorldJournal 11, 2509–2529 (2011).
Angajala, A. et al. Diverse roles of mitochondria in immune responses: novel insights into immuno-metabolism. Front Immunol. 9, 1605 (2018).
Wang, H., Liu, C., Zhao, Y. & Gao, G. Mitochondria regulation in ferroptosis. Eur. J. Cell Biol. 99, 151058 (2020).
Sumneang, N., Siri-Angkul, N., Kumfu, S., Chattipakorn, S. C. & Chattipakorn, N. The effects of iron overload on mitochondrial function, mitochondrial dynamics, and ferroptosis in cardiomyocytes. Arch. Biochem. Biophys. 680, 108241 (2020).
Tajima, S. et al. Iron reduction by deferoxamine leads to amelioration of adiposity via the regulation of oxidative stress and inflammation in obese and type 2 diabetes KKAy mice. Am. J. Physiol. Endocrinol. Metab. 302, E77–E86 (2012).
Green, A., Basile, R. & Rumberger, J. M. Transferrin and iron induce insulin resistance of glucose transport in adipocytes. Metabolism 55, 1042–1045 (2006).
Cooksey, R. C. et al. Dietary iron restriction or iron chelation protects from diabetes and loss of beta-cell function in the obese (ob/ob lep−/−) mouse. Am. J. Physiol. Endocrinol. Metab. 298, E1236–E1243 (2010).
Minamiyama, Y. et al. Iron restriction improves type 2 diabetes mellitus in Otsuka Long-Evans Tokushima fatty rats. Am. J. Physiol. Endocrinol. Metab. 298, E1140–E1149 (2010).
Valenti, L. et al. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: evidence from a case–control study. Am. J. Gastroenterol. 102, 1251–1258 (2007).
Valenti, L. et al. Venesection for non-alcoholic fatty liver disease unresponsive to lifestyle counselling—a propensity score-adjusted observational study. QJM 104, 141–149 (2011).
Gao, Y. et al. Adipocyte iron regulates leptin and food intake. J. Clin. Invest. 125, 3681–3691 (2015).
Tomay, F. et al. Purple corn extract induces long-lasting reprogramming and M2 phenotypic switch of adipose tissue macrophages in obese mice. J. Transl. Med 17, 237 (2019).
Weiss, G., Bogdan, C. & Hentze, M. W. Pathways for the regulation of macrophage iron metabolism by the anti-inflammatory cytokines IL-4 and IL-13. J. Immunol. 158, 420–425 (1997).
Brock, J. H., Djeha, A., Ismail, M., Oria, R. & Sinclair, R. H. Cellular responses to iron and iron compounds. Adv. Exp. Med Biol. 356, 91–100 (1994).
Weiss, G. et al. Iron modulates interferon-gamma effects in the human myelomonocytic cell line THP-1. Exp. Hematol. 20, 605–610 (1992).
Sugimoto, M. et al. MMMDB: Mouse Multiple Tissue Metabolome Database. Nucleic Acids Res. 40, D809–D814 (2012).
Fraenkel, P. G. Anemia of inflammation: a review. Med. Clin. North Am. 101, 285–296 (2017).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Peics, J. et al. Isolation of adipogenic and fibro-inflammatory stromal cell subpopulations from murine intra-abdominal adipose depots. J. Vis. Exp. https://doi.org/10.3791/61610 (2020).
Pinero, D. J., Li, N., Hu, J., Beard, J. L. & Connor, J. R. The intracellular location of iron regulatory proteins is altered as a function of iron status in cell cultures and rat brain. J. Nutr. 131, 2831–2836 (2001).
Gao, Y. et al. Iron downregulates leptin by suppressing protein O-GlcNAc modification in adipocytes, resulting in decreased levels of O-glycosylated CREB. J. Biol. Chem. 294, 5487–5495 (2019).
Acknowledgements
We thank the UTSW Animal Resource Center, Histology Core, Metabolic Phenotyping Core, the Live Cell Imaging Core, Transgenic Core and Flow Cytometry Facility for their excellent assistance with experiments performed in this paper. We also thank Shimadzu Scientific Instruments for the collaborative efforts in mass spectrometry technology resources. This study was supported by US National Institute of Health grants RC2-DK118620, R01-DK55758, R01-DK099110, R01-DK127274 and R01-DK131537 to P.E.S.; R01 DK108773 to D.Y.O.; C.C. is supported by K99-DK122019 and R00-DK122019. C.M.G. is supported by F32-DK-122623; V.A.P. was supported by an American Diabetes Association Minority Postdoctoral Fellowship (1-18-PMF-030). J.-B.F. was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; grant 414232833).
Author information
Authors and Affiliations
Contributions
Conceptualization, N.J., C.M.G, V.A.P., D.Y.O. and P.E.S.; methodology, N.J. and C.M.G; formal analysis, N.J., V.A.P. and C.C; investigation, N.J., C.M.G., J.-B.F., V.A.P., C.C., S.C., R.G. and C.M.K.; resources, W.J.G.; original draft writing, N.J. and P.E.S.; manuscript review and editing, N.J., C.M.G., J.-B.F. and P.E.S.; supervision, P.E.S.; funding acquisition, P.E.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Alyssa Hasty and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
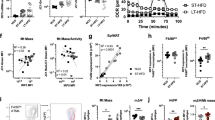
Extended Data Fig. 1 Validation of MitoNEET overexpression in macrophages.
(a-j) Six-week-old male control and Mac-MitoNEETTG mice were fed chow dox for 2 weeks. (a) Breeding strategy. (b) Cisd1 mRNA levels relative to control in monocytes (control n = 4, TG n = 3), ATMs (n = 3), and peritoneal macrophages (n = 6). (c) MitoNEET protein expression relative to β-actin in peritoneal macrophages (n = 6). (d) Representative Western blots chosen from 2 independent experiments. (e-f) Mitochondrial respiration in peritoneal macrophages (control n = 3, TG n = 5). (g) Mitochondrial electron transport chain protein expression relative to prohibitin in peritoneal macrophages (n = 6). (h) Representative Western blots. (i-j) Glycolysis stress test in peritoneal macrophages (control n = 6, TG n = 6). Significance in (b-c, f-g, j) between control and Mac-MitoNEETTG was calculated using a two-tailed Student’s t-test. Significance in (e, i) was calculated using a 2-way ANOVA with Sidak’s post-test for multiple comparisons. Error bars represent mean ± S.E.M. * (P < 0.05), ** (p < 0.01), *** (p < 0.0001), **** (p < 0.00001).
Extended Data Fig. 2 Validation of Ftmt overexpression in macrophages.
(a-j) Six-week-old male control and Mac-FtmtTG mice were fed chow dox for 2 weeks. (a) Breeding strategy. (b) Ftmt mRNA levels relative to control in peritoneal macrophages (control n = 7, TG n = 9). (c) FTMT protein expression relative to β-actin in peritoneal macrophages (control n = 5, TG n = 3). (d) Representative Western blots chosen from 2 independent experiments. (e-f) Mitochondrial respiration in peritoneal macrophages (control n = 6, TG n = 7). (g) Mitochondrial electron transport chain protein expression relative to prohibitin in peritoneal macrophages (control n = 5, TG n = 3). (h) Representative Western blots (control n = 5, TG n = 3). (i-j) Glycolysis stress test in peritoneal macrophages (control n = 4, TG n = 3). Significance in (b-c, f-g, j) between control and Mac-FtmtTG was calculated using a two-tailed Student’s t-test. Significance in (e, i) was calculated using a 2-way ANOVA with Sidak’s post-test for multiple comparisons. Error bars represent mean ± S.E.M. * (P < 0.05), ** (p < 0.01), *** (p < 0.0001), **** (p < 0.00001).
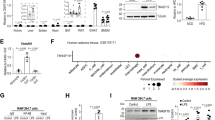
Extended Data Fig. 3 Macrophages respond to mitoNEET overexpression by up-regulating iron storage.
(a-q) Male control and Mac-MitoNEETTG mice were fed chow dox for 2 weeks. (a) Labile iron pool (LIP) in mitochondrial and cytosolic compartments of peritoneal macrophages (control n = 7, TG n = 6). (b) mRNA levels of iron metabolism genes relative to control in peritoneal macrophages (control n = 10, TG n = 7). (c) Ferritin (control n = 9, TG n = 12), (d) Ferroportin (control n = 3, TG n = 5), (e) CD163 (control n = 3, TG n = 5), (f) CD91 (control n = 3, TG n = 5) and (g) FTMT (control n = 3, TG n = 5) protein expression relative to β-actin in peritoneal macrophages. (h) Representative Western blots chosen from 2 independent experiments (i) mRNA levels of inflammation markers relative to control in peritoneal macrophages (control n = 7, TG n = 10). (j-l) Flow cytometric measurement of total (control n = 6, TG n = 5), M1-like control n = 6, TG n = 5) and M2-like macrophages in eWAT (n = 6). (m) Body weight (control n = 3, TG n = 5). (n-o) Oral glucose tolerance test (control n = 3, TG n = 5). (n) Blood glucose levels. (o) Serum insulin levels. (p) Blood glucose levels during insulin tolerance test (control n = 3, TG n = 5). (q) Serum triglyceride levels during triglyceride clearance (n = 6). Significance in (a-g, i-m) between control and Mac-MitoNEETTG was calculated using a two-tailed Student’s t-test. Significance in (n-q) was calculated using a 2-way ANOVA with Sidak’s post-test for multiple comparisons. Error bars represent mean ± S.E.M. * (P < 0.05), ** (p < 0.01), *** (p < 0.0001), **** (p < 0.00001).
Extended Data Fig. 4 Macrophages respond to Ftmt overexpression by up-regulating iron uptake.
(a-q) Male control and Mac-FtmtTG mice were fed chow dox for 2 weeks. (a) Labile iron pool (LIP) in mitochondrial and cytosolic compartments of peritoneal macrophages (n = 4). (b) mRNA levels of iron metabolism genes relative to control in peritoneal macrophages (control n = 5, TG n = 7). (c) CD91 (n = 8), (d) CD163 (n = 8), (e) Ferritin (n = 8), (f) Ferroportin (n = 4) and (g) MitoNEET (n = 8) protein expression relative to β-actin in peritoneal macrophages (n = 8). (h) Representative Western blots chosen from 2 independent experiments. (i) mRNA levels of inflammation markers relative to control in peritoneal macrophages (control n = 5; TG n = 7). (j-l) Flow cytometric measurement of total, M1-like and M2-like macrophages in eWAT (n = 6). (m) Body weight (n = 6). (n-o) Oral glucose tolerance test (n = 6). (n) Blood glucose levels (n = 6). (o) Serum insulin levels (control n = 7, TG n = 9). (p) Blood glucose levels during insulin tolerance test (control n = 6, TG n = 5). (q) Serum triglyceride levels during triglyceride clearance (n = 6). Significance in (a-g, i-m) between control and Mac-FtmtTG was calculated using a two-tailed Student’s t-test. Significance in (n-q) was calculated using a 2-way ANOVA with Sidak’s post-test for multiple comparisons. Error bars represent mean ± S.E.M. * (P < 0.05), ** (p < 0.01), *** (p < 0.0001), **** (p < 0.00001).
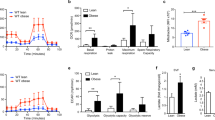
Extended Data Fig. 5 Mitochondrial iron depletion in macrophages during HFD increases iron recycling, protecting adipocytes from iron overload.
(a-x) Six-week-old male control and Mac-MitoNEETTG mice were fed HFD dox for 6 weeks. (a) Labile iron pool (LIP) in ATMs from eWAT (control n = 7, TG n = 7) (b) Total iron content measured by ICP-MS in mitochondrial and cytosolic compartments in peritoneal macrophages (control n = 7, TG n = 5). (c-d) LIP in (c) adipocytes and (d) SVF from eWAT (control n = 7, TG n = 5). (e-f) mRNA levels of iron metabolism genes relative to control (e) adipocytes (control n = 5, TG n = 6) and (f) ATMs (control n = 6, TG n = 5) from eWAT. (g) Ferroportin and (h) Ferritin protein expression relative to β-actin in adipocytes from eWAT (n = 6). (i) Representative Western blots chosen from 2 independent experiments. (j) CD163 (n = 9), (k) CD91, (l) Ferroportin, and (m) Ferritin protein expression relative to β-actin in ATMs from eWAT (control n = 9, TG n = 6). (n) Representative Western blots chosen from 2 independent experiments. (o) mRNA levels of mitochondrial iron metabolism genes relative to control in ATMs from eWAT (control n = 5, TG n = 6). (p) MitoNEET and (q) Mitoferritin protein expression relative to β-actin in ATMs from eWAT (n = 3). (r) MitoNEET protein expression relative to β-actin in adipocytes from eWAT (control n = 9, TG n = 6). (s) LIP in mitochondrial and cytosolic compartments of eWAT (control n = 5, TG n = 4). (t-u) mRNA levels of inflammatory marker genes relative to control in (t) ATMs and (u) adipocytes from eWAT (control n = 5, TG n = 6). (v) Representative fluorescence microscopic images of 4-HNE, Perilipin and DAPI staining of eWAT chosen from 3 independent experiments. (w) Quantification of 4-HNE fluorescence in eWAT relative to control (control n = 8, TG n = 7). (x) Protein carbonylation in adipocytes from eWAT (control n = 10, TG n = 11). Significance in (a-h, j-u, w-x) between control and Mac-MitoNEETTG was calculated using a two-tailed Student’s t-test. Error bars represent mean ± S.E.M. * (P < 0.05), ** (p < 0.01), *** (p < 0.0001), **** (p < 0.00001).
Extended Data Fig. 6 Mitochondrial iron overload in macrophages during HFD promotes adipocyte iron overload and inflammation.
(a-q) Six-week-old male control and Mac-FtmtTG mice were fed HFD dox for 6 weeks. (a) Total iron content measured by ICP-MS in mitochondrial (n = 8) and cytosolic (control n = 4, TG n = 5) compartments in peritoneal macrophages. (b-d) Labile iron pool (LIP) in (b) ATMs (control n = 4, TG n = 5), (c) adipocytes (control n = 9, TG n = 12) and (d) SVF (control n = 7, TG n = 5) from eWAT. (e-f) mRNA levels of iron metabolism genes relative to control in (e) adipocytes (n = 5) and (f) ATMs (n = 6) from eWAT. (g) Ferritin (control n = 7, TG n = 12) and (h) Ferroportin (n = 3) protein expression relative to β-actin in adipocytes from eWAT. (i) Representative Western blots chosen from 3 independent experiments. (j) Ferritin (control n = 7, TG n = 9) and (k) Ferroportin protein expression relative to β-actin in ATMs from eWAT (n = 3). (l) Representative Western blots chosen from 3 independent experiments. (m) mRNA levels of mitochondrial iron metabolism genes relative to control in ATMs from eWAT (n = 6). (i) MitoNEET protein expression relative to β-actin in adipocytes from eWAT (control n = 4, TG n = 10). (o) LIP in mitochondrial and cytosolic compartments of eWAT (n = 4). (p-q) mRNA levels of inflammatory marker genes relative to control in (p) ATMs and (q) adipocytes from eWAT (n = 5). Significance in (a-h, j-k, m-q) between control and Mac-FtmtTG was calculated using a two-tailed Student’s t-test. Error bars represent mean ± S.E.M. * (P < 0.05), ** (p < 0.01), *** (p < 0.0001), **** (p < 0.00001).
Extended Data Fig. 7 Eliminating MitoNEET in macrophages during HFD promotes insulin resistance and inflammation.
(a-k) Six-week-old male control and Mac-MitoNEETKO mice were fed HFD dox for 7 weeks. (a) Breeding strategy. (b) MitoNEET protein expression relative to β-actin in peritoneal macrophages (n = 3). (c) Representative Western blots (n = 3). (d) Body weight (control n = 9, TG n = 5). (e) Tissue weights (control n = 9, TG n = 5). (f-g) Oral glucose tolerance test. (f) Blood glucose levels (control n = 5, TG n = 3). (g) Serum insulin levels (control n = 9, TG n = 5). (h) Blood glucose levels during insulin tolerance test (control n = 9, TG n = 5). (i-k) Flow cytometric measurement of total, M1-like and M2-like macrophages in eWAT (control n = 5, TG n = 3). Significance in (b, e, i-k) between control and Mac-MitoNEETKO was calculated using a two-tailed Student’s t-test. Significance in (d, f-h) was calculated using a 2-way ANOVA with Sidak’s post-test for multiple comparisons. Error bars represent mean ± S.E.M. * (P < 0.05), ** (p < 0.01), *** (p < 0.0001), **** (p < 0.00001).
Extended Data Fig. 8 Metabolic analyses of Mac-MitoNEETTG mice.
(a-e) Six-week-old male control and Mac-MitoNEETTG mice were fed HFD for 20 weeks and then switched to HFD dox for 1 week. Metabolic analyses were performed at 3 d before and 7 d after diet change (n = 6). (a) Experimental design. (b) Body weight (n = 5). (c) Hourly food intake (n = 6). (d) Respiratory exchange ratio (n = 6). (e) Energy balance (n = 6). Significance in (b-e) between control and Mac-MitoNEETTG was calculated using a 2-way ANOVA with Sidak’s post-test for multiple comparisons. Error bars represent mean ± S.E.M. * (P < 0.05), ** (p < 0.01), *** (p < 0.0001), **** (p < 0.00001).
Extended Data Fig. 9 Validation of eWAT transplantation to wild-type mice.
(a-i) Six-week-old male control and Mac-MitoNEETTG mice were injected with PKH67 dye before transplantation of their eWAT to wild-type mice (n = 5). Recipient mice were allowed to recover 3 weeks and then fed HFD dox for 6 weeks. (a-b) Flow cytometric measurement of PKH67 fluorescence in (a) graft and (b) recipient eWAT (n = 5). (c-d) mRNA levels of Cisd1, Ftmt, and Tfam relative to control in (c) graft and (d) recipient eWAT (n = 5). (e-g) Flow cytometric measurement of total, M1-like, and M2-like macrophages in recipient eWAT (n = 5). (h-i) Wild-type mice were transplanted with either control or Mac-FtmtTG eWAT. Transplanted mice were allowed to recover 3 weeks and then fed HFD dox for 6 weeks. (h-i) mRNA levels of Cisd1, Ftmt and Tfam relative to control in (c) graft and (d) recipient eWAT (control n = 7, TG n = 8). Significance in (a-i) between control and transgenic fat-transplanted animals was calculated using a two-tailed Student’s t-test. Error bars represent mean ± S.E.M. * (P < 0.05), ** (p < 0.01), *** (p < 0.0001), **** (p < 0.00001).
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Supplementary Tables 1–3
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 1
Statistics and raw data.
Source Data Fig. 2
Statistics and raw data.
Source Data Fig. 3
Statistics and raw data.
Source Data Fig. 4
Statistics and raw data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 5
Statistics and raw data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 6
Statistics and raw data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Fig. 7
Statistics and raw data.
Source Data Fig. 8
Unprocessed western blots.
Source Data Fig. 8
Statistics and raw data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistics and raw data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistics and raw data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistics and raw data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistics and raw data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistics and raw data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistics and raw data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 7
Statistics and raw data.
Source Data Extended Data Fig. 8
Statistics and raw data.
Source Data Extended Data Fig. 9
Statistics and raw data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joffin, N., Gliniak, C.M., Funcke, JB. et al. Adipose tissue macrophages exert systemic metabolic control by manipulating local iron concentrations. Nat Metab 4, 1474–1494 (2022). https://doi.org/10.1038/s42255-022-00664-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00664-z
This article is cited by
-
Macrophage and T cell networks in adipose tissue
Nature Reviews Endocrinology (2024)
-
Unraveling the complex roles of macrophages in obese adipose tissue: an overview
Frontiers of Medicine (2024)
-
Macrophage mitochondrial levels of iron affect adipose tissue function in obesity
Nature Reviews Endocrinology (2023)
-
Obesity-induced and weight-loss-induced physiological factors affecting weight regain
Nature Reviews Endocrinology (2023)
-
Hepcidin deficiency in mice impairs white adipose tissue browning possibly due to a defect in de novo adipogenesis
Scientific Reports (2023)