Abstract
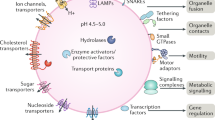
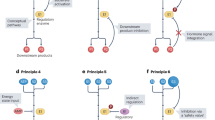
Metabolism has historically been studied at the levels of whole cells, whole tissues and whole organisms. As a result, our understanding of how compartmentalization—the spatial and temporal separation of pathways and components—shapes organismal metabolism remains limited. At its essence, metabolic compartmentalization fulfils three important functions or ‘pillars’: establishing unique chemical environments, providing protection from reactive metabolites and enabling the regulation of metabolic pathways. However, how these pillars are established, regulated and maintained at both the cellular and systemic levels remains unclear. Here we discuss how the three pillars are established, maintained and regulated within the cell and discuss the consequences of dysregulation of metabolic compartmentalization in human disease. Organelles are increasingly emerging as ‘command-and-control centres’ and the increased understanding of metabolic compartmentalization is revealing new aspects of metabolic homeostasis, with this knowledge being translated into therapies for the treatment of cancer and certain neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wishart, D. S. et al. HMDB 5.0: the Human Metabolome Database for 2022. Nucleic Acids Res. 50, D622–D631 (2022).
Ma, H. et al. The Edinburgh Human Metabolic Network reconstruction and its functional analysis. Mol. Syst. Biol. 3, 135 (2007).
Waters, D. et al. Global birth prevalence and mortality from inborn errors of metabolism: a systematic analysis of the evidence. J. Glob. Health 8, 021102 (2018).
Ferreira, C. R., van Karnebeek, C. D. M., Vockley, J. & Blau, N. A proposed nosology of inborn errors of metabolism. Genet. Med. 21, 102–106 (2019).
Inborn Errors of Metabolism Knowledgebase (IEMbase, accessed January 2022); http://www.iembase.org
Ovadi, J. & Saks, V. On the origin of intracellular compartmentation and organized metabolic systems. Mol. Cell. Biochem. 256–257, 5–12 (2004).
O’Flynn, B. G. & Mittag, T. The role of liquid–liquid phase separation in regulating enzyme activity. Curr. Opin. Cell Biol. 69, 70–79 (2021).
Prouteau, M. & Loewith, R. Regulation of cellular metabolism through phase separation of enzymes. Biomolecules 8, 160 (2018).
Sweetlove, L. J. & Fernie, A. R. The role of dynamic enzyme assemblies and substrate channelling in metabolic regulation. Nat. Commun. 9, 2136 (2018).
Go, Y.-M. & Jones, D. P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta 1780, 1273–1290 (2009).
Zeng, J., Shirihai, O. S. & Grinstaff, M. W. Modulating lysosomal pH: a molecular and nanoscale materials design perspective. J. Life Sci. (Westlake Village) 2, 25–37 (2020).
Huttanus, H. M. & Feng, X. Compartmentalized metabolic engineering for biochemical and biofuel production. Biotechnol. J. 12, 1700052 (2017).
Kulagina, N., Besseau, S., Papon, N. & Courdavault, V. Peroxisomes: a new hub for metabolic engineering in yeast. Front. Bioeng. Biotechnol. 9, 659431 (2021).
Tauqeer Alam, M. et al. The self-inhibitory nature of metabolic networks and its alleviation through compartmentalization. Nat. Commun. 8, 16018 (2017).
Alberty, R. A. Equilibrium concentrations for pyruvate dehydrogenase and the citric acid cycle at specified concentrations of certain coenzymes. Biophys. Chem. 109, 73–84 (2004).
Alberty, R. A. Standard molar entropies, standard entropies of formation, and standard transformed entropies of formation in the thermodynamics of enzyme-catalyzed reactions. J. Chem. Thermodyn. 38, 396–404 (2006).
Mindell, J. A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 74, 69–86 (2012).
Srere, P. A. The metabolon. Trends Biochem. Sci. 10, 109–110 (1985).
Castellana, M. et al. Enzyme clustering accelerates processing of intermediates through metabolic channeling. Nat. Biotechnol. 32, 1011–1018 (2014).
Maier, T., Jenni, S. & Ban, N. Architecture of mammalian fatty acid synthase at 4.5 Å resolution. Science 311, 1258–1262 (2006).
Maier, T., Leibundgut, M. & Ban, N. The crystal structure of a mammalian fatty acid synthase. Science 321, 1315–1322 (2008).
Miura, N. Condensate formation by metabolic enzymes in Saccharomyces cerevisiae. Microorganisms 10, 232 (2022).
Webb, B., Dosey, A., Wittmann, T., Kollman, J. & Barber, D. The glycolytic enzyme phosphofructokinase-1 assembles into filaments. J. Cell Biol. 216, 2305–2313 (2017).
Barry, R. M. et al. Large-scale filament formation inhibits the activity of CTP synthetase. eLife 3, e03638 (2014).
Lynch, E. M. et al. Human CTP synthase filament structure reveals the active enzyme conformation. Nat. Struct. Mol. Biol. 24, 507–514 (2017).
French, J. B. et al. Spatial colocalization and functional link of purinosomes with mitochondria. Science 351, 733–737 (2016).
Doigneaux, C. et al. Hypoxia drives the assembly of the multienzyme purinosome complex. J. Biol. Chem. 295, 9551–9566 (2020).
Yamada, S., Sato, A. & Sakakibara, S. Nwd1 regulates neuronal differentiation and migration through purinosome formation in the developing cerebral cortex. iScience 23, 101058 (2020).
Lippincott-Schwartz, J., Roberts, T. H. & Hirschberg, K. Secretory protein trafficking and organelle dynamics in living cells. Annu. Rev. Cell Dev. Biol. 16, 557–589 (2000).
Hao, T., Ma, H. W., Zhao, X. M. & Goryanin, I. Compartmentalization of the Edinburgh Human Metabolic Network. BMC Bioinformatics 11, 393 (2010).
Jennings, M. L. Carriers, exchangers, and cotransporters in the first 100 years of the Journal of General Physiology. J. Gen. Physiol. 150, 1063–1080 (2018).
Vasiliou, V., Vasiliou, K. & Nebert, D. W. Human ATP-binding cassette (ABC) transporter family. Hum. Genomics 3, 281–290 (2009).
Pizzagalli, M. D., Bensimon, A. & Superti-Furga, G. A guide to plasma membrane solute carrier proteins. FEBS J. 288, 2784–2835 (2021).
Ehinger, J. K. et al. Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat. Commun. 7, 12317 (2016).
Longo, N., Frigeni, M. & Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 1863, 2422–2435 (2016).
Cesar-Razquin, A. et al. A call for systematic research on solute carriers. Cell 162, 478–487 (2015).
Ruprecht, J. J. & Kunji, E. R. S. The SLC25 mitochondrial carrier family: structure and mechanism. Trends Biochem. Sci. 45, 244–258 (2020).
Xu, Y. et al. Cardiolipin remodeling enables protein crowding in the inner mitochondrial membrane. EMBO J. 40, e108428 (2021).
Paradies, G., Paradies, V., De Benedictis, V., Ruggiero, F. M. & Petrosillo, G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim. Biophys. Acta 1837, 408–417 (2014).
Dudek, J. Role of cardiolipin in mitochondrial signaling pathways. Front. Cell Dev. Biol. 5, 90 (2017).
Tyurina, Y. Y. et al. A mitochondrial pathway for biosynthesis of lipid mediators. Nat. Chem. 6, 542–552 (2014).
Liu, G.-Y. et al. The phospholipase iPLA2γ is a major mediator releasing oxidized aliphatic chains from cardiolipin, integrating mitochondrial bioenergetics and signaling. J. Biol. Chem. 292, 10672–10684 (2017).
Patwardhan, A. M., Scotland, P. E., Akopian, A. N. & Hargreaves, K. M. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc. Natl Acad. Sci. USA 106, 18820–18824 (2009).
Ku, G., Thomas, C. E., Akeson, A. L. & Jackson, R. L. Induction of interleukin 1 beta expression from human peripheral blood monocyte-derived macrophages by 9-hydroxyoctadecadienoic acid. J. Biol. Chem. 267, 14183–14188 (1992).
Alsalem, M. et al. The contribution of the endogenous TRPV1 ligands 9-HODE and 13-HODE to nociceptive processing and their role in peripheral inflammatory pain mechanisms. Br. J. Pharmacol. 168, 1961–1974 (2013).
Chu, C. T. et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell Biol. 15, 1197–1205 (2013).
Kagan, V. E. et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 1, 223–232 (2005).
Campbell, S. L. & Wellen, K. E. Metabolic signaling to the nucleus in cancer. Mol. Cell 71, 398–408 (2018).
Liu, X. J. et al. The existence of a nonclassical TCA cycle in the nucleus that wires the metabolic–epigenetic circuitry. Signal Transduct. Target. Ther. 6, 375 (2021).
Nagaraj, R. et al. Nuclear localization of mitochondrial TCA cycle enzymes as a critical step in mammalian zygotic genome activation. Cell 168, 210–223.e11 (2017).
Trefely, S. et al. Quantitative subcellular acyl-CoA analysis reveals distinct nuclear metabolism and isoleucine-dependent histone propionylation. Mol. Cell 82, 447–462 (2022).
Anderson, D. D., Woeller, C. F., Chiang, E. P., Shane, B. & Stover, P. J. Serine hydroxymethyltransferase anchors de novo thymidylate synthesis pathway to nuclear lamina for DNA synthesis. J. Biol. Chem. 287, 7051–7062 (2012).
MacFarlane, A. J. et al. Nuclear localization of de novo thymidylate biosynthesis pathway is required to prevent uracil accumulation in DNA. J. Biol. Chem. 286, 44015–44022 (2011).
Giardina, G. et al. The catalytic activity of serine hydroxymethyltransferase is essential for de novo nuclear dTMP synthesis in lung cancer cells. FEBS J. 285, 3238–3253 (2018).
Wagner, G. R. et al. A Class of Reactive Acyl-CoA Species Reveals the Non-enzymatic Origins of Protein Acylation. Cell Metab. 25, 823–837.e828 (2017).
Allaman, I., Belanger, M. & Magistretti, P. J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 9, 23 (2015).
Shi, Y. et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941–953 (2004).
Alfonso, L., Ai, G., Spitale, R. C. & Bhat, G. J. Molecular targets of aspirin and cancer prevention. Br. J. Cancer 111, 61–67 (2014).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 (2014).
Chen, X., Kang, R., Kroemer, G. & Tang, D. Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 18, 280–296 (2021).
Murphy, M. P. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 (2009).
Wang, Y., Branicky, R., Noe, A. & Hekimi, S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 217, 1915–1928 (2018).
Morgan, B. et al. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat. Chem. Biol. 12, 437–443 (2016).
Pak, V. V. et al. Ultrasensitive genetically encoded indicator for hydrogen peroxide identifies roles for the oxidant in cellmigration and mitochondrial function. Cell Metab. 31, 642–653.e646 (2020).
Hoehne, M. N. et al. Spatial and temporal control of mitochondrial H2O2 release in intact human cells. EMBO J. 41, e109169 (2022).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
Chouchani, E. T. et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532, 112–116 (2016).
Bar-Peled, L. et al. Chemical proteomics identifies druggable vulnerabilities in a genetically defined cancer. Cell 171, 696–709.e623 (2017).
Mills, E. L. et al. Cysteine 253 of UCP1 regulates energy expenditure and sex-dependent adipose tissue inflammation. Cell Metab. 34, 140–157.e148 (2022).
Dixon, S. J. & Stockwell, B. R. The hallmarks of ferroptosis. Annu. Rev. Cancer Biol. 3, 35–54 (2019).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Zou, Y. et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 585, 603–608 (2020).
Lodhi, I. J. & Semenkovich, C. F. Peroxisomes: a nexus for lipid metabolism and cellular signaling. Cell Metab. 19, 380–392 (2014).
Dixon, S. J. et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem. Biol. 10, 1604–1609 (2015).
Torii, S. et al. An essential role for functional lysosomes in ferroptosis of cancer cells. Biochem. J. 473, 769–777 (2016).
Tian, R. et al. Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis. Nat. Neurosci. 24, 1020–1034 (2021).
Yuan, H., Li, X., Zhang, X., Kang, R. & Tang, D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem. Biophys. Res. Commun. 478, 838–844 (2016).
Hangauer, M. J. et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250 (2017).
Cabanos, H. F. & Hata, A. N. Emerging insights into targeted therapy-tolerant persister cells in cancer. Cancers (Basel) 13, 2666 (2021).
Goldstein, J. L., DeBose-Boyd, R. A. & Brown, M. S. Protein sensors for membrane sterols. Cell 124, 35–46 (2006).
Sancak, Y. et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 (2008).
Zoncu, R. et al. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science 334, 678–683 (2011).
Wolfson, R. L. et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48 (2016).
Chantranupong, L. et al. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell 165, 153–164 (2016).
Bar-Peled, L. et al. A tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340, 1100–1106 (2013).
Castellano, B. M. et al. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science 355, 1306–1311 (2017).
Menon, S. et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell 156, 771–785 (2014).
Settembre, C. et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095–1108 (2012).
Abu-Remaileh, M. et al. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science 358, 807–813 (2017).
Torrence, M. E. et al. The mTORC1-mediated activation of ATF4 promotes protein and glutathione synthesis downstream of growth signals. eLife 10, e63326 (2021).
Condon, K. J. et al. Genome-wide CRISPR screens reveal multitiered mechanisms through which mTORC1 senses mitochondrial dysfunction. Proc. Natl Acad. Sci. USA 118, e2022120188 (2021).
Ye, J. B. et al. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 29, 2331–2336 (2015).
Quiros, P. M., Mottis, A. & Auwerx, J. Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Biol. 17, 213–226 (2016).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Herzig, S. & Shaw, R. J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 19, 121–135 (2018).
Biswas, G. et al. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. EMBO J. 18, 522–533 (1999).
Biswas, G., Anandatheerthavarada, Zaidi, M. & Avadhani, N. Mitochondria to nucleus stress signaling: a distinctive mechanism of NFkappaB/Rel activation through calcineurin-mediated inactivation of IkappaBbeta. J. Cell Biol. 161, 507–519 (2003).
Luo, Y., Bond, J. D. & Ingram, V. M. Compromised mitochondrial function leads to increased cytosolic calcium and to activation of MAP kinases. Proc. Natl Acad. Sci. USA 94, 9705–9710 (1997).
Guo, X. Y. et al. Mitochondrial stress is relayed to the cytosol by an OMA1-DELE1-HRI pathway. Nature 579, 427–432 (2020).
Fessler, E. et al. A pathway coordinated by DELE1 relays mitochondrial stress to the cytosol. Nature 579, 433–437 (2020).
Wu, W. & Papagiannakopoulos, T. The pleiotropic role of the KEAP1/NRF2 pathway in cancer. Annu. Rev. Cancer Biol. 4, 413–435 (2020).
Sporn, M. B. & Liby, K. T. NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev. Cancer 12, 564–571 (2012).
Imhoff, B. R. & Hansen, J. M. Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem. J. 424, 491–500 (2009).
Cullinan, S. B. & Diehl, J. A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 279, 20108–20117 (2004).
Guo, Z., Kozlov, S., Lavin, M., Person, M. & Paull, T. ATM activation by oxidative stress. Science 330, 517–521 (2010).
Zhang, Y. et al. Mitochondrial redox sensing by the kinase ATM maintains cellular antioxidant capacity. Sci. Signal. 11, eaaq0702 (2018).
Cambronne, X. A. & Kraus, W. L. Location, location, location: compartmentalization of NAD(+) synthesis and functions in mammalian cells. Trends Biochem. Sci. 45, 858–873 (2020).
Chang, A. Y. & Marshall, W. F. Organelles – understanding noise and heterogeneity in cell biology at an intermediate scale. J. Cell Sci. 130, 819–826 (2017).
Korolchuk, V. I. et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 13, 453–460 (2011).
Wang, X. & Schwarz, T. L. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell 136, 163–174 (2009).
Shi, X. et al. Combinatorial GxGxE CRISPR screen identifies SLC25A39 in mitochondrial glutathione transport linking iron homeostasis to OXPHOS. Nat. Commun. 13, 2483 (2022).
Ito, T. et al. Paralog knockout profiling identifies DUSP4 and DUSP6 as a digenic dependence in MAPK pathway-driven cancers. Nat. Genet. 53, 1664 (2021).
Scorrano, L. et al. Coming together to define membrane contact sites. Nat. Commun. 10, 1287 (2019).
Burgoyne, T., Patel, S. & Eden, E. R. Calcium signaling at ER membrane contact sites. Biochim. Biophys. Acta Mol. Cell Res. 1853, 2012–2017 (2015).
Tatsuta, T., Scharwey, M. & Langer, T. Mitochondrial lipid trafficking. Trends Cell Biol. 24, 44–52 (2014).
Huang, X., Jiang, C., Yu, L. & Yang, A. Current and emerging approaches for studying inter-organelle membrane contact sites. Front. Cell Dev. Biol. 8, 195 (2020).
Birchmeier, W. Orchestrating Wnt signalling for metabolic liver zonation. Nat. Cell Biol. 18, 463–465 (2016).
Garcia-Rocha, M. et al. Intracellular distribution of glycogen synthase and glycogen in primary cultured rat hepatocytes. Biochem. J. 357, 17–24 (2001).
Siess, E., Brocks, D. & Wieland, O. Distribution of metabolites between the cytosolic and mitochondrial compartments of hepatocytes isolated from fed rats. Hoppe Seylers Z. Physiol. Chem. 359, 785–798 (1978).
Chen, W. W., Freinkman, E., Wang, T., Birsoy, K. & Sabatini, D. M. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell 166, 1324–1337.e11 (2016).
Adelmann, C. H. et al. MFSD12 mediates the import of cysteine into melanosomes and lysosomes. Nature 588, 699–704 (2020).
Jordan Ray, G. et al. A PEROXO-Tag enables rapid isolation of peroxisomes from human cells. iScience 23, 101109 (2020).
Koveal, D., Diaz-Garcia, C. M. & Yellen, G. Fluorescent biosensors for neuronal metabolism and the challenges of quantitation. Curr. Opin. Neurobiol. 63, 111–121 (2020).
Zhao, Y. et al. SoNar, a highly responsive NAD+/NADH sensor, allows high-throughput metabolic screening of anti-tumor agents. Cell Metab. 21, 777–789 (2015).
Tao, R. et al. Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nat. Methods 14, 720–728 (2017).
Cracan, V., Titov, D. V., Shen, H., Grabarek, Z. & Mootha, V. K. A genetically encoded tool for manipulation of NADP(+)/NADPH in living cells. Nat. Chem. Biol. 13, 1088–1095 (2017).
Titov, D. V. et al. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 352, 231–235 (2016).
McElroy, G. et al. NAD+ regeneration rescues lifespan, but not ataxia, in a mouse model of brain mitochondrial complex I dysfunction. Cell Metab. 32, 301–308 (2020).
Seo, B. B. et al. Molecular remedy of complex I defects: rotenone-insensitive internal NADH-quinone oxidoreductase of Saccharomyces cerevisiae mitochondria restores the NADH oxidase activity of complex I-deficient mammalian cells. Proc. Natl Acad. Sci. USA 95, 9167–9171 (1998).
Steinhorn, B. et al. Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction. Nat. Commun. 9, 4044 (2018).
Pollegioni, L., Langkau, B., Tischer, W., Ghisla, S. & Pilone, M. S. Kinetic mechanism of d-amino acid oxidases from Rhodotorula gracilis and Trigonopsis variabilis. J. Biol. Chem. 268, 13850–13857 (1993).
Goodman, R. P. et al. Hepatic NADH reductive stress underlies common variation in metabolic traits. Nature 583, 122–126 (2020).
Luengo, A. et al. Increased demand for NAD(+) relative to ATP drives aerobic glycolysis. Mol. Cell 81, 691–707.e696 (2021).
Chen, T., Yavuz, A. & Wang, M. C. Dissecting lipid droplet biology with coherent Raman scattering microscopy. J. Cell Sci. 135, jcs252353 (2022).
Shirshin, E. A. et al. Label-free sensing of cells with fluorescence lifetime imaging: the quest for metabolic heterogeneity. Proc. Natl Acad. Sci. USA 119, e2118241119 (2022).
Datta, S. et al. Snx14 proximity labeling reveals a role in saturated fatty acid metabolism and ER homeostasis defective in SCAR20 disease. Proc. Natl Acad. Sci. USA 117, 33282–33294 (2020).
Bersuker, K. et al. A Proximity Labeling Strategy Provides Insights into the Composition and Dynamics of Lipid Droplet Proteomes. Dev. Cell 44, 97–112.e117 (2018).
Thomen, A. et al. Subcellular mass spectrometry imaging and absolute quantitative analysis across organelles. ACS Nano 14, 4316–4325 (2020).
Gorman, G. et al. Mitochondrial diseases. Nat. Rev. Dis. Primers 2, 16080 (2016).
Lake, N. J., Bird, M. J., Isohanni, P. & Paetau, A. Leigh syndrome: neuropathology and pathogenesis. J. Neuropathol. Exp. Neurol. 74, 482–492 (2015).
McFarland, R., Taylor, R. W. & Turnbull, D. M. A neurological perspective on mitochondrial disease. Lancet Neurol. 9, 829–840 (2010).
Bax, B. E. Mitochondrial neurogastrointestinal encephalomyopathy: approaches to diagnosis and treatment. J. Transl. Genet. Genom. 4, 1–16 (2020).
Platt, F., d'Azzo, A., Davidson, B., Neufeld, E. & Tifft, C. Lysosomal storage diseases. Nat. Rev. Dis. Primers 4, 27 (2018).
Parenti, G., Andria, G. & Ballabio, A. Lysosomal storage diseases: from pathophysiology to therapy. Annu. Rev. Med. 66, 471–486 (2015).
Shayman, J. A. ELIGLUSTAT TARTRATE: glucosylceramide synthase inhibitor treatment of type 1 Gaucher disease. Drugs Future 35, 613–620 (2010).
Fujiki, Y. et al. Recent insights into peroxisome biogenesis and associated diseases. J. Cell Sci. 133, jcs236943 (2020).
Klouwer, F. C. et al. Zellweger spectrum disorders: clinical overview and management approach. Orphanet J. Rare Dis. 10, 151 (2015).
Berendse, K. et al. Cholic acid therapy in Zellweger spectrum disorders. J. Inherit. Metab. Dis. 39, 859–868 (2016).
Lo Giudice, T., Lombardi, F., Santorelli, F. M., Kawarai, T. & Orlacchio, A. Hereditary spastic paraplegia: clinical–genetic characteristics and evolving molecular mechanisms. Exp. Neurol. 261, 518–539 (2014).
Bellofatto, M., De Michele, G., Iovino, A., Filla, A. & Santorelli, F. M. Management of hereditary spastic paraplegia: a systematic review of the literature. Front. Neurol. 10, 3 (2019).
Agarwal, A. K., Barnes, R. I. & Garg, A. Genetic basis of congenital generalized lipodystrophy. Int. J. Obes. Relat. Metab. Disord. 28, 336–339 (2004).
Herzig, S. et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science 337, 93–96 (2012).
Bricker, D. K. et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, drosophila, and humans. Science 337, 96–100 (2012).
Tavoulari, S. et al. The yeast mitochondrial pyruvate carrier is a hetero-dimer in its functional state. EMBO J. 38, e100785 (2019).
Papa, S., Francavilla, A., Paradies, G. & Meduri, B. The transport of pyruvate in rat liver mitochondria. FEBS Lett. 12, 285–288 (1971).
Muoio, D. M. Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell 159, 1253–1262 (2014).
Vacanti, N. et al. The role of the mitochondrial pyruvate carrier in substrate regulation. Mol. Cell 56, 425–435 (2014).
Zhang, Y. et al. Mitochondrial pyruvate carriers are required for myocardial stress adaptation. Nat. Metab. 2, 1248–1264 (2020).
Bensard, C. L. et al. Regulation of tumor initiation by the mitochondrial pyruvate carrier. Cell Metab. 31, 284–300.e287 (2020).
Sharma, A. et al. Impaired skeletal muscle mitochondrial pyruvate uptake rewires glucose metabolism to drive whole-body leanness. eLife 8, e45873 (2019).
De La Rossa, A. et al. Paradoxical neuronal hyperexcitability in a mouse model of mitochondrial pyruvate import deficiency. eLife 11, e72595 (2022).
Stein, L. R. & Imai, S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol. Metab. 23, 420–428 (2012).
Luongo, T. S. et al. SLC25A51 is a mammalian mitochondrial NAD+ transporter. Nature 588, 174–179 (2020).
Kory, N. et al. MCART1/SLC25A51 is required for mitochondrial NAD transport. Sci. Adv. 6, eabe5310 (2020).
Girardi, E. et al. Epistasis-driven identification of SLC25A51 as a regulator of human mitochondrial NAD import. Nat. Commun. 11, 6145 (2020).
Cambronne, X. A. et al. Biosensor reveals multiple sources for mitochondrial NAD+.Science 352, 1474–1477 (2016).
Pittelli, M. et al. Inhibition of nicotinamide phosphoribosyltransferase: cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J. Biol. Chem. 285, 34106–34114 (2010).
Bai, L. et al. Overexpression of SLC25A51 promotes hepatocellular carcinoma progression by driving aerobic glycolysis through activation of SIRT5. Free Radic. Biol. Med. 182, 11–22 (2022).
Fu, Z. et al. The mitochondrial NAD+ transporter SLC25A51 is a fasting-induced gene affecting SIRT3 functions. Metabolism 135, 155275 (2022).
Wang, Y. et al. SLC25A39 is necessary for mitochondrial glutathione import in mammalian cells. Nature 599, 136–140 (2021).
Rouault, T. A. & Tong, W. H. Iron–sulfur cluster biogenesis and human disease. Trends Genet. 24, 398–407 (2008).
Schulz, J., Lindenau, J., Seyfried, J. & Dichgans, J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 267, 4904–4911 (2000).
Alkazemi, D., Rahman, A. & Habra, B. Alterations in glutathione redox homeostasis among adolescents with obesity and anemia. Sci. Rep. 11, 3034 (2021).
Slabbaert, J. R. et al. Shawn, the Drosophila homolog of SLC25A39/40, is a mitochondrial carrier that promotes neuronal survival. J. Neurosci. 36, 1914–1929 (2016).
Acknowledgements
We thank M. Abu-Remaileh, H. Adelman, W. Chen, S. Hui, D. Lamming, B. Manning, D. Sabatini and T. Walther for comments and suggestions. We thank J. Gosse for editing. We apologize to our colleagues in the field for not being able to cite important contributions owing to space limitations.
Author information
Authors and Affiliations
Contributions
N.K. and L.B.-P. conceived and prepared the original draft and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
N.K. is funded by National Institutes of Health (NIH) R00 CA241332 and the Damon Runyon Cancer Research Foundation (73-22). L.B.-P. is funded by the Damon Runyon Cancer Research Foundation (62-20), the American Association for Cancer Research (19-20-45-BARP), the American Cancer Society, the Melanoma Research Alliance, the Ludwig Cancer Center of Harvard Medical School, Lungevity, ALK Positive, V-Foundation, Mary Kay Foundation, Paula and Rodger Riney Foundation and the NIH/National Cancer Institute (1R21CA226082-01, R37CA260062). L.B-P. is a founder and consultant of and holds privately held equity in Scorpion Therapeutics.
Peer review
Peer review information
Nature Metabolism thanks Giuseppe Fiermonte and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bar-Peled, L., Kory, N. Principles and functions of metabolic compartmentalization. Nat Metab 4, 1232–1244 (2022). https://doi.org/10.1038/s42255-022-00645-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00645-2
This article is cited by
-
Metabolic rewiring of macrophages by epidermal-derived lactate promotes sterile inflammation in the murine skin
The EMBO Journal (2024)
-
Regulators of mitonuclear balance link mitochondrial metabolism to mtDNA expression
Nature Cell Biology (2023)
-
Functional-metabolic coupling in distinct renal cell types coordinates organ-wide physiology and delays premature ageing
Nature Communications (2023)
-
Energiemetabolismus des Immunsystems
Zeitschrift für Rheumatologie (2023)